Loteprednol: Difference between revisions
m →Further reading: Journal cites, Added 1 doi to a journal cite |
Removed biased statement left uncited for over a year. Removed biased statement cited only with information from the drug manufacturer. DO NOT REVERT WITHOUT PROPER CITATION. |
||
| Line 69: | Line 69: | ||
==Adverse effects== |
==Adverse effects== |
||
Common adverse effects include foreign body sensation in the eye, dry eye and [[epiphora (medicine)|epiphora]] (overflow of tears), [[chemosis]] (swelling of the [[conjunctiva]]), headache, and itching. |
Common adverse effects include foreign body sensation in the eye, dry eye and [[epiphora (medicine)|epiphora]] (overflow of tears), [[chemosis]] (swelling of the [[conjunctiva]]), headache, and itching. |
||
==Interactions== |
==Interactions== |
||
| Line 103: | Line 103: | ||
==References== |
==References== |
||
{{reflist|refs= |
{{reflist|refs= |
||
<ref name="Drugs.com">Loteprednol {{Drugs.com|ppa|loteprednol}}.</ref> |
|||
<ref name="AC">{{cite book|title=Austria-Codex|editor=Haberfeld, H.|publisher=Österreichischer Apothekerverlag|location=Vienna|year=2015|language=German}}</ref> |
<ref name="AC">{{cite book|title=Austria-Codex|editor=Haberfeld, H.|publisher=Österreichischer Apothekerverlag|location=Vienna|year=2015|language=German}}</ref> |
||
<ref name="Dinnendahl">{{cite book|title=Arzneistoff-Profile|editor1=Dinnendahl, V.|editor2=Fricke, U.|publisher=Govi Pharmazeutischer Verlag|location=Eschborn, Germany|date=2008|edition=22|volume=6|isbn=978-3-7741-9846-3|language=German}}</ref> |
|||
<ref name="Druzgala">{{cite journal |author1=Druzgala, P. |author2=Hochhaus, G. |author3=Bodor, N. | title = Soft drugs—10. Blanching activity and receptor binding affinity of a new type of glucocorticoid: Loteprednol etabonate | journal = J. Steroid Biochem. Mol. Biol. | date = 1991 | volume = 38 | issue = 2 | pages = 149–54 | doi = 10.1016/0960-0760(91)90120-T| pmid = 2004037 }}</ref> |
<ref name="Druzgala">{{cite journal |author1=Druzgala, P. |author2=Hochhaus, G. |author3=Bodor, N. | title = Soft drugs—10. Blanching activity and receptor binding affinity of a new type of glucocorticoid: Loteprednol etabonate | journal = J. Steroid Biochem. Mol. Biol. | date = 1991 | volume = 38 | issue = 2 | pages = 149–54 | doi = 10.1016/0960-0760(91)90120-T| pmid = 2004037 }}</ref> |
||
<ref name="Dekker">{{cite book|title=Inhaled Steroids in Asthma. Optimizing Effects in the Airways|year=2002|publisher=Marcel Dekker, New York|pages=541–564|author=Bodor, N.|chapter=Design and development of a soft corticosteroid, loteprednol etabonate|author2=Buchwald, P. |editor=Schleimer, R.P. |editor2=O'Byrne, P.M. |editor3=Szefler, S.J. |editor4=Brattsand, R.|work=Lung Biology in Health and Disease, Vol. 163}}</ref> |
<ref name="Dekker">{{cite book|title=Inhaled Steroids in Asthma. Optimizing Effects in the Airways|year=2002|publisher=Marcel Dekker, New York|pages=541–564|author=Bodor, N.|chapter=Design and development of a soft corticosteroid, loteprednol etabonate|author2=Buchwald, P. |editor=Schleimer, R.P. |editor2=O'Byrne, P.M. |editor3=Szefler, S.J. |editor4=Brattsand, R.|work=Lung Biology in Health and Disease, Vol. 163}}</ref> |
||
Revision as of 12:58, 4 October 2018
 | |
| Clinical data | |
|---|---|
| Trade names | Lotemax |
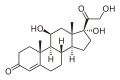
| Other names | 11β,17α,Dihydroxy-21-oxa-21-chloromethylpregna-1,4-diene-3,20-dione 17α-ethylcarbonate |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | Eye drops |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | None |
| Protein binding | 95% |
| Metabolism | Ester hydrolysis |
| Metabolites | Δ1-cortienic acid and its etabonate |
| Onset of action | ≤2 hrs (allergic conjunctivitis) |
| Elimination half-life | 2.8 hrs |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.167.120 |
| Chemical and physical data | |
| Formula | C24H31ClO7 |
| Molar mass | 466.951 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 220.5 to 223.5 °C (428.9 to 434.3 °F) |
| Solubility in water | 0.0005 mg/mL (20 °C) |
| |
| |
| | |
Loteprednol (as the ester loteprednol etabonate) is a corticosteroid used to treat inflammations of the eye. It is marketed by Bausch and Lomb as Lotemax[1] and Loterex.
Medical uses
Applications for this drug include the reduction of inflammation after eye surgery,[1] seasonal allergic conjunctivitis, uveitis,[2] as well as chronic forms of keratitis (e.g. adenoviral and Thygeson's keratitis), vernal keratoconjunctivitis, pingueculitis, and episcleritis.[citation needed]
Contraindications
As corticosteroids are immunosuppressive, loteprednol is contraindicated in patients with viral, fungal or mycobacterial infections of the eye.[1][2][3]
Adverse effects
Common adverse effects include foreign body sensation in the eye, dry eye and epiphora (overflow of tears), chemosis (swelling of the conjunctiva), headache, and itching.
Interactions
The effect of drugs lowering intraocular pressure may be reduced. Loteprednol is not detectable in the bloodstream; so interactions with systemic drugs are highly unlikely.[1]
Pharmacology
Mechanism of action
Pharmacokinetics
Neither loteprednol etabonate nor its inactive metabolites Δ1-cortienic acid and Δ1-cortienic acid etabonate are detectable in the bloodstream, even after oral administration. A study with patients receiving loteprednol eye drops over 42 days showed no adrenal suppression, which would be a sign of the drug reaching the bloodstream to a clinically relevant extent.[1]
Steroid receptor affinity was 4.3 times that of dexamethasone in animal studies.[1]
Retrometabolic drug design
Loteprednol etabonate was developed using retrometabolic drug design. It is a so-called soft drug, meaning its structure was designed so that it is predictably metabolised to inactive substances. These metabolites, Δ1-cortienic acid and its etabonate, are derivatives of cortienic acid, itself an inactive metabolite of hydrocortisone.[1][3][4]
-
Δ1-Cortienic acid, inactive metabolite of loteprednol
-
Cortienic acid, inactive metabolite of hydrocortisone
-
Hydrocortisone, the "parent compound" of corticosteroids
Chemistry
Loteprednol etabonate is an ester of loteprednol with etabonate (ethyl carbonate), with a melting point between 220.5 °C (428.9 °F) and 223.5 °C (434.3 °F). Its solubility in water is 1:2,000,000.[3] The ketone in the side chain of classical corticosteroids such as hydrocortisone is replaced by a cleavable ester, which accounts for the rapid inactivation.[5] (This is not the same as the etabonate ester.)
Chemical synthesis
This section needs expansion. You can help by adding to it. (June 2016) |
References
- ^ a b c d e f g Haberfeld, H., ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
- ^ a b Cite error: The named reference
Drugs.comwas invoked but never defined (see the help page). - ^ a b c Cite error: The named reference
Dinnendahlwas invoked but never defined (see the help page). - ^ Bodor, N.; Buchwald, P. (2002). "Design and development of a soft corticosteroid, loteprednol etabonate". In Schleimer, R.P.; O'Byrne, P.M.; Szefler, S.J.; Brattsand, R. (eds.). Inhaled Steroids in Asthma. Optimizing Effects in the Airways. Marcel Dekker, New York. pp. 541–564.
{{cite book}}:|work=ignored (help) - ^ Pavesio, C.E.; Decory, H.H. (2008). "Treatment of ocular inflammatory conditions with loteprednol etabonate". Br J Ophthalmol. 92 (4): 455–459. doi:10.1136/bjo.2007.132621. PMID 18245274.
- ^ Druzgala, P.; Hochhaus, G.; Bodor, N. (1991). "Soft drugs—10. Blanching activity and receptor binding affinity of a new type of glucocorticoid: Loteprednol etabonate". J. Steroid Biochem. Mol. Biol. 38 (2): 149–54. doi:10.1016/0960-0760(91)90120-T. PMID 2004037.
Further reading
- Steward, R.; et al. (November 1998). "Double-masked, placebo-controlled evaluation of loteprednol etabonate 0.5% for postoperative inflammation. Loteprednol Etabonate Post-operative Inflammation Study Group 1". J Cataract Refract Surg. 24 (11): 1480–1489. doi:10.1016/s0886-3350(98)80170-3. PMID 9818338.