Ammonium lauryl sulfate: Difference between revisions
Unicodifying |
+ nl |
||
| Line 51: | Line 51: | ||
[[Category:Surfactants]] |
[[Category:Surfactants]] |
||
[[Category:Organosulfates]] |
[[Category:Organosulfates]] |
||
[[nl:Ammoniumlaurylsulfaat]] |
|||
Revision as of 11:28, 21 November 2006
| Ammonium lauryl sulfate | |
|---|---|
| |
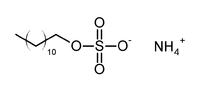
| Systematic name | Ammonium lauryl sulfate |
| Chemical formula | CxHxNxOx |
| Molecular mass | xx.xx g/mol |
| Density | x.xxx g/cm³ |
| Melting point | xx.x °C |
| Boiling point | xx.x °C |
| CAS number | [xx-xx-xx] |
| SMILES | xxxxx |
| Disclaimer and references | |
Ammonium lauryl sulfate (ALS), CH3(CH2)10CH2OSO3NH4, is an anionic surfactant found in cosmetic and toiletry formulations, with some shampoos containing up to 30% ALS. It is most widely used in the United States. In Europe sodium laureth sulfate is used instead because ammonium lauryl sulfate gives off an ammonia odor when the pH is alkaline.
The chemical can cause skin and eye irritation, and is generally only found in products that are on the skin for a short period.
Ammonium Lauryl Sulfate, aka Ammonium Dodecyl sulfate, is simply a cheaper form of sodium Dodecyl sulfate, and has essentially equivalent properties. Contrary to the above, the laureth sulfate is used not because of the lack of ammonia odor, but rather because it is even cheaper.
The lauryl sulfates, ammonium or sodium, are the primary ingredients in most shampoos, and notably are used as a known "positive" in the Draise eye irritation tests. The lauryl sulfates are used because they are very high foam, but not nearly as effective a cleaner, thus will not "de-oil" the hair, as would the more common Dodecyl benzene sulfonates found in most US hand dishwashing compounds.
Ammonium lauryl sulfate, like any other surfactant, makes a good base for cleansers because of the way it disrupts the hydrogen bonding in water. Hydrogen bonding is the cause of "water tension". In solution, the lauryl sulfate molecules ionize, meaning that the ammonium or sodium separates from the rest of the molecule as a +1 ion. The rest of the molecule aligns itself with others like it and forms what is known as a micelle. The molecules align themselves in a sphere, with the polar heads (the sulfate) on the surface of the sphere and the polar hydrophopic tails pointing inwards towards the center. The water molecules around the micelle arrange themselves around the polar heads, but this disrupts their hydrogen bonding with the water surrounding them. The overall effect of having these micelles in an aqueous (water) environment is that the water becomes more able to penetrate things like cloth fibers or hair, and also becomes more readily available to solvate anything coming off the before mentioned substance.
