Fluorouracil: Difference between revisions
moving to talk |
|||
| Line 174: | Line 174: | ||
== External links == |
== External links == |
||
*[https://www.ncbi.nlm.nih.gov/books/NBK395610/ U.S. National Center for Biotechnology Information: Medical Genetics Summaries - Fluorouracil Therapy and DPYD Genotype] |
|||
*{{cite journal|last1=Latchman|first1=Jessica|last2=Guastella|first2=Ann|last3=Tofthagen|first3=Cindy|title=5-Fluorouracil Toxicity and Dihydropyrimidine Dehydrogenase Enzyme: Implications for Practice|journal=[[Clinical Journal of Oncology Nursing]].|date=1 October 2014|volume=18|issue=5|pages=581–585|doi=10.1188/14.CJON.581-585}} |
*{{cite journal|last1=Latchman|first1=Jessica|last2=Guastella|first2=Ann|last3=Tofthagen|first3=Cindy|title=5-Fluorouracil Toxicity and Dihydropyrimidine Dehydrogenase Enzyme: Implications for Practice|journal=[[Clinical Journal of Oncology Nursing]].|date=1 October 2014|volume=18|issue=5|pages=581–585|doi=10.1188/14.CJON.581-585}} |
||
Revision as of 09:34, 17 January 2019
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌflʊəroʊˈjʊərəsɪl/[1] |
| Trade names | Adrucil, Carac, Efudex, Efudix, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682708 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | IV (infusion or bolus) and topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 28 to 100% |
| Protein binding | 8 to 12% |
| Metabolism | Intracellular and liver (CYP-mediated) |
| Elimination half-life | 16 minutes |
| Excretion | kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.078 |
| Chemical and physical data | |
| Formula | C4H3FN2O2 |
| Molar mass | 130.077 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 282–283 °C (540–541 °F) |
| |
| |
| (verify) | |
Fluorouracil (5-FU), sold under the brand name Adrucil among others, is a medication used to treat cancer.[2] By injection into a vein it is used for colon cancer, esophageal cancer, stomach cancer, pancreatic cancer, breast cancer, and cervical cancer.[2] As a cream it is used for actinic keratosis, basal cell carcinoma, and skin warts.[3][4]
When used by injection most people develop side effects.[2] Common side effects include inflammation of the mouth, loss of appetite, low blood cell counts, hair loss, and inflammation of the skin.[2] When used as a cream, irritation at the site of application may occur.[3] Use of either form in pregnancy may harm the baby.[2] Fluorouracil is in the antimetabolite and pyrimidine analog families of medications.[5][6] How it works is not entirely clear but believed to involve blocking the action of thymidylate synthase and thus stopping the production of DNA.[2]
Fluorouracil was patented in 1956 and came into medical use in 1962.[7] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[8] The wholesale cost in the developing world is about US$1.18–3.40 per 500 mg vial.[9] In the United Kingdom this amount costs the NHS about 6.40 pounds.[5] In the United States it costs about $18.71.[10]
Medical uses
Fluorouracil has been given systemically for anal, breast, colorectal, oesophageal, stomach, pancreatic and skin cancers (especially head and neck cancers).[11] It has also been given topically (on the skin) for actinic keratoses, skin cancers and Bowen's disease[11] and as eye drops for treatment of ocular surface squamous neoplasia.[12] Other uses include ocular injections into a previously created trabeculectomy bleb to inhibit healing and cause scarring of tissue, thus allowing adequate aqueous humor flow to reduce intraocular pressure.
Contraindications
It is contraindicated in patients that are severely debilitated or in patients with bone marrow suppression due to either radiotherapy or chemotherapy.[13] It is likewise contraindicated in pregnant or breastfeeding women.[13] It should also be avoided in patients that do not have malignant illnesses.[13]
Adverse effects
Adverse effects by frequency include:[11][13][14][15][16][17][18][19][20]
During systemic use
Common (> 1% frequency):
- Nausea
- Vomiting
- Diarrhea (see below for details)
- Mucositis
- Headache
- Myelosuppression (see below for details)
- Alopecia (hair loss)
- Photosensitivity
- Hand-foot syndrome
- Maculopapular eruption
- Itch
- Cardiotoxicity (see below for details)
- Persistent hiccups
- Mood disorders (irritability, anxiety, depression)
Uncommon (0.1–1% frequency):
- Oesophagitis
- GI ulceration and bleeding
- Proctitis
- Nail disorders
- Vein pigmentation
- Confusion
- Cerebellar syndrome
- Encephalopathy
- Visual changes
- Photophobia
- Lacrimation (the expulsion of tears without any emotional or physiologic reason)
Rare (< 0.1% frequency):
- Anaphylaxis
- Allergic reactions
- Fever without signs of infection
Diarrhea is severe and may be dose-limiting and is exacerbated by co-treatment with calcium folinate.[11] Neutropenia tends to peak about 9–14 days after beginning treatment.[11] Thrombocytopenia tends to peak about 7–17 days after the beginning of treatment and tends to recover about 10 days after its peak.[11] Cardiotoxicity is a fairly common side effect, but usually this cardiotoxicity is just angina or symptoms associated with coronary artery spasm, but in about 0.55% of those receiving the drug will develop life-threatening cardiotoxicity.[21] Life-threatening cardiotoxicity includes: arrhythmias, ventricular tachycardia and cardiac arrest, secondary to transmural ischaemia.[21]
During topical use
Common (> 1% frequency):
- Local pain
- Itchiness
- Burning
- Stinging
- Crusting
- Weeping
- Dermatitis
- Photosensitivity
Uncommon (0.1–1% frequency):
- hyper- or hypopigmentation
- Scarring
Neurological damage
5-FU injection and topical application, even in small doses, cause both acute central nervous system (CNS) damage and progressively worsening delayed degeneration of the CNS in mice. This latter effect is caused by 5-FU-induced damage to the oligodendrocytes that produce the insulating myelin sheaths. [22] [23]
The United States package insert warns that acute cerebellar syndrome has been observed following injection of fluorouracil and may persist after cessation of treatment. Symptoms include ataxia, nystagmus, and dysmetria.[24]
Potential overdose
There is very little difference between the minimum effective dose and maximum tolerated dose of 5-FU, and the drug exhibits marked individual pharmacokinetic variability.[25][26][27] Therefore, an identical dose of 5-FU may result in a therapeutic response with acceptable toxicity in some patients and unacceptable and possibly life-threatening toxicity in others.[25] Both overdosing and underdosing are of concern with 5-FU, although several studies have shown that the majority of colorectal cancer patients treated with 5-FU are underdosed based on today's dosing standard, body surface area (BSA).[28][29][30][31] The limitations of BSA-based dosing prevent oncologists from being able to accurately titer the dosage of 5-FU for the majority of individual patients, which results in sub-optimal treatment efficacy or excessive toxicity.[28][29]
Numerous studies have found significant relationships between concentrations of 5-FU in blood plasma and both desirable or undesirable effects on patients.[32][33] Studies have also shown that dosing based on the concentration of 5-FU in plasma can greatly increase desirable outcomes while minimizing negative side effects of 5-FU therapy.[28][34] One such test that has been shown to successfully monitor 5-FU plasma levels and which "may contribute to improved efficacy and safety of commonly used 5-FU-based chemotherapies" is the My5-FU test.[30][35][36]
Interactions
Its use should be avoided in patients receiving drugs known to modulate dihydropyrimidine dehydrogenase (such as the antiviral drug sorivudine).[13] It may also increase the INR and prothrombin times in patients on warfarin.[13] Fluorouracil's efficacy is decreased when used alongside allopurinol, which can be used to decrease fluorouracil induced stomatitis through use of allopurinol mouthwash.[37]
Pharmacology
Pharmacogenetics
The dihydropyrimidine dehydrogenase (DPD) enzyme is responsible for the detoxifying metabolism of fluoropyrimidines, a class of drugs that includes 5-fluorouracil, capecitabine, and tegafur.[38] Genetic variations within the DPD gene (DPYD) can lead to reduced or absent DPD activity, and individuals who are heterozygous or homozygous for these variations may have partial or complete DPD deficiency; an estimated 0.2% of individuals have complete DPD deficiency.[38][39] Those with partial or complete DPD deficiency have a significantly increased risk of severe or even fatal drug toxicities when treated with fluoropyrimidines; examples of toxicities include myelosuppression, neurotoxicity and hand-foot syndrome.[38][39]
Mechanism of action
5-FU acts in several ways, but principally as a thymidylate synthase (TS) inhibitor. Interrupting the action of this enzyme blocks synthesis of the pyrimidine thymidine, which is a nucleoside required for DNA replication. Thymidylate synthase methylates deoxyuridine monophosphate (dUMP) to form thymidine monophosphate (dTMP). Administration of 5-FU causes a scarcity in dTMP, so rapidly dividing cancerous cells undergo cell death via thymineless death.[40] Calcium folinate provides an exogenous source of reduced folinates and hence stabilises the 5-FU-TS complex, hence enhancing 5-FU's cytotoxicity.[41]
History
In 1954 Abraham Cantarow and Karl Paschkis found liver tumors absorbed radioactive uracil more readily than normal liver cells. Charles Heidelberger, who had earlier found that fluorine in fluoroacetic acid inhibited a vital enzyme, asked Robert Duschinsky and Robert Schnitzer at Hoffman-La Roche to synthesize fluorouracil.[42] Some credit Heidelberger and Duschinsky with the discovery that 5-fluorouracil markedly inhibited tumors in mice.[43] The original 1957 report[44][45] in Nature has Heidelberger as lead author, along with N. K. Chaudhuri, Peter Danneberg, Dorothy Mooren, Louis Griesbach, Robert Duschinsky, R. J. Schnitzer, E. Pleven, and J. Scheiner.[46]
Natural analogues
In 2003 scientists isolated 5-Fluorouracil derivatives, closely related compounds, from the marine sponge, Phakellia fusca collected around the Yongxing Island of the Xisha Islands in the South China Sea. This is significant because fluorine-containing organic compounds exist only rarely in nature, and also because manmade anticancer drugs are not frequently found to have analogues in nature.[47]
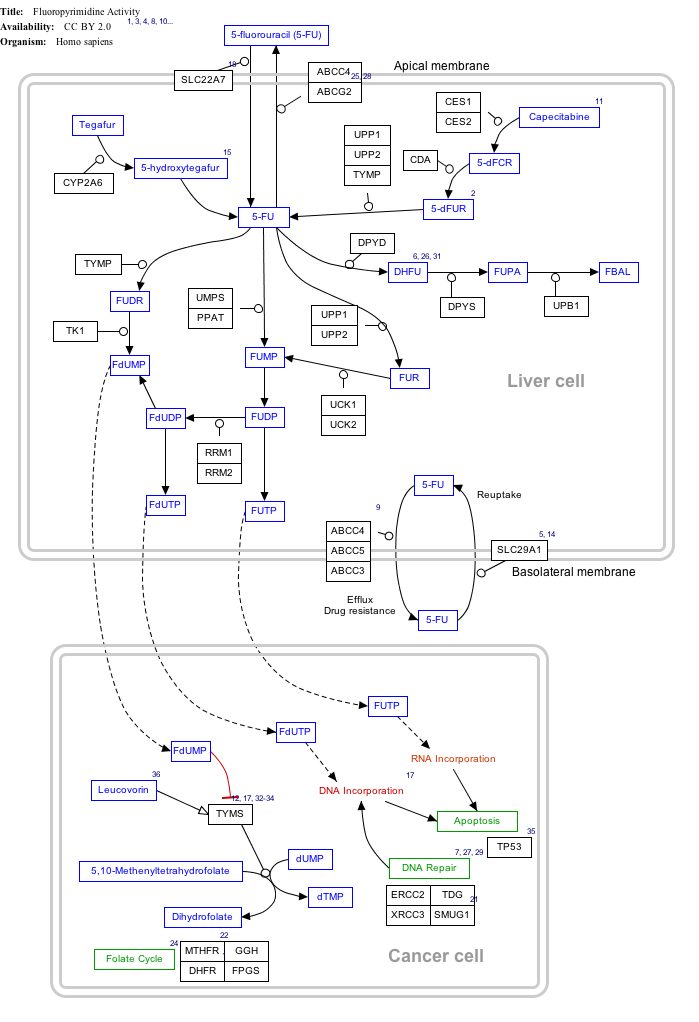
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.[§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "FluoropyrimidineActivity_WP1601".
Names
The name "fluorouracil" is the INN, USAN, USP name, and BAN. The form "5-fluorouracil" is often used; it shows that there is a fluorine atom on the 5th carbon of a uracil ring.
References
- ^ "Fluorouracil – Definition and More from the Free Merriam-Webster Dictionary". Archived from the original on 2014-11-29. Retrieved 2014-11-19.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c d e f "Fluorouracil". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b "Fluorouracil topical". The American Society of Health-System Pharmacists. Archived from the original on 26 December 2016. Retrieved 8 December 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Moore, AY (2009). "Clinical applications for topical 5-fluorouracil in the treatment of dermatological disorders". The Journal of dermatological treatment. 20 (6): 328–35. doi:10.3109/09546630902789326. PMID 19954388.
- ^ a b British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 590. ISBN 9780857111562.
- ^ Airley, Rachel (2009). Cancer Chemotherapy: Basic Science to the Clinic. John Wiley & Sons. p. 76. ISBN 9780470092569. Archived from the original on 2017-11-05.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 511. ISBN 9783527607495. Archived from the original on 2017-09-11.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived from the original (PDF) on 13 December 2016. Retrieved 8 December 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Fluorouracil". International Drug Price Indicator Guide. Retrieved 8 December 2016.
- ^ "Fluorouracil Prices, Coupons & Patient Assistance Programs - Drugs.com". Drugs.com. Archived from the original on 5 November 2017. Retrieved 27 June 2017.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c d e f g Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ^ Joag, Madhura G.; Sise, Adam; Murillo, Juan Carlos; Sayed-Ahmed, Ibrahim Osama; Wong, James R.; Mercado, Carolina; Galor, Anat; Karp, Carol L. (2016-03-27). "Topical 5-Fluorouracil 1% as Primary Treatment for Ocular Surface Squamous Neoplasia". Ophthalmology. doi:10.1016/j.ophtha.2016.02.034. ISSN 1549-4713. PMID 27030104.
- ^ a b c d e f "Fluorouracil 50 mg/ml Injection – Summary of Product Characteristics". electronic Medicines Compendium. Hospira UK Ltd. 24 August 2011. Archived from the original on 1 February 2014. Retrieved 24 January 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "DBL Fluorouracil Injection BP". TGA eBusiness Services. Hospira Australia Pty Ltd. 21 June 2012. Archived from the original (PDF) on 28 January 2017. Retrieved 24 January 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "ADRUCIL (fluorouracil) injection [Teva Parenteral Medicines, Inc.]". DailyMed. Teva Parenteral Medicines, Inc. August 2012. Archived from the original on 1 February 2014. Retrieved 24 January 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b "Efudex, Carac (fluorouracil topical) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 2 February 2014. Retrieved 24 January 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Adrucil (fluorouracil) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 2 February 2014. Retrieved 24 January 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Ha, JH; Hwang, DY; Yu, J; Park, DH; Ryu, SH (2011). "Onset of Manic Episode during Chemotherapy with 5-Fluorouracil". Psychiatry Investig. 8: 71–3. doi:10.4306/pi.2011.8.1.71. PMC 3079190. PMID 21519541.
- ^ Park H. J., Choi Y. T., Kim I. H., Hah J. C.; A case of reversible dementia associated with depression in a patient on 5-FU or its analogue drugs. J. Korean Neuropsychiatr. Assoc. 1987;30:199–202.
- ^ MedsFacts meta-analysis covering adverse side effect reports of 5fu(fluorouracil) patients who developed hiccups Archived 2014-09-05 at the Wayback Machine at MedsFact, 2013
- ^ a b Brayfield, A, ed. (9 January 2017). "Fluorouracil: Martindale: The Complete Drug Reference". MedicinesComplete. London, UK: Pharmaceutical Press. Retrieved 14 August 2017.
- ^ "Researchers Detail Chemotherapy's Damage to the Brain". Archived from the original on 2014-08-14.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Common Cancer Treatments Toxic to Healthy Brain Cells". Archived from the original on 2014-08-14.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Adrucil (Fluorouracil) Injection [TEVA Parenteral Medicines, Inc.]". Archived from the original on 2014-08-14.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Gamelin, E.; Boisdron-Celle, M. (1999). "Dose monitoring of 5-fluorouracil in patients with colorectal or head and neck cancer—status of the art". Critical Reviews in Oncology/Hematology. 30 (1): 71–79. doi:10.1016/s1040-8428(98)00036-5.
- ^ Felici A.; J. Verweij; Sparreboom A. (2002). "Dosing strategies for anticancer drugs: the good, the bad and body-surface area". Eur J Cancer. 38 (13): 1677–84. doi:10.1016/s0959-8049(02)00151-x.
- ^ Baker S. D.; Verweij J.; Rowinsky E. K.; Donehower R. C.; Schellens J. H.; Grochow L. B.; Sparreboom A. (2002). "Role of body surface area in dosing of investigational anticancer agents in adults, 1991–2001". J Natl Cancer Inst. 94 (24): 1883–8. doi:10.1093/jnci/94.24.1883.
- ^ a b c Capitain O.; Asevoaia A.; Boisdron-Celle M.; Poirier A. L.; Morel A.; Gamelin E. (2012). "Individual Fluorouracil Dose Adjustment in FOLFOX Based on Pharmacokinetic Follow-Up Compared With Conventional Body-Area-Surface Dosing: A Phase II, Proof-of-Concept Study". Clin. Colorectal Cancer. 11 (4): 263–267. doi:10.1016/j.clcc.2012.05.004.
- ^ a b Saam, J; Critchfield G. C.; Hamilton S. A.; Roa B. B.; Wenstrup R. J.; Kaldate R. R. (2011). "Body surface area-based dosing of 5-fluoruracil results in extensive interindividual variability in 5-fluorouracil exposure in colorectal cancer patients on FOLFOX regimens". Clin Colorectal Cancer. 10 (3): 203–6. doi:10.1016/j.clcc.2011.03.015.
- ^ a b Beumer J. H.; Boisdron-Celle M.; Clarke W.; Courtney J. B.; Egorin M. J.; Gamelin E; Harney R. L.; Hammett-Stabler C; Lepp S.; Li Y.; Lundell G. D.; McMillin G.; Milano G.; Salamone S. J. (2009). "Multicenter evaluation of a novel nanoparticle immunoassay for 5-fluorouracil on the Olympus AU400 analyzer". Ther. Drug Monit. 31 (6): 688–94.
- ^ Goldberg R. M.; Rothenberg M. L.; Van Cutsem E.; Benson A. B. 3rd; Blanke C. D.; Diasio R. B.; Grothey A.; Lenz H. J.; Meropol N. J.; Ramanathan R. K.; Becerra C. H.; Wickham R.; Armstrong D.; Viele C. (2007). "The Continuum of Care: A Paradigm for the Management of Metastatic Colorectal Cancer". The Oncologist. 12: 38–50. doi:10.1634/theoncologist.12-1-38.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ Ploylearmsaeng, S.-a.; Fuhr U.; Jetter A. (2006). "How may Anticancer Chemotherapy with Fluorouracil be Individualised?". Clin Pharmacokinet. 45 (6): 567–92. doi:10.2165/00003088-200645060-00002.
- ^ van Kuilenburg, A. B.; Maring J. G. (2013). "Evaluation of 5-fluorouracil pharmacokinetic models and therapeutic drug monitoring in cancer patients". Pharmacogenomics. 14 (7): 799–811. doi:10.2217/pgs.13.54.
- ^ Gamelin E. C.; Delva R.; Jacob J.; Merrouche Y; Raoul J. L.; Pezet D.; Dorval E.; Piot G.; Morel A.; Boisdron-Celle M. (2008). "Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: Results of a multicenter randomized trial of patients with metastatic colorectal cancer". J. Clin. Oncol. 26 (13): 2099–2105. doi:10.1200/jco.2007.13.3934. PMID 18445839.
- ^ "Customizing Chemotherapy for Better Cancer Care". MyCare Diagnostics. Archived from the original on 2014-04-28.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "A Brief History of BSA Dosing". MyCare Diagnostics. Archived from the original on 2014-04-28.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Porta C, Moroni M, Nastasi G (1994). "Allopurinol mouthwashes in the treatment of 5-fluorouracil-induced stomatitis". Am. J. Clin. Oncol. 17 (3): 246–7. doi:10.1097/00000421-199406000-00014. PMID 8192112.
- ^ a b c Caudle, KE; Thorn, CF; Klein, TE; Swen, JJ; McLeod, HL; Diasio, RB; Schwab, M (December 2013). "Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing". Clinical Pharmacology and Therapeutics. 94 (6): 640–5. doi:10.1038/clpt.2013.172. PMC 3831181. PMID 23988873.
- ^ a b Amstutz, U; Froehlich, TK; Largiadèr, CR (September 2011). "Dihydropyrimidine dehydrogenase gene as a major predictor of severe 5-fluorouracil toxicity". Pharmacogenomics. 12 (9): 1321–36. doi:10.2217/pgs.11.72. PMID 21919607.
- ^ Longley D. B.; Harkin D. P.; Johnston P. G. (May 2003). "5-fluorouracil: mechanisms of action and clinical strategies". Nat. Rev. Cancer. 3 (5): 330–8. doi:10.1038/nrc1074. PMID 12724731. Archived from the original on 2011-05-14.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Álvarez, P.; Marchal, J. A.; Boulaiz, H.; Carrillo, E.; Vélez, C.; Rodríguez-Serrano, F.; Melguizo, C.; Prados, J.; Madeddu, R.; Aranega, A. (February 2012). "5-Fluorouracil derivatives: a patent review". Expert Opinion on Therapeutic Patents. 22 (2): 107–123. doi:10.1517/13543776.2012.661413. PMID 22329541.
- ^ Sneader W. (2005). Drug Discovery, p. 255.
- ^ Cohen, Seymour (30 January 2008). "50 years ago in cell biology: A virologist recalls his work on cell growth inhibition". The Scientist. Archived from the original on 19 September 2010.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Chu E (September 2007). "Ode to 5-Fluorouracil". Clinical Colorectal Cancer. 6 (9): 609. doi:10.3816/CCC.2007.n.029. Archived from the original on 2012-07-14.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ National Academy of Sciences, Biographical Memoirs,80:135
- ^ Heidelberger C.; Chaudhuri N. K.; Danneberg P.; et al. (March 1957). "Fluorinated pyrimidines, a new class of tumour-inhibitory compounds". Nature. 179 (4561): 663–6. doi:10.1038/179663a0. PMID 13418758.
- ^ Xu, Xiao-Hua; Yao, Guang-Min; Li, Yan-Ming; Lu, Jian-Hua; Lin, Chang-Jiang; Wang, Xin; Kong, Chui-Hua (2003-02-01). "5-Fluorouracil derivatives from the sponge Phakellia fusca". Journal of Natural Products. 66 (2): 285–288. doi:10.1021/np020034f. ISSN 0163-3864. PMID 12608868.
External links
- U.S. National Center for Biotechnology Information: Medical Genetics Summaries - Fluorouracil Therapy and DPYD Genotype
- Latchman, Jessica; Guastella, Ann; Tofthagen, Cindy (1 October 2014). "5-Fluorouracil Toxicity and Dihydropyrimidine Dehydrogenase Enzyme: Implications for Practice". Clinical Journal of Oncology Nursing. 18 (5): 581–585. doi:10.1188/14.CJON.581-585.