Chromium(III) acetylacetonate: Difference between revisions
removed Category:Chromium compounds; added Category:Chromium complexes using HotCat |
IUPAC name was incorrect |
||
| Line 9: | Line 9: | ||
| ImageAlt1 = Solid Chromium (III) acetylacetonate |
| ImageAlt1 = Solid Chromium (III) acetylacetonate |
||
| ImageCaption1 = Solid chromium(III) acetylacetonate |
| ImageCaption1 = Solid chromium(III) acetylacetonate |
||
| IUPACName = |
| IUPACName = Tris(acetylacetonato)chromium(III) |
||
| OtherNames = Tris(2,4-pentanediono)chromium(III), Cr(acac)<sub>3</sub>, Cr(pd)<sub>3</sub> |
| OtherNames = Tris(2,4-pentanediono)chromium(III), Cr(acac)<sub>3</sub>, Cr(pd)<sub>3</sub> |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
Revision as of 12:37, 7 March 2019
| |
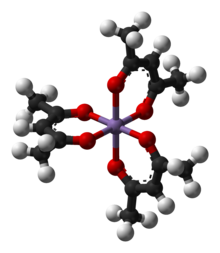
 Solid chromium(III) acetylacetonate
| |
| Names | |
|---|---|
| IUPAC name
Tris(acetylacetonato)chromium(III)
| |
| Other names
Tris(2,4-pentanediono)chromium(III), Cr(acac)3, Cr(pd)3
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.040.463 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Cr(C5H7O2)3 | |
| Molar mass | 349.32 |
| Appearance | deep maroon |
| Density | 1.34 g/cm3 |
| Melting point | 210 °C (410 °F; 483 K) |
| Boiling point | 340 °C (644 °F; 613 K) (sublimes near 100 °C) |
| Solubility in non-polar organic solvents | soluble |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chromium(III) acetylacetonate is the coordination compound with the formula Cr(C5H7O2)3, sometimes designated as Cr(acac)3. This purplish coordination complex is used in NMR spectroscopy as a relaxation agent because of its solubility in nonpolar organic solvents and its paramagnetism.
Synthesis and structure
The compound is prepared by the reaction of chromium(III) oxide with acetylacetone (Hacac):[2]
- Cr2O3 + 6 Hacac → 2 Cr(acac)3 + 3 H2O
The complex has idealized D3 symmetry. The Cr-O distances are 1.93 Å.[3] The complex has been resolved into individual enantiomers by separation of its adduct with dibenzoyltartrate.[4]
Like many other Cr(III) compounds, it has a quartet ground state.
The complex is relatively inert toward substitution but undergoes bromination at the 3-positions of the chelate rings.
References
- ^ Chromium acetylacetonate at American Elements
- ^ W. Conard Fernelius, Julian E. Blanch “Chromium(III) Acetylacetonate: [Tris(2,4-Pentanediono)Chromium(III)]” Inorganic Syntheses, 1957, Volume 5, 130-131.doi:10.1002/9780470132364.ch35
- ^ B. Morosin "The crystal structure of trisacetylacetonatochromium(III)" Acta Crystallogr. 1965. vol. 19, 131-137. doi:10.1107/S0365110X65002876
- ^ "The optical resolution of tris(pentane-2,4-dionato)metal(III) complexes". Polyhedron. 2: 537–538. 1983. doi:10.1016/S0277-5387(00)87108-9.
{{cite journal}}: Unknown parameter|authors=ignored (help)
