Rayon: Difference between revisions
JustAMuggle (talk | contribs) Copy edit. |
No edit summary |
||
| Line 3: | Line 3: | ||
[[File:Rayon synthesis.webm|thumb|When a solution of cellulose in cuprammonium hydroxide comes into contact with sulfuric acid, the cellulose begins to precipitate from the solution. The sulfuric acid reacts with a complex compound of copper and dissolves it. Thin blue fibers of rayon are formed. After some time, sulfuric acid reacts with the complex compound and washes out the copper salts from the fibers. The fibers become colorless.]] |
[[File:Rayon synthesis.webm|thumb|When a solution of cellulose in cuprammonium hydroxide comes into contact with sulfuric acid, the cellulose begins to precipitate from the solution. The sulfuric acid reacts with a complex compound of copper and dissolves it. Thin blue fibers of rayon are formed. After some time, sulfuric acid reacts with the complex compound and washes out the copper salts from the fibers. The fibers become colorless.]] |
||
'''Rayon''' is a manufactured [[fiber]] made from |
'''Rayon''' is a manufactured [[fiber]] made from poulomi [[cellulose fiber]]. The many types and grades of rayon can imitate the feel and texture of [[natural fiber]]s such as [[silk]], [[wool]], [[cotton]], and [[linen]]. The types that resemble silk are often called [[art silk|artificial silk]]. |
||
Since rayon is manufactured from naturally occurring [[polymer]]s, it is not considered to be synthetic<ref>{{cite journal |doi= 10.1021/ed070p887|bibcode=1993JChEd..70..887K |title=Rayon: the first semi-synthetic fiber product |year= 1993 |last1= Kauffman |first1= George B. |journal= Journal of Chemical Education|volume= 70 |issue= 11 |page= 887}}</ref>. Technically, the term [[synthetic fiber]] is reserved for [[total synthesis|fully synthetic]] fibers. In manufacturing terms, rayon is classified as "a fiber formed by regenerating natural materials into a usable form". <ref name="Swicofil">{{cite web|url=https://www.swicofil.com/commerce/products/viscose/278/introduction.html |title=Viscose CV }}</ref> Specific types of rayon include [[viscose]], [[Modal (textile)|modal]] and [[lyocell]], each of which differs in manufacturing process and properties of the finished product. |
Since rayon is manufactured from naturally occurring [[polymer]]s, it is not considered to be synthetic<ref>{{cite journal |doi= 10.1021/ed070p887|bibcode=1993JChEd..70..887K |title=Rayon: the first semi-synthetic fiber product |year= 1993 |last1= Kauffman |first1= George B. |journal= Journal of Chemical Education|volume= 70 |issue= 11 |page= 887}}</ref>. Technically, the term [[synthetic fiber]] is reserved for [[total synthesis|fully synthetic]] fibers. In manufacturing terms, rayon is classified as "a fiber formed by regenerating natural materials into a usable form". <ref name="Swicofil">{{cite web|url=https://www.swicofil.com/commerce/products/viscose/278/introduction.html |title=Viscose CV }}</ref> Specific types of rayon include [[viscose]], [[Modal (textile)|modal]] and [[lyocell]], each of which differs in manufacturing process and properties of the finished product. |
||
Revision as of 05:04, 26 March 2019
This article needs additional citations for verification. (March 2019) |
Rayon is a manufactured fiber made from poulomi cellulose fiber. The many types and grades of rayon can imitate the feel and texture of natural fibers such as silk, wool, cotton, and linen. The types that resemble silk are often called artificial silk.
Since rayon is manufactured from naturally occurring polymers, it is not considered to be synthetic[1]. Technically, the term synthetic fiber is reserved for fully synthetic fibers. In manufacturing terms, rayon is classified as "a fiber formed by regenerating natural materials into a usable form". [2] Specific types of rayon include viscose, modal and lyocell, each of which differs in manufacturing process and properties of the finished product.
Rayon is made from purified cellulose, harvested primarily from wood pulp, which is chemically converted into a soluble compound. It is then dissolved and forced through a spinneret to produce filaments which are chemically solidified, resulting in fibers of nearly pure cellulose.[3] Unless the chemicals are handled carefully, workers can be seriously harmed by the carbon disulfide used to manufacture most rayon.[4][5]
Rayon and its variants
Nitrocellulose
This section needs additional citations for verification. (March 2019) |
The solubility of nitrocellulose in organic solvents such as ether and acetone was the basis for the first "artificial silk" by Georges Audemars in about 1855. Although it is chemically distinct from rayon, cellulose was successfully solubilized albeit with chemical modification. Commercial production started in 1891, but the result was flammable and more expensive than cellulose acetate or cuprammonium rayon. Because of this expense, production ceased early in the 1900s. Nitrocellulose was briefly known as "mother-in-law silk".[6] Frank Hastings Griffin invented the double-godet, a special stretch-spinning process that changed artificial silk to rayon, rendering it usable in many industrial products such as tire cords and clothing. Nathan Rosenstein invented the "spunize process" by which he turned rayon from a hard fiber to a fabric. This allowed rayon to become a popular raw material in textiles.
Acetate
Rayon should not be confused with acetate (cellulose acetate) or triacetate. Although these terms were sometimes used interchangeably in the past, they are now distinct. The difference is that while the rayon process reconstitutes the natural cellulose polymer, the older acetate process reacts cellulose with acetic anhydride to form cellulose acetate. Furthermore, rayon production requires carbon disulfide as a solvent, while acetate uses considerably safer solvents such as acetone. Because rayon is a more robust fiber than the otherwise similar acetate, it has come to dominate the market.
Cuprammonium method
Swiss chemist Matthias Eduard Schweizer (1818–1860) discovered that cellulose dissolves in tetraaminecopper dihydroxide. Max Fremery and Johann Urban developed a method to produce carbon fibers for use in light bulbs in 1897.[7] Production of cuprammonium rayon for textiles started in 1899 in the Vereinigte Glanzstoff Fabriken AG in Oberbruch near Aachen.[citation needed][8] Improvement by J. P. Bemberg AG in 1904 made the artificial silk a product comparable to real silk.[citation needed][9]
Viscose method

English chemist Charles Frederick Cross and his collaborators, Edward John Bevan and Clayton Beadle, patented their artificial silk in 1894. They named their material "viscose" because its production involved the intermediacy of a highly viscous solution. The process built on the reaction of cellulose with a strong base, followed by treatment of that solution with carbon disulfide to give a xanthate derivative. The xanthate is then converted back to a cellulose fiber in a subsequent step. The first commercial viscose rayon was produced by the UK company Courtaulds Fibres in 1905. Courtaulds formed an American division, American Viscose (later known as Avtex Fibers), to produce their formulation in the United States in 1910.[10] The name "rayon" was adopted in 1924, with "viscose" being used for the viscous organic liquid used to make both rayon and cellophane. In Europe, though, the fabric itself became known as "viscose", which has been ruled an acceptable alternative term for rayon by the US Federal Trade Commission (FTC).[citation needed]
The viscose method can use wood as a source of cellulose, whereas other routes to rayon require lignin-free cellulose as starting material. The use of woody sources of cellulose makes viscose cheaper, so it was traditionally used on a larger scale than the other methods. On the other hand, the viscose process affords large amounts of contaminated wastewater. Rayon was produced only as a filament fiber until the 1930s, when methods were developed to utilize "broken waste rayon" as staple fiber.
The physical properties of rayon remained unchanged until the development of high-tenacity rayon in the 1940s. Further research and development led to high-wet-modulus rayon (HWM rayon) in the 1950s.[11] Research in the UK was centred on the government-funded British Rayon Research Association.
Industrial applications of rayon emerged around 1935. Substituting cotton fiber in tires and belts, industrial types of rayon developed a totally different set of properties, amongst which tensile strength (elasticity) was paramount.
Lyocell
The Lyocell process relies on dissolution of cellulose products in a solvent, N-methylmorpholine N-oxide. The process starts with woody sources of cellulose and involves dry jet-wet spinning. It was developed at the now defunct American Enka and Courtaulds Fibres. As of 2013, Lenzing's Tencel brand is perhaps the most widely known lyocell fiber producer.[citation needed]
Modal
Modal is a type of rayon, a semi-synthetic cellulose fiber made by spinning reconstituted cellulose, in this case often from beech trees. Modal is used alone or with other fibers (often cotton or spandex) in clothing and household items such as pajamas, underwear, bathrobes, towels, and bedsheets.
Modal is processed under different conditions to produce a fiber that is stronger and more stable when it is wet than standard rayon, yet has a soft feel, similar to cotton. It can be tumble dried without damage due to its increased molecular alignment.[12] The fabric has been known to pill less than cotton due to fiber properties and lower surface friction.[13]
Micro-Modal is a variant of Modal textiles. The material is softer than cotton and has desirable properties that include higher resistance to shrinking and moisture wicking.
Major fiber properties
Rayon is a versatile fiber and is widely claimed to have the same comfort properties as natural fibers, although the drape and slipperiness of rayon textiles are often more like nylon. It can imitate the feel and texture of silk, wool, cotton and linen. The fibers are easily dyed in a wide range of colors. Rayon fabrics are soft, smooth, cool, comfortable, and highly absorbent, but they do not insulate body heat, making them ideal for use in hot and humid climates, although also making their "hand" (feel) cool and sometimes almost slimy to the touch.[14]
The durability and appearance retention of regular viscose rayon are low, especially when wet; also, rayon has the lowest elastic recovery of any fiber. However, HWM rayon (high-wet-modulus rayon) is much stronger and exhibits higher durability and appearance retention. Recommended care for regular viscose rayon is dry-cleaning only. HWM rayon can be machine-washed.[11]
Rayon industrial yarns outperform polyester and are produced for belts in high performance tires (e.g. Cordenka, Germany).[citation needed]
Gallery of textures
-
A sample of rayon from a skirt, photographed with a macro lens.
-
Another skirt with a different texture.
-
A blouse with a texture similar to the second.
Physical structure
Regular rayon has lengthwise lines called striations and its cross-section is an indented circular shape. The cross-sections of HWM and cupra rayon are rounder. Filament rayon yarns vary from 80 to 980 filaments per yarn and vary in size from 40 to 5000 denier. Staple fibers range from 1.5 to 15 denier and are mechanically or chemically crimped. Rayon fibers are naturally very bright, but the addition of delustering pigments cuts down on this natural brightness.[11]
Production method
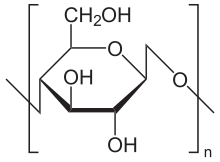
Regular rayon (or viscose) is the most widely produced form of rayon. This method of rayon production has been used since the early 1900s and it has the ability to produce either filament or staple fibers. The process is as follows:
The steps
- Cellulose. Production begins with processed cellulose (obtained from wood pulp and plant fibers). The cellulose content in the pulp should be around 87% to 95%.
- Immersion. The cellulose is dissolved in caustic soda:
(C6H10O5)n + nNaOH → (C6H9O4ONa)n + nH2O
is the chemical reaction. The reaction converts cellulose to alkali cellulose and removes impurities. - Pressing. The solution is then pressed between rollers to remove excess liquid. The pressing is done in accordance with the press-weight ratio. Press weight ratio is the ratio between the pressed alkali cellulose sheet weight to the weight of air dried pulp. It is adjusted to 2.7-3.[15]
- "White Crumb." The pressed sheets are crumbled or shredded to produce what is known as "white crumb."
- Ageing. The "white crumb" is aged through exposure to oxygen. This is a depolymerization step and is avoided in case of polynosics.
- "Xanthation." The aged "white crumb" is mixed with carbon disulfide in a process known as "Xanthation," in which the aged alkali cellulose crumbs are placed in vats and are allowed to react with carbon disulfide under controlled temperature (20 to 30 °C) to form cellulose xanthate:
- (C6H9O4ONa)n + nCS2 → (C6H9O4O−SC−SNa)n
is the reaction.
- (C6H9O4ONa)n + nCS2 → (C6H9O4O−SC−SNa)n
- "Orange-Yellow Crumb." The Xanthation changes the chemical makeup of the cellulose mixture, and the resulting product is now called "yellow crumb." This orange coloration is due to the formation of sodium trithiocarbonate (Na2CS3).[16]
- Viscose. The "yellow crumb" is dissolved in a caustic solution to form viscose.
- Ripening. The viscose is set to stand for a period of time, allowing it to ripen:
- (C6H9O4O−SC−SNa)n + nH2O → (C6H10O5)n + nCS2 + nNaOH
is the reaction.
- (C6H9O4O−SC−SNa)n + nH2O → (C6H10O5)n + nCS2 + nNaOH
- Filtering. After ripening, the viscose is filtered to remove any undissolved particles.
- Degassing. Any bubbles of air are pressed from the viscose in a degassing process.
- Extruding. The viscose solution is extruded through a spinneret, which resembles a shower head with many small holes.
- Acid Bath. As the viscose exits the spinneret, it lands in a bath of sulfuric acid, resulting in the formation of rayon filaments. The acid is used as a regenerating agent. It converts cellulose xanthate back to cellulose. The regeneration step is rapid which doesn't allow proper orientation of cellulose molecules. So to delay the process of regeneration, zinc sulphate is used in the bath which converts cellulose xanthate to zinc cellulose xanthate thus providing time for proper orientation to take place before regeneration.
- (C6H9O4O−SC−SNa)n + 1/2nH2SO4 → (C6H10O5)n + nCS2 + 1/2nNa2SO4
is the reaction.[17]
- (C6H9O4O−SC−SNa)n + 1/2nH2SO4 → (C6H10O5)n + nCS2 + 1/2nNa2SO4
- Spinning. The spinning of viscose rayon fiber is done using a wet spinning process. The filaments are allowed to pass through a coagulation bath after extrusion from the spinneret holes. Two-way mass transfer takes place.
- Drawing. The rayon filaments are stretched, in a procedure known as drawing, to straighten out the fibers.
- Washing. The fibers are then washed to remove any residual chemicals from them.
- Cutting. If filament fibers are desired, then the process ends here. The filaments are cut down when producing staple fibers.[3]
High wet modulus rayon (HWM) is a modified version of viscose that is stronger when wet. It also has the ability to be mercerized like cotton. HWM rayons are also known as "polynosic." Polynosic fibers are dimensionally stable, and do not shrink or get pulled out of shape when wet like many rayons. They are also wear resistant and strong while maintaining a soft, silky feel. They are sometimes identified by the trade name Modal.[18]
High-tenacity rayon is another modified version of viscose that has almost twice the strength of HWM. This type of rayon is typically used for industrial purposes such as tire cord.[18]
Cuprammonium rayon has properties similar to viscose; however, during its production, the cellulose is combined with copper and ammonia (Schweizer's reagent). Due to the detrimental environmental effects of this production method, cuprammonium rayon is no longer produced in the United States.[18]
Manufacturing health hazards
See also Viscose#Pollution and harm to workers
Highly toxic carbon disulfide is used in the production of viscose, leading to many incidents and legal cases.[19] However, the volatile carbon disulfide is lost before the rayon gets to the consumer; the rayon itself is basically pure cellulose.[4] Studies from the 1930s show that 30% of American rayon workers suffered severe effects. Rates of disability in modern factories (mainly in China, Indonesia and India) are unknown.[5][20]
Disposal and biodegradability
The biodegradability of various fibers in soil burial and sewage sludge was evaluated by Korean researchers. Rayon was found to be more biodegradable than cotton, and cotton more than acetate. The more water-repellent the rayon-based fabric, the more slowly it will decompose.[21] Silverfish can eat rayon.[citation needed]
A recent ocean survey found that rayon contributed to 56.9% of the total fibers found in deep ocean areas, the rest being polyester, polyamides, acetate and acrylic.[22]
Alternative to cotton
Rising cotton prices in 2010 led clothing makers to begin replacing cotton with rayon in their fabrics. As demand for rayon increases, companies such as Fortress Paper have been investing in pulp mills to increase production. Rayon now sells for as much as $2.70 per pound, which has led to an increase in the retail price of clothing made with rayon, yet rayon has a price advantage over cotton.[23]
Mislabelling
See also Bamboo textile
In 2010, the U.S. Federal Trade Commission issued letters informing over 100 companies that they were mislabeling products made of rayon as being made from bamboo, deceiving environmentally conscious consumers.[24] In 2015, the FTC filed complaints against Bed Bath & Beyond, Nordstrom, J.C. Penney, Backcountry.com, and their subsidiaries, for continuing to deceptively sell rayon mislabeled as bamboo. The four companies were required to pay civil penalties totaling US$1.3 million for violating the "Textile Act and the Textile Rules" and Section 5(m)(1)(B) of the FTC Act.[25] Similar action took place in Canada.[26]
Impact on U.S. textile industry
Rayon contributed partly to the decline of the US textile industry in the 1920s.[27] It is far cheaper to produce than wool, cotton, or silk. It also requires less processing and hence fewer workers. In addition, it was 50% cheaper than silk during the 1920s in the US.[27] Then, it was used initially for men's socks but later for lingerie and women's stockings.[27]
Producers
Trade names are used within the rayon industry to label the type of rayon in the product. Viscose Rayon was first produced in Coventry England in 1905 by Courtaulds.
Bemberg is a trade name for cupramonium rayon developed by J. P. Bemberg. Bemberg performs much like viscose but has a smaller diameter and comes closest to silk in feel. Bemberg is no longer produced in Italy, but is still produced in Japan, due to United States Environmental Protection Agency regulations in the US. The fibers are finer than viscose rayon.[9]
Modal and Tencel are widely used forms of rayon produced by Lenzing AG. Tencel, generic name lyocell, is made by a slightly different solvent recovery process, and is considered a different fiber by the US FTC. Tencel lyocell was first produced commercially by Courtaulds' Grimsby plant in England. The process, which dissolves cellulose without a chemical reaction, was developed by Courtaulds Research.
Accordis was a major manufacturer of cellulose based fibers and yarns. Production facilities can be found throughout Europe, the U.S. and Brazil.[28]
Visil rayon is a flame retardant form of viscose that has silica embedded in the fiber during manufacturing.
North American Rayon Corporation of Tennessee produced viscose rayon until its closure in the year 2000.[29][30]
Grasim of India is the largest producer of rayon in the world (claiming 24% market share). It has plants in Nagda, Kharach and Harihar – all in India, as well as joint ventures in Canada, Laos and China.[31]
See also
- Viscose
- Cellophane (sheet-extruded viscose rayon)
- Hilaire de Chardonnet
- Ray P. Dinsmore - pioneered use of Rayon in tires
References
- ^ Kauffman, George B. (1993). "Rayon: the first semi-synthetic fiber product". Journal of Chemical Education. 70 (11): 887. Bibcode:1993JChEd..70..887K. doi:10.1021/ed070p887.
- ^ "Viscose CV".
- ^ a b "Rayon Fiber (Viscose)". afma.org. Archived from the original on April 6, 2008.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Blanc, Paul D (2017). Fake silk: the lethal history of viscose rayon. Yale University Press. ISBN 978-0-300-20466-7. OCLC 961828769.
- ^ a b Monosson, Emily (2016). "Toxic textiles". Science. 354 (6315): 977. Bibcode:2016Sci...354..977M. doi:10.1126/science.aak9834. PMID 27884997.
- ^ Editors, Time-Life (1991). Inventive Genius. New York: Time-Life Books. p. 52. ISBN 978-0-8094-7699-2.
{{cite book}}:|last=has generic name (help) - ^ Over 100 years old and still going strong From Glanzstoff (artificial silk) factory to industry park. industriepark-oberbruch.de
- ^ Verinigte Glanstoff Fabriken merged with the Nederlandse Kunstzijdefabrieken in 1929 to form the Algemene Kunstzijde Unie, AkzoNobel's predecessor.[citation needed]
- ^ a b J. P. Bemberg AG was one of the Vereinigte Glanzstoff-Fabriken which merged into the Dutch based Algemene Kunstzijde Unie (AKU)--AkzoNobel today.[citation needed]
- ^ Owen, Geoffrey (2010). The Rise and Fall of Great Companies: Courtaulds and the Reshaping of the Man-Made Fibres Industry. OUP/Pasold Research Fund.
- ^ a b c Kadolph, Sara J.; Langford, Anna L. (2001). Textiles (9 ed.). Prentice Hall. ISBN 978-0-13-025443-6.
{{cite book}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ "Viscose vs. Modal vs. Lyocell - Difference?". Robert Owen Undershirts Co. Retrieved 2018-06-11.
- ^ "How to Wash Modal Clothes". The Spruce. Retrieved 2018-06-11.
- ^ LaBat, Karen L.; Salusso, Carol J. (2003). Classifications & Analysis of Textiles: A Handbook. University of Minnesota.
{{cite book}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ Gupta, p. 484
- ^ Gupta, p. 485
- ^ Gupta, p. 487
- ^ a b c Joyce A. Smith. Rayon – The Multi-Faceted Fiber. Ohio State University Rayon Fact Sheet
- ^ Supreme Court of Alabama. COURTAULDS FIBERS, INC. v. Horace L. LONG, Jr., et al.
Horace L. Long, Jr., et al. v. Courtaulds Fibers, Inc. 1971996 and 1972028.
Decided: September 15, 2000 - ^ Nijhuis, Michelle (2009). "Bamboo Boom: Is This Material for You?". Scientific American. 19 (2): 60–65. Bibcode:2009SciAm..19f..60N. doi:10.1038/scientificamericanearth0609-60.
- ^ Park, Chung Hee; Kang, Yun Kyung; Im, Seung Soon (2004). "Biodegradability of cellulose fabrics". Journal of Applied Polymer Science. 94: 248. doi:10.1002/app.20879.
- ^ Abundance of microplastics in the world's deep seas (2014-12-16). Retrieved on 2014-12-17.
- ^ Wirz, Matt (January 7, 2011) "The Touch, The Feel – Of Rayon," Wall Street Journal, Money and Investment section, p. c1
- ^ Lipka, Mitch (2010-02-03). "Bamboo-zled: FTC Says Retailers Fibbed about Bamboo Product Claims". AOL.com. Retrieved 2017-09-12.
- ^ "Nordstrom, Bed Bath & Beyond, Backcountry.com, and J.C. Penney to Pay Penalties Totaling $1.3 Million for Falsely Labeling Rayon Textiles as Made of "Bamboo". www.ftc.gov. 2015-12-09. Retrieved 2017-09-12.
- ^ "Bamboo textiles no more 'natural' than rayon". CBC News. 2010-02-01. Retrieved 2017-09-12.
- ^ a b c Waugh, Steve; Wright, John (2009). The USA 1919-1941, GCSE Modern World History for Edexcel. Hodder Education. p. 24. ISBN 978-0-340-98441-3.
- ^ Colbond History. colbond.us. Acordis was a spinoff by AkzoNobel in 2000 after it had acquired Courtaulds. It was through AkzoNobel's original parent company's, the Nederlandse Kunstzijdefabriek (ENKA), joint venture with Rento Hofstede Crull 's De Vijf, named De Internationale Spinpot Exploitatie Maatschappij (ISEM) that the commercial production of rayon was made viable. Hofstede Crull had supplied the solution for the problem of manufacturing rayon with his Driving Device For A Centrifugal Spinning Machine in 1925 (1931 U.S. patent 1,798,312). The ISEM was fully integrated with the Algemene Kunstzijde Unie, the Nederlandse Kunstzijdefabriek's successor, with the death of Hofstede Crull in 1938. (See AkzoNobel, American Enka Company, and also Rento Hofstede Crull.)
- ^ "North American Rayon Corporation and American Bemberg Corporation" in the Tennessee Encyclopedia
- ^ North American Rayon Corporation of Tennessee was an American subsidiary of J. P. Bemburg A.G. which was part of the Vereinigte Glanstoff Fabriken that were absorbed into the Dutch AKU, AkzoNobel today
- ^ viscose staple fibre. Grasim (2004-08-06). Retrieved on 2012-08-22.
Cited sources
- Gupta, VB; Kothari, VK and Sengupta, AK eds. (1997) Manufactured Fibre Technology. Chapman & Hall, London. ISBN 9780412540301.
Further reading
- For a review of all rayon production methods and markets see "Regenerated Cellulose Fibres" (book – Edited by C R Woodings) Hardback 2001, ISBN 1-85573-459-1, Woodhead Publishing Ltd.
- For a description of the production method at a factory in Germany in World War II, see Agnès Humbert (tr. Barbara Mellor) Résistance: Memoirs of Occupied France, London, Bloomsbury Publishing PLC, 2008 ISBN 978-0-7475-9597-7 (American title: Resistance: A Frenchwoman's Journal of the War, Bloomsbury, USA, 2008) pp. 152–155
- For a complete set of photographs of the process see "The Story of Rayon" published by Courtaulds Ltd (1948)



