Adipose tissue: Difference between revisions
No edit summary |
No edit summary |
||
| Line 140: | Line 140: | ||
A '''body fat meter''' is a widely available tool used to measure the percentage of fat in the human body. Different meters use various methods to determine the [[Body fat percentage|body fat to weight ratio]]. They tend to under-read body fat percentage. |
A '''body fat meter''' is a widely available tool used to measure the percentage of fat in the human body. Different meters use various methods to determine the [[Body fat percentage|body fat to weight ratio]]. They tend to under-read body fat percentage. |
||
In contrast with clinical tools, one relatively inexpensive type of body fat meter uses the principle of [[bioelectrical impedance analysis]] (BIA) in order to determine an individual's body fat percentage. To achieve this, the meter passes a small, harmless, [[electric current]] through the body and measures the [[electrical resistance|resistance]], then uses information on the person's weight, height, age, and sex to calculate an approximate value for the person's body fat percentage. The calculation measures the total volume of water in the body (lean tissue and muscle contain a higher percentage of water than fat), and estimates the percentage of fat based on this information. The result can fluctuate several percentage points depending on what has been eaten and how much water has been drunk before the analysis. Before [[bioelectrical impedance analysis]] machines such as were developed, there were many different ways in analyzing body composition such as [[Skin fold caliper]], [[ |
In contrast with clinical tools, one relatively inexpensive type of body fat meter uses the principle of [[bioelectrical impedance analysis]] (BIA) in order to determine an individual's body fat percentage. To achieve this, the meter passes a small, harmless, [[electric current]] through the body and measures the [[electrical resistance|resistance]], then uses information on the person's weight, height, age, and sex to calculate an approximate value for the person's body fat percentage. The calculation measures the total volume of water in the body (lean tissue and muscle contain a higher percentage of water than fat), and estimates the percentage of fat based on this information. The result can fluctuate several percentage points depending on what has been eaten and how much water has been drunk before the analysis. Before [[bioelectrical impedance analysis]] machines such as were developed, there were many different ways in analyzing body composition such as [[Skin fold caliper]], [[underwater weighing]] & [[BodPod ]]and the ual energy X-Ray absorpsiometry [[(DEXA]]) - the gold standard in measuring body composition. |
||
Analysing the right amount of body fat and muscle mass is very important for everyone to prevent injuries as well as have a better body composition. |
Analysing the right amount of body fat and muscle mass is very important for everyone to prevent injuries as well as have a better body composition. |
||
Revision as of 10:30, 22 April 2019
| Adipose tissue | |
|---|---|
 Adipose tissue is one of the main types of connective tissue. | |
| Pronunciation | /ˈædɪˌpoʊs/ |
| Identifiers | |
| MeSH | D000273 |
| FMA | 20110 |
| Anatomical terminology | |
In biology, adipose tissue, body fat, or simply fat is a loose connective tissue composed mostly of adipocytes.[1] In addition to adipocytes, adipose tissue contains the stromal vascular fraction (SVF) of cells including preadipocytes, fibroblasts, vascular endothelial cells and a variety of immune cells such as adipose tissue macrophages. Adipose tissue is derived from preadipocytes. Its main role is to store energy in the form of lipids, although it also cushions and insulates the body. Far from being hormonally inert, adipose tissue has, in recent years, been recognized as a major endocrine organ,[2] as it produces hormones such as leptin, estrogen, resistin, and the cytokine TNFα. The two types of adipose tissue are white adipose tissue (WAT), which stores energy, and brown adipose tissue (BAT), which generates body heat. The formation of adipose tissue appears to be controlled in part by the adipose gene. Adipose tissue – more specifically brown adipose tissue – was first identified by the Swiss naturalist Conrad Gessner in 1551.[3]
Anatomical features
In humans, adipose tissue is located: beneath the skin (subcutaneous fat), around internal organs (visceral fat), in bone marrow (yellow bone marrow), intermuscular (Muscular system) and in the breast tissue. Adipose tissue is found in specific locations, which are referred to as adipose depots. Apart from adipocytes, which comprise the highest percentage of cells within adipose tissue, other cell types are present, collectively termed stromal vascular fraction (SVF) of cells. SVF includes preadipocytes, fibroblasts, adipose tissue macrophages, and endothelial cells. Adipose tissue contains many small blood vessels. In the integumentary system, which includes the skin, it accumulates in the deepest level, the subcutaneous layer, providing insulation from heat and cold. Around organs, it provides protective padding. However, its main function is to be a reserve of lipids, which can be oxidised to meet the energy needs of the body and to protect it from excess glucose by storing triglycerides produced by the liver from sugars, although some evidence suggests that most lipid synthesis from carbohydrates occurs in the adipose tissue itself.[4] Adipose depots in different parts of the body have different biochemical profiles. Under normal conditions, it provides feedback for hunger and diet to the brain.
Mice

Mice have eight major adipose depots, four of which are within the abdominal cavity.[1] The paired gonadal depots are attached to the uterus and ovaries in females and the epididymis and testes in males; the paired retroperitoneal depots are found along the dorsal wall of the abdomen, surrounding the kidney, and, when massive, extend into the pelvis. The mesenteric depot forms a glue-like web that supports the intestines and the omental depot (which originates near the stomach and spleen) and - when massive - extends into the ventral abdomen. Both the mesenteric and omental depots incorporate much lymphoid tissue as lymph nodes and milky spots, respectively.
The two superficial depots are the paired inguinal depots, which are found anterior to the upper segment of the hind limbs (underneath the skin) and the subscapular depots, paired medial mixtures of brown adipose tissue adjacent to regions of white adipose tissue, which are found under the skin between the dorsal crests of the scapulae. The layer of brown adipose tissue in this depot is often covered by a "frosting" of white adipose tissue; sometimes these two types of fat (brown and white) are hard to distinguish. The inguinal depots enclose the inguinal group of lymph nodes. Minor depots include the pericardial, which surrounds the heart, and the paired popliteal depots, between the major muscles behind the knees, each containing one large lymph node.[5] Of all the depots in the mouse, the gonadal depots are the largest and the most easily dissected,[6] comprising about 30% of dissectible fat.[7]
Obesity
In an obese person, excess adipose tissue hanging downward from the abdomen is referred to as a panniculus. A panniculus complicates surgery of the morbidly obese individual. It may remain as a literal "apron of skin" if a severely obese person quickly loses large amounts of fat (a common result of gastric bypass surgery). This condition cannot be effectively corrected through diet and exercise alone, as the panniculus consists of adipocytes and other supporting cell types shrunken to their minimum volume and diameter.[citation needed] Reconstructive surgery is one method of treatment.[citation needed]
Visceral fat

Visceral fat or abdominal fat[8] (also known as organ fat or intra-abdominal fat) is located inside the abdominal cavity, packed between the organs (stomach, liver, intestines, kidneys, etc.). Visceral fat is different from subcutaneous fat underneath the skin, and intramuscular fat interspersed in skeletal muscles. Fat in the lower body, as in thighs and buttocks, is subcutaneous and is not consistently spaced tissue, whereas fat in the abdomen is mostly visceral and semi-fluid.[9] Visceral fat is composed of several adipose depots, including mesenteric, epididymal white adipose tissue (EWAT), and perirenal depots. Visceral fat is often expressed in terms of its area in cm2 (VFA, visceral fat area).[10]
An excess of visceral fat is known as central obesity, or "belly fat", in which the abdomen protrudes excessively. New developments such as the Body Volume Index (BVI) are specifically designed to measure abdominal volume and abdominal fat. Excess visceral fat is also linked to type 2 diabetes,[11] insulin resistance,[12] inflammatory diseases,[13] and other obesity-related diseases.[14] Likewise, the accumulation of neck fat (or cervical adipose tissue) has been shown to be associated with mortality.[15] Several studies have suggested that visceral fat can be predicted from simple anthropometric measures,[16] and predicts mortality more accurately than body mass index or waist circumference.[17]
Men are more likely to have fat stored in the abdomen due to sex hormone differences. Female sex hormone causes fat to be stored in the buttocks, thighs, and hips in women.[18][19] When women reach menopause and the estrogen produced by the ovaries declines, fat migrates from the buttocks, hips and thighs to the waist;[20] later fat is stored in the abdomen.[9]
High-intensity exercise is one way to effectively reduce total abdominal fat.[21][22] One study suggests at least 10 MET-hours per week of aerobic exercise is required for visceral fat reduction.[23]
Epicardial fat
Epicardial adipose tissue (EAT) is a particular form of visceral fat deposited around the heart and found to be a metabolically active organ that generates various bioactive molecules, which might significantly affect cardiac function.[24] Marked component differences have been observed in comparing EAT with subcutaneous fat, suggesting a depot specific impact of stored fatty acids on adipocyte function and metabolism.[25]
Subcutaneous fat

Most of the remaining nonvisceral fat is found just below the skin in a region called the hypodermis.[26] This subcutaneous fat is not related to many of the classic obesity-related pathologies, such as heart disease, cancer, and stroke, and some evidence even suggests it might be protective.[27] The typically female (or gynecoid) pattern of body fat distribution around the hips, thighs, and buttocks is subcutaneous fat, and therefore poses less of a health risk compared to visceral fat.[28]
Like all other fat organs, subcutaneous fat is an active part of the endocrine system, secreting the hormones leptin and resistin.[26]
The relationship between the subcutaneous adipose layer and total body fat in a person is often modelled by using regression equations. The most popular of these equations was formed by Durnin and Wormersley, who rigorously tested many types of skinfold, and, as a result, created two formulae to calculate the body density of both men and women. These equations present an inverse correlation between skinfolds and body density—as the sum of skinfolds increases, the body density decreases.[29]
Factors such as sex, age, population size or other variables may make the equations invalid and unusable, and, as of 2012[update], Durnin and Wormersley's equations remain only estimates of a person's true level of fatness. New formulae are still being created.[29]
Marrow fat
Marrow fat, also known as marrow adipose tissue (MAT), is a poorly understood adipose depot that resides in the bone and is interspersed with hematopoietic cells as well as bony elements. The adipocytes in this depot are derived from mesenchymal stem cells (MSC) which can give rise to fat cells, bone cells as well as other cell types. The fact that MAT increases in the setting of calorie restriction/ anorexia is a feature that distinguishes this depot from other fat depots.[30][31][32] Exercise regulates MAT, decreasing MAT quantity and diminishing the size of marrow adipocytes.[33][34][35] The exercise regulation of marrow fat suggests that it bears some physiologic similarity to other white adipose depots. Moreover, increased MAT in obesity further suggests a similarity to white fat depots.[33]
Ectopic fat
Ectopic fat is the storage of triglycerides in tissues other than adipose tissue, that are supposed to contain only small amounts of fat, such as the liver, skeletal muscle, heart, and pancreas.[1] This can interfere with cellular functions and hence organ function and is associated with insulin resistance in type-2 diabetes.[36] It is stored in relatively high amounts around the organs of the abdominal cavity, but is not to be confused with visceral fat.
The specific cause for the accumulation of ectopic fat is unknown. The cause is likely a combination of genetic, environmental, and behavioral factors that are involved in excess energy intake and decreased physical activity. Substantial weight loss can reduce ectopic fat stores in all organs and this is associated with an improvement of the function of that organ.[36]
In the latter case, non-invasive weight loss interventions like diet or exercise can decrease ectopic fat (particularly in heart and liver) in overweight or obese children and adults.[37][38]
Physiology
Free fatty acids (FFAs) are liberated from lipoproteins by lipoprotein lipase (LPL) and enter the adipocyte, where they are reassembled into triglycerides by esterifying them onto glycerol. Human fat tissue contains about 87% lipids.[citation needed]
There is a constant flux of FFAs entering and leaving adipose tissue. The net direction of this flux is controlled by insulin and leptin—if insulin is elevated, then there is a net inward flux of FFA, and only when insulin is low can FFA leave adipose tissue. Insulin secretion is stimulated by high blood sugar, which results from consuming carbohydrates.[citation needed]
In humans, lipolysis (hydrolysis of triglycerides into free fatty acids) is controlled through the balanced control of lipolytic B-adrenergic receptors and a2A-adrenergic receptor-mediated antilipolysis.
Fat cells have an important physiological role in maintaining triglyceride and free fatty acid levels, as well as determining insulin resistance. Abdominal fat has a different metabolic profile—being more prone to induce insulin resistance. This explains to a large degree why central obesity is a marker of impaired glucose tolerance and is an independent risk factor for cardiovascular disease (even in the absence of diabetes mellitus and hypertension).[39] Studies of female monkeys at Wake Forest University (2009) discovered that individuals suffering from higher stress have higher levels of visceral fat in their bodies. This suggests a possible cause-and-effect link between the two, wherein stress promotes the accumulation of visceral fat, which in turn causes hormonal and metabolic changes that contribute to heart disease and other health problems.[40]
Recent advances in biotechnology have allowed for the harvesting of adult stem cells from adipose tissue, allowing stimulation of tissue regrowth using a patient's own cells. In addition, adipose-derived stem cells from both human and animals reportedly can be efficiently reprogrammed into induced pluripotent stem cells without the need for feeder cells.[41] The use of a patient's own cells reduces the chance of tissue rejection and avoids ethical issues associated with the use of human embryonic stem cells.[42] A growing body of evidence also suggests that different fat depots (i.e. abdominal, omental, pericardial) yield adipose-derived stem cells with different characteristics.[42][43] These depot-dependent features include proliferation rate, immunophenotype, differentiation potential, gene expression, as well as sensitivity to hypoxic culture conditions.[44]
Adipose tissue is the greatest peripheral source of aromatase in both males and females,[citation needed] contributing to the production of estradiol.
Adipose derived hormones include:
- Adiponectin
- Resistin
- Plasminogen activator inhibitor-1 (PAI-1)
- TNFα
- IL-6
- Leptin
- Estradiol (E2)
Adipose tissues also secrete a type of cytokines (cell-to-cell signalling proteins) called adipokines (adipocytokines), which play a role in obesity-associated complications. Perivascular adipose tissue releases adipokines such as adiponectin that affect the contractile function of the vessels that they surround.[1][45]
Brown fat
Brown fat or brown adipose tissue (BAT) is a specialized form of adipose tissue important for adaptive thermogenesis in humans and other mammals. BAT can generate heat by "uncoupling" the respiratory chain of oxidative phosphorylation within mitochondria through tissue-specific expression of uncoupling protein 1 (UCP1).[46] BAT is primarily located around the neck and large blood vessels of the thorax, where may effectively act in heat exchange. BAT is robustly activated upon cold exposure by the release of catecholamines from sympathetic nerves that results in UCP1 activation. BAT activation may also occur in response to overfeeding.[47] UCP1 activity is stimulated by long chain fatty acids that are produced subsequent to β-adrenergic receptor activation.[46] UCP1 is proposed to function as a fatty acid proton symporter, although the exact mechanism has yet to be elucidated.[48] In contrast, UCP1 is inhibited by ATP, ADP, and GTP.[49]
Attempts to simulate this process pharmacologically have so far been unsuccessful. Techniques to manipulate the differentiation of "brown fat" could become a mechanism for weight loss therapy in the future, encouraging the growth of tissue with this specialized metabolism without inducing it in other organs.
Until recently, brown adipose tissue was thought to be primarily limited to infants in humans, but new evidence has now overturned that belief. Metabolically active tissue with temperature responses similar to brown adipose was first reported in the neck and trunk of some human adults in 2007,[50] and the presence of brown adipose in human adults was later verified histologically in the same anatomical regions.[51][52][53]
Beige fat and WAT browning

Browning of WAT, also referred to as "beiging", occurs when adipocytes within WAT depots develop features of BAT. Beige adipocytes take on a multilocular appearance (containing several lipid droplets) and increase expression of uncoupling protein 1 (UCP1).[54] In doing so, these normally energy-storing adipocytes become energy-releasing adipocytes.
The calorie-burning capacity of brown and beige fat has been extensively studied as research efforts focus on therapies targeted to treat obesity and diabetes. The drug 2,4-dinitrophenol, which also acts as a chemical uncoupler similarly to UCP1, was used for weight loss in the 1930s. However, it was quickly discontinued when excessive dosing led to adverse side effects including hyperthermia and death.[54] β3 agonists, like CL316,243, have also been developed and tested in humans. However, the use of such drugs has proven largely unsuccessful due to several challenges, including varying species receptor specificity and poor oral bioavailability.[55]
Cold is a primary regulator of BAT processes and induces WAT browning. Browning in response to chronic cold exposure has been well documented and is a reversible process. A study in mice demonstrated that cold-induced browning can be completely reversed in 21 days, with measurable decreases in UCP1 seen within a 24-hour period.[56] A study by Rosenwald et al. revealed that when the animals are re-exposed to a cold environment, the same adipocytes will adopt a beige phenotype, suggesting that beige adipocytes are retained.[57]
Transcriptional regulators, as well as a growing number of other factors, regulate the induction of beige fat. Four regulators of transcription are central to WAT browning and serve as targets for many of the molecules known to influence this process.[58] These include peroxisome proliferator-activated receptor gamma (PPARγ), PR domain containing 16 (PRDM16),[59] peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α), and Early B-Cell Factor-2 (EBF2).[60][61][62]
The list of molecules that influence browning has grown in direct proportion to the popularity of this topic and is constantly evolving as more knowledge is acquired. Among these molecules are irisin and fibroblast growth factor 21 (FGF21), which have been well-studied and are believed to be important regulators of browning. Irisin is secreted from muscle in response to exercise and has been shown to increase browning by acting on beige preadipocytes.[63] FGF21, a hormone secreted mainly by the liver, has garnered a great deal of interest after being identified as a potent stimulator of glucose uptake and a browning regulator through its effects on PGC-1α.[54] It is increased in BAT during cold exposure and is thought to aid in resistance to diet-induced obesity[64] FGF21 may also be secreted in response to exercise and a low protein diet, although the latter has not been thoroughly investigated.[65][66] Data from these studies suggest that environmental factors like diet and exercise may be important mediators of browning. In mice, it was found that beiging can occur through the production of methionine-enkephalin peptides by type 2 innate lymphoid cells in response to interleukin 33.[67]
Genomics and bioinformatics tools to study browning
Due to the complex nature of adipose tissue and a growing list of browning regulatory molecules, great potential exists for the use of bioinformatics tools to improve study within this field. Studies of WAT browning have greatly benefited from advances in these techniques, as beige fat is rapidly gaining popularity as a therapeutic target for the treatment of obesity and diabetes.
DNA microarray is a bioinformatics tool used to quantify expression levels of various genes simultaneously, and has been used extensively in the study of adipose tissue. One such study used microarray analysis in conjunction with Ingenuity IPA software to look at changes in WAT and BAT gene expression when mice were exposed to temperatures of 28 and 6 °C.[68] The most significantly up- and downregulated genes were then identified and used for analysis of differentially expressed pathways. It was discovered that many of the pathways upregulated in WAT after cold exposure are also highly expressed in BAT, such as oxidative phosphorylation, fatty acid metabolism, and pyruvate metabolism.[68] This suggests that some of the adipocytes switched to a beige phenotype at 6 °C. Mössenböck et al. also used microarray analysis to demonstrate that insulin deficiency inhibits the differentiation of beige adipocytes but does not disturb their capacity for browning.[69] These two studies demonstrate the potential for the use of microarray in the study of WAT browning.
RNA sequencing (RNA-Seq) is a powerful computational tool that allows for the quantification of RNA expression for all genes within a sample. Incorporating RNA-Seq into browning studies is of great value, as it offers better specificity, sensitivity, and a more comprehensive overview of gene expression than other methods. RNA-Seq has been used in both human and mouse studies in an attempt characterize beige adipocytes according to their gene expression profiles and to identify potential therapeutic molecules that may induce the beige phenotype. One such study used RNA-Seq to compare gene expression profiles of WAT from wild-type (WT) mice and those overexpressing Early B-Cell Factor-2 (EBF2). WAT from the transgenic animals exhibited a brown fat gene program and had decreased WAT specific gene expression compared to the WT mice.[70] Thus, EBF2 has been identified as a potential therapeutic molecule to induce beiging.
Chromatin immunoprecipitation with sequencing (ChIP-seq) is a method used to identify protein binding sites on DNA and assess histone modifications. This tool has enabled examination of epigenetic regulation of browning and helps elucidate the mechanisms by which protein-DNA interactions stimulate the differentiation of beige adipocytes. Studies observing the chromatin landscapes of beige adipocytes have found that adipogenesis of these cells results from the formation of cell specific chromatin landscapes, which regulate the transcriptional program and, ultimately, control differentiation. Using ChIP-seq in conjunction with other tools, recent studies have identified over 30 transcriptional and epigenetic factors that influence beige adipocyte development.[70]
Genetics
The thrifty gene hypothesis (also called the famine hypothesis) states that in some populations the body would be more efficient at retaining fat in times of plenty, thereby endowing greater resistance to starvation in times of food scarcity. This hypothesis, originally advanced in the context of glucose metabolism and insulin resistance, has been discredited by physical anthropologists, physiologists, and the original proponent of the idea himself with respect to that context, although according to its developer it remains "as viable as when [it was] first advanced" in other contexts.[71][72]
In 1995, Jeffrey Friedman, in his residency at the Rockefeller University, together with Rudolph Leibel, Douglas Coleman et al. discovered the protein leptin that the genetically obese mouse lacked.[73][74][75] Leptin is produced in the white adipose tissue and signals to the hypothalamus. When leptin levels drop, the body interprets this as a loss of energy, and hunger increases. Mice lacking this protein eat until they are four times their normal size.
Leptin, however, plays a different role in diet-induced obesity in rodents and humans. Because adipocytes produce leptin, leptin levels are elevated in the obese. However, hunger remains, and—when leptin levels drop due to weight loss—hunger increases. The drop of leptin is better viewed as a starvation signal than the rise of leptin as a satiety signal.[76] However, elevated leptin in obesity is known as leptin resistance. The changes that occur in the hypothalamus to result in leptin resistance in obesity are currently the focus of obesity research.[77]
Gene defects in the leptin gene (ob) are rare in human obesity.[78] As of July 2010[update], only 14 individuals from five families have been identified worldwide who carry a mutated ob gene (one of which was the first ever identified cause of genetic obesity in humans)—two families of Pakistani origin living in the UK, one family living in Turkey, one in Egypt, and one in Austria[79][80][81][82][83]—and two other families have been found that carry a mutated ob receptor.[84][85] Others have been identified as genetically partially deficient in leptin, and, in these individuals, leptin levels on the low end of the normal range can predict obesity.[86]
Several mutations of genes involving the melanocortins (used in brain signaling associated with appetite) and their receptors have also been identified as causing obesity in a larger portion of the population than leptin mutations.[87]
Physical properties
Adipose tissue has a density of ~0.9 g/ml.[88] Thus, a person with more adipose tissue will float more easily than a person of the same weight with more muscular tissue, since muscular tissue has a density of 1.06 g/ml.[89]
Body fat meter
A body fat meter is a widely available tool used to measure the percentage of fat in the human body. Different meters use various methods to determine the body fat to weight ratio. They tend to under-read body fat percentage.
In contrast with clinical tools, one relatively inexpensive type of body fat meter uses the principle of bioelectrical impedance analysis (BIA) in order to determine an individual's body fat percentage. To achieve this, the meter passes a small, harmless, electric current through the body and measures the resistance, then uses information on the person's weight, height, age, and sex to calculate an approximate value for the person's body fat percentage. The calculation measures the total volume of water in the body (lean tissue and muscle contain a higher percentage of water than fat), and estimates the percentage of fat based on this information. The result can fluctuate several percentage points depending on what has been eaten and how much water has been drunk before the analysis. Before bioelectrical impedance analysis machines such as were developed, there were many different ways in analyzing body composition such as Skin fold caliper, underwater weighing & BodPod and the ual energy X-Ray absorpsiometry (DEXA) - the gold standard in measuring body composition. Analysing the right amount of body fat and muscle mass is very important for everyone to prevent injuries as well as have a better body composition.
Animal studies
Within the fat (adipose) tissue of CCR2 deficient mice, there is an increased number of eosinophils, greater alternative Macrophage activation, and a propensity towards type 2 cytokine expression. Furthermore, this effect was exaggerated when the mice became obese from a high fat diet.[90]
Gallery
-
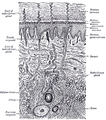
Diagrammatic sectional view of the skin (magnified).
-
White adipose tissue in paraffin section
-
Electronic instrument of body fat meter
See also
- Adipose differentiation-related protein
- Adiposopathy
- Apelin
- Bioelectrical impedance analysis – a method to measure body fat percentage.
- Blubber – an extra thick form of adipose tissue found in some marine mammals.
- Body fat percentage
- Cellulite
- Lipolysis
- Lipodystrophy
- Human fat used as pharmaceutical in traditional medicine
- Obesity
- Starvation
- Steatosis (also called fatty change, fatty degeneration or adipose degeneration)
- Stem cells
- Subcutaneous fat
- Bariatrics
- Classification of obesity
- Classification of childhood obesity
- EPODE International Network, the world's largest obesity-prevention network
- World Fit A program of the United States Olympic Committee (USOC), and the United States Olympians and Paralympians Association (USOP)
- Obesity and walking
- Social stigma of obesity
References
- ^ a b c d Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (August 2013). "Role of pericytes in skeletal muscle regeneration and fat accumulation". Stem Cells and Development. 22 (16): 2298–314. doi:10.1089/scd.2012.0647. PMC 3730538. PMID 23517218.
- ^ Kershaw EE, Flier JS (June 2004). "Adipose tissue as an endocrine organ". The Journal of Clinical Endocrinology and Metabolism. 89 (6): 2548–56. doi:10.1210/jc.2004-0395. PMID 15181022.
- ^ Cannon B, Nedergaard J (August 2008). "Developmental biology: Neither fat nor flesh". Nature. 454 (7207): 947–48. Bibcode:2008Natur.454..947C. doi:10.1038/454947a. PMID 18719573.
- ^ Aarsland A, Chinkes D, Wolfe RR (June 1997). "Hepatic and whole-body fat synthesis in humans during carbohydrate overfeeding". The American Journal of Clinical Nutrition. 65 (6): 1774–82. doi:10.1093/ajcn/65.6.1774. PMID 9174472.
- ^ Pond, Caroline M. (1998). The Fats of Life. Cambridge University Press. ISBN 978-0-521-63577-6.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Cinti S (July 2005). "The adipose organ". Prostaglandins, Leukotrienes, and Essential Fatty Acids. 73 (1): 9–15. doi:10.1016/j.plefa.2005.04.010. PMID 15936182.
- ^ Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK (March 2001). "Nutrient preference and diet-induced adiposity in C57BL/6ByJ and 129P3/J mice". Physiology & Behavior. 72 (4): 603–13. doi:10.1016/S0031-9384(01)00412-7. PMC 3341942. PMID 11282146.
- ^ Fat on the Inside: Looking Thin is Not Enough, By Fiona Haynes, About.com
- ^ a b "Abdominal fat and what to do about it". President & Fellows of Harvard College. September 2005.
Visceral fat more of a health concern than subcutaneous fat
- ^ Nagai M, Komiya H, Mori Y, Ohta T, Kasahara Y, Ikeda Y (May 2010). "Estimating visceral fat area by multifrequency bioelectrical impedance". Diabetes Care. 33 (5): 1077–79. doi:10.2337/dc09-1099. PMC 2858179. PMID 20150289.
- ^ Montague CT, O'Rahilly S (June 2000). "The perils of portliness: causes and consequences of visceral adiposity". Diabetes. 49 (6): 883–88. doi:10.2337/diabetes.49.6.883. PMID 10866038.
- ^ Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G (May 2001). "Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance". American Journal of Physiology. Endocrinology and Metabolism. 280 (5): E745–51. doi:10.1152/ajpendo.2001.280.5.e745. PMID 11287357.
- ^ Marette A (December 2003). "Molecular mechanisms of inflammation in obesity-linked insulin resistance". International Journal of Obesity and Related Metabolic Disorders. 27 Suppl 3: S46–48. doi:10.1038/sj.ijo.0802500. PMID 14704744.
- ^ Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS (January 2003). "Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001". JAMA. 289 (1): 76–79. doi:10.1001/jama.289.1.76. PMID 12503980.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Maresky HS, Sharfman Z, Ziv-Baran T, Gomori JM, Copel L, Tal S (November 2015). "Anthropometric Assessment of Neck Adipose Tissue and Airway Volume Using Multidetector Computed Tomography: An Imaging Approach and Association With Overall Mortality". Medicine. 94 (45): e1991. doi:10.1097/MD.0000000000001991. PMC 4912280. PMID 26559286.
- ^ Brown JC, Harhay MO, Harhay MN (2016). "Anthropometrically predicted visceral adipose tissue and blood-based biomarkers: a cross-sectional analysis". European Journal of Nutrition. 57 (1): 191–198. doi:10.1007/s00394-016-1308-8. PMC 5513780. PMID 27614626.
- ^ Brown JC, Harhay MO, Harhay MN (2017). "Anthropometrically-predicted visceral adipose tissue and mortality among men and women in the third national health and nutrition examination survey (NHANES III)". American Journal of Human Biology. 29 (1): e22898. doi:10.1002/ajhb.22898. PMC 5241265. PMID 27427402.
- ^ "Reduce Abdominal Fat".
Estrogen causes fat to be stored around the pelvic region, hips, butt and thighs (pelvic region)
- ^ "Waistline Worries: Turning Apples Back Into Pears". healthywomen.org. Archived from the original on 2009-06-09.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Researchers think that the lack of estrogen at menopause plays a role in driving our fat northward. See: Andrews, Michelle (2006-12-01). "A Matter of Fat". Yahoo Health. Women's Health. Archived from the original on 2007-03-15.
- ^ Irving BA, Davis CK, Brock DW, Weltman JY, Swift D, Barrett EJ, Gaesser GA, Weltman A (November 2008). "Effect of exercise training intensity on abdominal visceral fat and body composition". Medicine and Science in Sports and Exercise. 40 (11): 1863–72. doi:10.1249/MSS.0b013e3181801d40. PMC 2730190. PMID 18845966.
- ^ Coker RH, Williams RH, Kortebein PM, Sullivan DH, Evans WJ (August 2009). "Influence of exercise intensity on abdominal fat and adiponectin in elderly adults". Metabolic Syndrome and Related Disorders. 7 (4): 363–68. doi:10.1089/met.2008.0060. PMC 3135883. PMID 19196080.
- ^ Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I (December 2007). "A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials". International Journal of Obesity. 31 (12): 1786–97. doi:10.1038/sj.ijo.0803683. PMID 17637702.
- ^ Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O'Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y (November 2003). "Human epicardial adipose tissue is a source of inflammatory mediators". Circulation. 108 (20): 2460–66. doi:10.1161/01.CIR.0000099542.57313.C5. PMID 14581396.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Pezeshkian M, Noori M, Najjarpour-Jabbari H, Abolfathi A, Darabi M, Darabi M, Shaaker M, Shahmohammadi G (April 2009). "Fatty acid composition of epicardial and subcutaneous human adipose tissue". Metabolic Syndrome and Related Disorders. 7 (2): 125–31. doi:10.1089/met.2008.0056. PMID 19422139.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b Hoehn, Katja; Marieb, Elaine N. (2008). Anatomy & Physiology (3rd ed.). San Francisco, Calif.: Pearson/Benjamin Cummings. ISBN 978-0-8053-0094-9.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ, Fox CS (June 2009). "Abdominal subcutaneous adipose tissue: a protective fat depot?". Diabetes Care. 32 (6): 1068–75. doi:10.2337/dc08-2280. PMC 2681034. PMID 19244087.
- ^ "Belly fat in women: Taking – and keeping – it off". MayoClinic.com. 2013-06-08. Retrieved 2013-12-02.
- ^ a b Brodie D, Moscrip V, Hutcheon R (March 1998). "Body composition measurement: a review of hydrodensitometry, anthropometry, and impedance methods". Nutrition. 14 (3): 296–310. doi:10.1016/S0899-9007(97)00474-7. PMID 9583375.
- ^ Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML (September 2010). "Caloric restriction leads to high marrow adiposity and low bone mass in growing mice". Journal of Bone and Mineral Research. 25 (9): 2078–88. doi:10.1002/jbmr.82. PMC 3127399. PMID 20229598.
- ^ Cawthorn WP, Scheller EL, Parlee SD, Pham HA, Learman BS, Redshaw CM, Sulston RJ, Burr AA, Das AK, Simon BR, Mori H, Bree AJ, Schell B, Krishnan V, MacDougald OA (February 2016). "Expansion of Bone Marrow Adipose Tissue During Caloric Restriction Is Associated With Increased Circulating Glucocorticoids and Not With Hypoleptinemia". Endocrinology. 157 (2): 508–21. doi:10.1210/en.2015-1477. PMC 4733126. PMID 26696121.
- ^ Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A (June 2009). "Increased bone marrow fat in anorexia nervosa". The Journal of Clinical Endocrinology and Metabolism. 94 (6): 2129–36. doi:10.1210/jc.2008-2532. PMC 2690416. PMID 19318450.
- ^ a b Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, Xie Z, Zong X, Styner MA, Rubin CT, Rubin J (August 2017). "Exercise Decreases Marrow Adipose Tissue Through ß-Oxidation in Obese Running Mice". Journal of Bone and Mineral Research. 32 (8): 1692–702. doi:10.1002/jbmr.3159. PMC 5550355. PMID 28436105.
- ^ Styner M, Pagnotti GM, Galior K, Wu X, Thompson WR, Uzer G, Sen B, Xie Z, Horowitz MC, Styner MA, Rubin C, Rubin J (August 2015). "Exercise Regulation of Marrow Fat in the Setting of PPARγ Agonist Treatment in Female C57BL/6 Mice". Endocrinology. 156 (8): 2753–61. doi:10.1210/en.2015-1213. PMC 4511140. PMID 26052898.
- ^ Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, Case N, Xie Z, Sen B, Romaine A, Pagnotti GM, Rubin CT, Styner MA, Horowitz MC, Rubin J (July 2014). "Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise". Bone. 64: 39–46. doi:10.1016/j.bone.2014.03.044. PMC 4041820. PMID 24709686.
- ^ a b Snel M, Jonker JT, Schoones J, Lamb H, de Roos A, Pijl H, Smit JW, Meinders AE, Jazet IM (2012). "Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions". International Journal of Endocrinology. 2012: 1–18. doi:10.1155/2012/983814. PMC 3366269. PMID 22675355.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Hens W, Vissers D, Hansen D, Peeters S, Gielen J, Van Gaal L, Taeymans J (2017). "The effect of diet or exercise on ectopic adiposity in children and adolescents with obesity: a systematic review and meta-analysis". Obesity Reviews. 18 (11): 1310–22. doi:10.1111/obr.12577. PMID 28913977.
- ^ Hens W, Taeyman J, Cornelis J, Gielen J, Van Gaal L, Vissers D (2016). "The Effect of Lifestyle Interventions on Excess Ectopic Fat Deposition Measured by Noninvasive Techniques in Overweight and Obese Adults: A Systematic Review and Meta-Analysis". Journal of Physical Activity & Health. 13 (6): 671–94. doi:10.1123/jpah.2015-0560. PMID 26694194.
- ^ Dhaliwal SS, Welborn TA (2009). "Central obesity and multivariable cardiovascular risk as assessed by the Framingham prediction scores". Am J Cardiol. 103 (10): 1403–07. doi:10.1016/j.amjcard.2008.12.048. PMID 19427436.
- ^ Park, Alice (2009-08-08). "Fat-Bellied Monkeys Suggest Why Stress Sucks". Time. Archived from the original on December 20, 2013. Retrieved 2013-12-19.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help); Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Sugii S, Kida Y, Kawamura T, Suzuki J, Vassena R, Yin YQ, Lutz MK, Berggren WT, Izpisúa Belmonte JC, Evans RM (February 2010). "Human and mouse adipose-derived cells support feeder-independent induction of pluripotent stem cells". Proceedings. 107 (8): 3558–63. Bibcode:2010PNAS..107.3558S. doi:10.1073/pnas.0910172106. PMC 2840462. PMID 20133714.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b Atzmon G, Yang XM, Muzumdar R, Ma XH, Gabriely I, Barzilai N (November 2002). "Differential gene expression between visceral and subcutaneous fat depots". Hormone and Metabolic Research = Hormon- und Stoffwechselforschung = Hormones et Metabolisme. 34 (11–12): 622–28. doi:10.1055/s-2002-38250. PMID 12660871.
- ^ Baglioni S, Cantini G, Poli G, Francalanci M, Squecco R, Di Franco A, Borgogni E, Frontera S, Nesi G, Liotta F, Lucchese M, Perigli G, Francini F, Forti G, Serio M, Luconi M (4 May 2012). "Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell". PLOS One. 7 (5): e36569. Bibcode:2012PLoSO...736569B. doi:10.1371/journal.pone.0036569. PMC 3344924. PMID 22574183.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Russo V, Yu C, Belliveau P, Hamilton A, Flynn LE (February 2014). "Comparison of human adipose-derived stem cells isolated from subcutaneous, omental, and intrathoracic adipose tissue depots for regenerative applications". Stem Cells Translational Medicine. 3 (2): 206–17. doi:10.5966/sctm.2013-0125. PMC 3925056. PMID 24361924.
- ^ Löhn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM (July 2002). "Periadventitial fat releases a vascular relaxing factor". FASEB Journal. 16 (9): 1057–63. doi:10.1096/fj.02-0024com. PMID 12087067.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Cannon B, Nedergaard J (January 2004). "Brown adipose tissue: function and physiological significance". Physiological Reviews. 84 (1): 277–359. doi:10.1152/physrev.00015.2003. PMID 14715917.
- ^ Busiello RA, Savarese S, Lombardi A (2015). "Mitochondrial uncoupling proteins and energy metabolism". Frontiers in Physiology. 6 (36): 36. doi:10.3389/fphys.2015.00036. PMC 4322621. PMID 25713540.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Fedorenko A, Lishko PV, Kirichok Y (October 2012). "Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria". Cell. 151 (2): 400–13. doi:10.1016/j.cell.2012.09.010. PMC 3782081. PMID 23063128.
- ^ Azzu V, Brand MD (May 2010). "The on-off switches of the mitochondrial uncoupling proteins". Trends in Biochemical Sciences. 35 (5): 298–307. doi:10.1016/j.tibs.2009.11.001. PMC 3640847. PMID 20006514.
- ^ Nedergaard J, Bengtsson T, Cannon B (August 2007). "Unexpected evidence for active brown adipose tissue in adult humans". American Journal of Physiology. Endocrinology and Metabolism. 293 (2): E444–52. doi:10.1152/ajpendo.00691.2006. PMID 17473055.
- ^ Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P (April 2009). "Functional brown adipose tissue in healthy adults". The New England Journal of Medicine. 360 (15): 1518–25. doi:10.1056/NEJMoa0808949. PMID 19357407.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ (April 2009). "Cold-activated brown adipose tissue in healthy men". The New England Journal of Medicine. 360 (15): 1500–08. doi:10.1056/NEJMoa0808718. PMID 19357405.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR (April 2009). "Identification and importance of brown adipose tissue in adult humans". The New England Journal of Medicine. 360 (15): 1509–17. doi:10.1056/NEJMoa0810780. PMC 2859951. PMID 19357406.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b c Harms M, Seale P (October 2013). "Brown and beige fat: development, function and therapeutic potential". Nature Medicine. 19 (10): 1252–63. doi:10.1038/nm.3361. PMID 24100998.
- ^ Cypess AM, Kahn CR (April 2010). "Brown fat as a therapy for obesity and diabetes". Current Opinion in Endocrinology, Diabetes and Obesity. 17 (2): 143–49. doi:10.1097/MED.0b013e328337a81f. PMC 3593105. PMID 20160646.
- ^ Gospodarska E, Nowialis P, Kozak LP (March 2015). "Mitochondrial turnover: a phenotype distinguishing brown adipocytes from interscapular brown adipose tissue and white adipose tissue". The Journal of Biological Chemistry. 290 (13): 8243–55. doi:10.1074/jbc.M115.637785. PMC 4375480. PMID 25645913.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Rosenwald M, Perdikari A, Rülicke T, Wolfrum C (June 2013). "Bi-directional interconversion of brite and white adipocytes". Nature Cell Biology. 15 (6): 659–67. doi:10.1038/ncb2740. PMID 23624403.
- ^ Lo KA, Sun L (September 2013). "Turning WAT into BAT: a review on regulators controlling the browning of white adipocytes". Bioscience Reports. 33 (5): 711–19. doi:10.1042/BSR20130046. PMC 3764508. PMID 23895241.
- ^ Harms MJ, Ishibashi J, Wang W, Lim HW, Goyama S, Sato T, Kurokawa M, Won KJ, Seale P (April 2014). "Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice". Cell Metabolism. 19 (4): 593–604. doi:10.1016/j.cmet.2014.03.007. PMC 4012340. PMID 24703692.
- ^ Wang W, Kissig M, Rajakumari S, Huang L, Lim HW, Won KJ, Seale P (October 2014). "Ebf2 is a selective marker of brown and beige adipogenic precursor cells". Proceedings. 111 (40): 14466–71. Bibcode:2014PNAS..11114466W. doi:10.1073/pnas.1412685111. PMC 4209986. PMID 25197048.
- ^ Kissig M, Shapira SN, Seale P (June 2016). "SnapShot: Brown and Beige Adipose Thermogenesis". Cell. 166 (1): 258–258.e1. doi:10.1016/j.cell.2016.06.038. PMC 5478388. PMID 27368105.
- ^ Shapira SN, Lim HW, Rajakumari S, Sakers AP, Ishibashi J, Harms MJ, Won KJ, Seale P (April 2017). "EBF2 transcriptionally regulates brown adipogenesis via the histone reader DPF3 and the BAF chromatin remodeling complex". Genes & Development. 31 (7): 660–73. doi:10.1101/gad.294405.116. PMC 5411707. PMID 28428261.
- ^ Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM (January 2012). "A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis". Nature. 481 (7382): 463–68. Bibcode:2012Natur.481..463B. doi:10.1038/nature10777. PMC 3522098. PMID 22237023.
- ^ Ohta H, Itoh N (2014). "Roles of FGFs as Adipokines in Adipose Tissue Development, Remodeling, and Metabolism". Frontiers in Endocrinology. 5 (18): 18. doi:10.3389/fendo.2014.00018. PMC 3932445. PMID 24605108.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Fenzl A, Kiefer FW (July 2014). "Brown adipose tissue and thermogenesis". Hormone Molecular Biology and Clinical Investigation. 19 (1): 25–37. doi:10.1515/hmbci-2014-0022. PMID 25390014.
- ^ Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Münzberg H, Hutson SM, Gettys TW, Schwartz MW, Morrison CD (September 2014). "FGF21 is an endocrine signal of protein restriction". The Journal of Clinical Investigation. 124 (9): 3913–22. doi:10.1172/JCI74915. PMC 4153701. PMID 25133427.
- ^ Brestoff, Jonathan R.; Kim, Brian S.; Saenz, Steven A.; Stine, Rachel R.; Monticelli, Laurel A.; Sonnenberg, Gregory F.; Thome, Joseph J.; Farber, Donna L.; Lutfy, Kabirullah (12 March 2015). "Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity". Nature. 519 (7542): 242–246. Bibcode:2015Natur.519..242B. doi:10.1038/nature14115. ISSN 1476-4687. PMC 4447235. PMID 25533952.
- ^ a b Rosell M, Kaforou M, Frontini A, Okolo A, Chan YW, Nikolopoulou E, Millership S, Fenech ME, MacIntyre D, Turner JO, Moore JD, Blackburn E, Gullick WJ, Cinti S, Montana G, Parker MG, Christian M (April 2014). "Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice". American Journal of Physiology. Endocrinology and Metabolism. 306 (8): E945–64. doi:10.1152/ajpendo.00473.2013. PMC 3989735. PMID 24549398.
- ^ Inagaki T, Sakai J, Kajimura S (August 2016). "Transcriptional and epigenetic control of brown and beige adipose cell fate and function". Nature Reviews Molecular Cell Biology. 17 (8): 480–95. doi:10.1038/nrm.2016.62. PMC 4956538. PMID 27251423.
- ^ a b Stine RR, Shapira SN, Lim HW, Ishibashi J, Harms M, Won KJ, Seale P (January 2016). "EBF2 promotes the recruitment of beige adipocytes in white adipose tissue". Molecular Metabolism. 5 (1): 57–65. doi:10.1016/j.molmet.2015.11.001. PMC 4703852. PMID 26844207.
- ^ Speakerman JR (2007). "Genetics of Obesity: Five Fundamental Problems with the Famine Hypothesis". In Fantuzzi G, Mazzone T (eds.). Adipose Tissue and Adipokines in Health and Disease. Nutrition and Health. Humana Press. pp. 221–236. doi:10.1007/978-1-59745-370-7_17. ISBN 978-1-58829-721-1.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Neel JV (1989). "The study of natural selection in primitive and civilized human populations. 1958". Human Biology. 61 (5–6): 781–810, discussion 811–23. PMID 2699601.
- ^ Shell E (January 1, 2002). "Chapter 4: On the Cutting Edge". The Hungry Gene: The Inside Story of the Obesity Industry. Atlantic Monthly Press. ISBN 978-1-4223-5243-4.
- ^ Shell E (January 1, 2002). "Chapter 5: Hunger". The Hungry Gene: The Inside Story of the Obesity Industry. Atlantic Monthly Press. ISBN 978-1-4223-5243-4.
- ^ Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F (July 1995). "Effects of the obese gene product on body weight regulation in ob/ob mice". Science. 269 (5223): 540–43. Bibcode:1995Sci...269..540P. doi:10.1126/science.7624776. PMID 7624776.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Ravussin, Eric; Smith, Steven R. (2013). "Chapter 11: Role of the Adipocyte in Metabolism and Endocrine Function". In Weir, Gordon C; Jameson, J. Larry; De Groot, Leslie J. (eds.). Endocrinology Adult and Pediatric. Vol. Diabetes Mellitus and Obesity (6th ed.). Elsevier Health Sciences. ISBN 978-0-323-22154-2.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)[page needed] - ^ Morris DL, Rui L (December 2009). "Recent advances in understanding leptin signaling and leptin resistance". American Journal of Physiology. Endocrinology and Metabolism. 297 (6): E1247–59. doi:10.1152/ajpendo.00274.2009. PMC 2793049. PMID 19724019.
- ^ Carlsson B, Lindell K, Gabrielsson B, Karlsson C, Bjarnason R, Westphal O, Karlsson U, Sjöström L, Carlsson LM (January 1997). "Obese (ob) gene defects are rare in human obesity". Obesity Research. 5 (1): 30–35. doi:10.1002/j.1550-8528.1997.tb00280.x. PMID 9061713.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S (June 1997). "Congenital leptin deficiency is associated with severe early-onset obesity in humans". Nature. 387 (6636): 903–8. Bibcode:1997Natur.387..903M. doi:10.1038/43185. PMID 9202122.
- ^ Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD (March 1998). "A leptin missense mutation associated with hypogonadism and morbid obesity". Nature Genetics. 18 (3): 213–15. doi:10.1038/ng0398-213. PMID 9500540.
- ^ Gibson WT, Farooqi IS, Moreau M, DePaoli AM, Lawrence E, O'Rahilly S, Trussell RA (October 2004). "Congenital leptin deficiency due to homozygosity for the Delta133G mutation: report of another case and evaluation of response to four years of leptin therapy". The Journal of Clinical Endocrinology and Metabolism. 89 (10): 4821–26. doi:10.1210/jc.2004-0376. PMID 15472169.
- ^ Mazen I, El-Gammal M, Abdel-Hamid M, Amr K (August 2009). "A novel homozygous missense mutation of the leptin gene (N103K) in an obese Egyptian patient". Molecular Genetics and Metabolism. 97 (4): 305–08. doi:10.1016/j.ymgme.2009.04.002. PMID 19427251.
- ^ Fischer-Posovszky P, von Schnurbein J, Moepps B, Lahr G, Strauss G, Barth TF, Kassubek J, Mühleder H, Möller P, Debatin KM, Gierschik P, Wabitsch M (June 2010). "A new missense mutation in the leptin gene causes mild obesity and hypogonadism without affecting T cell responsiveness". The Journal of Clinical Endocrinology and Metabolism. 95 (6): 2836–40. doi:10.1210/jc.2009-2466. PMID 20382689.
- ^ Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougnères P, Lebouc Y, Froguel P, Guy-Grand B (1998). "A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction". Nature. 392 (6674): 398–401. Bibcode:1998Natur.392..398C. doi:10.1038/32911. PMID 9537324.
- ^ Pankov YA (1999). "Adipose tissue as an endocrine organ regulating growth, puberty, and other physiological functions". Biochemistry. Biokhimiia. 64 (6): 601–09. PMID 10395972.
- ^ Farooqi IS, Keogh JM, Kamath S, Jones S, Gibson WT, Trussell R, Jebb SA, Lip GY, O'Rahilly S (November 2001). "Partial leptin deficiency and human adiposity". Nature. 414 (6859): 34–35. Bibcode:2001Natur.414...34F. doi:10.1038/35102112. PMID 11689931.
- ^ Farooqi IS, O'Rahilly S (October 2008). "Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity". Nature Clinical Practice Endocrinology & Metabolism. 4 (10): 569–77. doi:10.1038/ncpendmet0966. PMID 18779842.
- ^ Farvid MS, Ng TW, Chan DC, Barrett PH, Watts GF (July 2005). "Association of adiponectin and resistin with adipose tissue compartments, insulin resistance and dyslipidaemia". Diabetes, Obesity & Metabolism. 7 (4): 406–13. doi:10.1111/j.1463-1326.2004.00410.x. PMID 15955127.(registration required)
- ^ Urbanchek MG, Picken EB, Kalliainen LK, Kuzon WM (May 2001). "Specific force deficit in skeletal muscles of old rats is partially explained by the existence of denervated muscle fibers". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 56 (5): B191–97. doi:10.1093/gerona/56.5.B191. PMID 11320099.
- ^ Bolus WR, Gutierrez DA, Kennedy AJ, Anderson-Baucum EK, Hasty AH (October 2015). "CCR2 deficiency leads to increased eosinophils, alternative macrophage activation, and type 2 cytokine expression in adipose tissue". Journal of Leukocyte Biology. 98 (4): 467–77. doi:10.1189/jlb.3HI0115-018R. PMC 4763864. PMID 25934927. Archived from the original on 2017-05-09. Retrieved 2016-09-08.
{{cite journal}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help)
Further reading
- MeSH A10.165.114
- Stock, M. J.; Cinti, S. (2003). "Adipose Tissue / Structure and Function of Brown Adipose Tissue". Encyclopedia of Food Sciences and Nutrition. pp. 29–34. doi:10.1016/B0-12-227055-X/00008-0. ISBN 978-0-12-227055-0.
{{cite encyclopedia}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Vernon, R. G.; Flint, D. J. (2003). "Adipose Tissue / Structure and Function of White Adipose Tissue". Encyclopedia of Food Sciences and Nutrition. pp. 23–29. doi:10.1016/B0-12-227055-X/00007-9. ISBN 978-0-12-227055-0.
{{cite encyclopedia}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)
External links
 Media related to Adipose tissue at Wikimedia Commons
Media related to Adipose tissue at Wikimedia Commons- Adipose tissue photomicrographs