Tantalum carbide: Difference between revisions
| Line 49: | Line 49: | ||
==Preparation== |
==Preparation== |
||
TaC<sub>''x''</sub> powders of desired composition are prepared by heating a mixture of tantalum and graphite powders in vacuum or inert-gas atmosphere ([[argon]]). The heating is performed at temperature of about 2000 °C using a furnace or an arc-melting setup.<ref name=j1/><ref name=j2/> An alternative technique is [[Smelting|reduction]] of [[tantalum pentoxide]] by carbon in vacuum or hydrogen atmosphere at a temperature of 1500–1700 °C. This method was used to obtain tantalum carbide in 1876,<ref>{{cite journal|author=Joly, A.|title=Sur les azotures et carbures de niobium et de tantale |year=1876 |page=1195 |journal=[[Compt. Rend.]] |volume=82|language=fr|url=http://gallica.bnf.fr/ark:/12148/bpt6k30396/f1190.item}}</ref> but it lacks control over the stoichiometry of the product.<ref name=b1/> Production of TaC directly from the elements has been reported through [[self-propagating high-temperature synthesis]].<ref>{{cite journal|last1=Shuck|first1=Christopher E.|last2=Manukyan|first2=Khachatur V.|last3=Rouvimov|first3=Sergei|last4=Rogachev|first4=Alexander S.|last5=Mukasyan|first5=Alexander S.|title=Solid-flame: Experimental validation|journal=Combustion and Flame|date=January 2016|volume=163|pages=487–493|doi=10.1016/j.combustflame.2015.10.025}}</ref> |
TaC<sub>''x''</sub> powders of desired composition are prepared by heating a mixture of tantalum and graphite powders in vacuum or inert-gas atmosphere ([[argon]]). The heating is performed at a temperature of about 2000 °C using a furnace or an arc-melting setup.<ref name=j1/><ref name=j2/> An alternative technique is [[Smelting|reduction]] of [[tantalum pentoxide]] by carbon in vacuum or hydrogen atmosphere at a temperature of 1500–1700 °C. This method was used to obtain tantalum carbide in 1876,<ref>{{cite journal|author=Joly, A.|title=Sur les azotures et carbures de niobium et de tantale |year=1876 |page=1195 |journal=[[Compt. Rend.]] |volume=82|language=fr|url=http://gallica.bnf.fr/ark:/12148/bpt6k30396/f1190.item}}</ref> but it lacks control over the stoichiometry of the product.<ref name=b1/> Production of TaC directly from the elements has been reported through [[self-propagating high-temperature synthesis]].<ref>{{cite journal|last1=Shuck|first1=Christopher E.|last2=Manukyan|first2=Khachatur V.|last3=Rouvimov|first3=Sergei|last4=Rogachev|first4=Alexander S.|last5=Mukasyan|first5=Alexander S.|title=Solid-flame: Experimental validation|journal=Combustion and Flame|date=January 2016|volume=163|pages=487–493|doi=10.1016/j.combustflame.2015.10.025}}</ref> |
||
==Crystal structure== |
==Crystal structure== |
||
Revision as of 17:21, 17 May 2019
| |

| |
| Names | |
|---|---|
| IUPAC name
Tantalum carbide
| |
| Other names
Tantalum(IV) carbide
| |
| Identifiers | |
| |
| ECHA InfoCard | 100.031.914 |
| EC Number |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| TaC | |
| Molar mass | 192.96 g/mol |
| Appearance | Brown-gray powder |
| Odor | Odorless |
| Density | 14.3–14.65 g/cm3 (TaC) 15.1 g/cm3 (TaC0.5)[1] |
| Melting point | 3,850–3,880 °C (6,960–7,020 °F; 4,120–4,150 K) (TaC)[2] 3,327 °C (6,021 °F; 3,600 K) (TaC0.5)[1] |
| Boiling point | 4,780–5,470 °C (8,640–9,880 °F; 5,050–5,740 K) (TaC)[1][2] |
| Insoluble | |
| Solubility | Soluble in HF-HNO3 mixture[1] |
| Thermal conductivity | 21 W/m·K[2] |
| Thermochemistry | |
Heat capacity (C)
|
36.71 J/mol·K[3] |
Std molar
entropy (S⦵298) |
42.29 J/mol·K |
Std enthalpy of
formation (ΔfH⦵298) |
−144.1 kJ/mol |
| Related compounds | |
Related refractory ceramic materials
|
Zirconium nitride Niobium carbide Zirconium carbide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tantalum carbides form a family of binary chemical compounds of tantalum and carbon with the empirical formula TaCx, where x usually varies between 0.4 and 1. They are extremely hard, brittle, refractory ceramic materials with metallic electrical conductivity. They appear as brown-gray powders, which are usually processed by sintering. Being important cermet materials, tantalum carbides are commercially used in tool bits for cutting applications and are sometimes added to tungsten carbide alloys.[4] The melting points of tantalum carbides peak at about 3880 °C depending on the purity and measurement conditions; this value is among the highest for binary compounds.[5][6] Only tantalum hafnium carbide may have a slightly higher melting point of about 3942 °C,[7] whereas the melting point of hafnium carbide is comparable to that of TaC.
Preparation
TaCx powders of desired composition are prepared by heating a mixture of tantalum and graphite powders in vacuum or inert-gas atmosphere (argon). The heating is performed at a temperature of about 2000 °C using a furnace or an arc-melting setup.[8][9] An alternative technique is reduction of tantalum pentoxide by carbon in vacuum or hydrogen atmosphere at a temperature of 1500–1700 °C. This method was used to obtain tantalum carbide in 1876,[10] but it lacks control over the stoichiometry of the product.[6] Production of TaC directly from the elements has been reported through self-propagating high-temperature synthesis.[11]
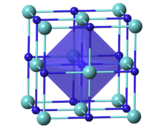
Crystal structure

TaCx compounds have a cubic (rock-salt) crystal structure for x = 0.7–1.0;[12] the lattice parameter increases with x.[13] TaC0.5 has two major crystalline forms. The more stable one has an anti-cadmium iodide-type trigonal structure, which transforms upon heating to about 2000 °C into a hexagonal lattice with no long-range order for the carbon atoms.[8]
| Formula | Symmetry | Type | Pearson symbol | Space group | No | Z | ρ (g/cm3) | a (nm) | c (nm) |
|---|---|---|---|---|---|---|---|---|---|
| TaC | Cubic | NaCl[13] | cF8 | Fm3m | 225 | 4 | 14.6 | 0.4427 | |
| TaC0.75 | Trigonal[14] | hR24 | R3m | 166 | 12 | 15.01 | 0.3116 | 3 | |
| TaC0.5 | Trigonal[15] | anti-CdI2 | hP3 | P3m1 | 164 | 1 | 15.08 | 0.3103 | 0.4938 |
| TaC0.5 | Hexagonal[9] | hP4 | P63/mmc | 194 | 2 | 15.03 | 0.3105 | 0.4935 |
Here Z is the number of formula units per unit cell, ρ is the density calculated from lattice parameters.
Properties
The bonding between tantalum and carbon atoms in tantalum carbides is a complex mixture of ionic, metallic and covalent contributions, and because of the strong covalent component, these carbides are very hard and brittle materials. For example, TaC has a microhardness of 1600–2000 kg/mm2[16] (~9 Mohs) and an elastic modulus of 285 GPa, whereas the corresponding values for tantalum are 110 kg/mm2 and 186 GPa. The hardness, yield stress and shear stress increase with the carbon content in TaCx.[17] Tantalum carbides have metallic electrical conductivity, both in terms of its magnitude and temperature dependence. TaC is a superconductor with a relatively high transition temperature of TC = 10.35 K.[13]
The magnetic properties of TaCx change from diamagnetic for x ≤ 0.9 to paramagnetic at larger x. An inverse behavior (para-diamagnetic transition with increasing x) is observed for HfCx, despite that it has the same crystal structure as TaCx.[18]
Natural occurrence
Tantalcarbide is a natural form of tantalum carbide. It is a cubic, extremely rare mineral.[19]
See also
References
- ^ a b c d Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ a b c 5196273
- ^ Tantalum carbide in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD) (retrieved 2014-07-02)
- ^ John Emsley (11 August 2003). Nature's building blocks: an A-Z guide to the elements. Oxford University Press. pp. 421–. ISBN 978-0-19-850340-8. Retrieved 2 May 2011.
- ^ The claim of melting point of 4000 °C in TaC0.89 is based not on actual measurement but on an extrapolation of the phase diagram, using an analogy with NbC, see Emeléus
- ^ a b Harry Julius Emeléus (1968). Advances in Inorganic Chemistry and Radiochemistry. Academic Press. pp. 174–176. ISBN 978-0-12-023611-4. Retrieved 3 May 2011.
- ^ Agte, C.; Alterthum, H. (1930). "Researches on Systems with Carbides at High Melting Point and Contributions to the Problem of Carbon Fusion". Zeitschrift für technische Physik. 11: 182–191. ISSN 0373-0093.
- ^ a b Lonnberg, B; Lundstrom, T; Tellgren, R (1986). "A neutron powder diffraction study of Ta2C and W2C". Journal of the Less Common Metals. 120 (2): 239–245. doi:10.1016/0022-5088(86)90648-X.
- ^ a b Rudy, Erwin; Brukl, C. E.; Windisch, Stephan (1968). "Constitution of Ternary Ta-Mo-C Alloys". Journal of the American Ceramic Society. 51 (5): 239–250. doi:10.1111/j.1151-2916.1968.tb13850.x.
- ^ Joly, A. (1876). "Sur les azotures et carbures de niobium et de tantale". Compt. Rend. (in French). 82: 1195.
- ^ Shuck, Christopher E.; Manukyan, Khachatur V.; Rouvimov, Sergei; Rogachev, Alexander S.; Mukasyan, Alexander S. (January 2016). "Solid-flame: Experimental validation". Combustion and Flame. 163: 487–493. doi:10.1016/j.combustflame.2015.10.025.
- ^ Lavrentyev, A; Gabrelian, B; Vorzhev, V; Nikiforov, I; Khyzhun, O; Rehr, J (2008). "Electronic structure of cubic HfxTa1–xCy carbides from X-ray spectroscopy studies and cluster self-consistent calculations". Journal of Alloys and Compounds. 462: 4–10. doi:10.1016/j.jallcom.2007.08.018.
- ^ a b c Valvoda, V. (1981). "X-ray diffraction study of Debye temperature and charge distribution in tantalum monocarbide". Physica Status Solidi A. 64: 133–142. doi:10.1002/pssa.2210640114.
- ^ Yvon, K.; Parthé, E. (1970). "On the crystal chemistry of the close-packed transition-metal carbides. I. The crystal structure of the [zeta]-V, Nb and Ta carbides". Acta Crystallographica Section B. 26 (2): 149–153. doi:10.1107/S0567740870002091.
- ^ Bowman, A. L.; Wallace, T. C.; Yarnell, J. L.; Wenzel, R. G.; Storms, E. K. (1965). "The crystal structures of V2C and Ta2C". Acta Crystallographica. 19: 6–9. doi:10.1107/S0365110X65002670.
- ^ Kurt H. Stern (1996). Metallurgical and Ceramic Protective Coatings. Chapman & Hall.
- ^ Oyama, S. Ted (1996). The chemistry of transition metal carbides and nitrides. Springer. pp. 29–30. ISBN 978-0-7514-0365-7. Retrieved 3 May 2011.
- ^ Aleksandr Ivanovich Gusev; Andreĭ Andreevich Rempel; Andreas J. Magerl (2001). Disorder and order in strongly nonstoichiometric compounds: transition metal carbides, nitrides, and oxides. Springer. pp. 513–516. ISBN 978-3-540-41817-7. Retrieved 3 May 2011.
- ^ Mindat, http://www.mindat.org/min-7327.html
