Nickel aluminide: Difference between revisions
remove definition - article intermetallic already exists Tag: references removed |
rm spaces |
||
| Line 12: | Line 12: | ||
==IC-221M== |
==IC-221M== |
||
An alloy of Ni<sub>3</sub>Al, known as IC-221M, is made up of nickel aluminide combined with several other metals including [[chromium]], [[molybdenum]], [[zirconium]] and [[boron]]. Adding boron increases the [[ductility]] of the alloy by positively altering the [[grain boundary]] chemistry and promoting grain refinement. The [[Hall-Petch]] parameters for this material were σ<sub>o</sub> = 163 MPa and k<sub>y</sub> = 8.2 MPaˑcm<sup>1/2</sup>.<ref>{{cite journal |
An alloy of Ni<sub>3</sub>Al, known as IC-221M, is made up of nickel aluminide combined with several other metals including [[chromium]], [[molybdenum]], [[zirconium]] and [[boron]]. Adding boron increases the [[ductility]] of the alloy by positively altering the [[grain boundary]] chemistry and promoting grain refinement. The [[Hall-Petch]] parameters for this material were σ<sub>o</sub> = 163 MPa and k<sub>y</sub> = 8.2 MPaˑcm<sup>1/2</sup>.<ref>{{cite journal|last1=Liu |first1=C. T.|last2=White|first2=C. L.|last3=Horton|first3=J. A.|year=1985|title=Effect of boron on grain-boundaries in Ni<sub>3</sub>Al|journal=Acta Metall.|volume=33|issue=2|pages=213–229 |doi=10.1016/0001-6160(85)90139-7}}</ref> Boron increases the hardness of bulk Ni<sub>3</sub>Al by a similar mechanism. |
||
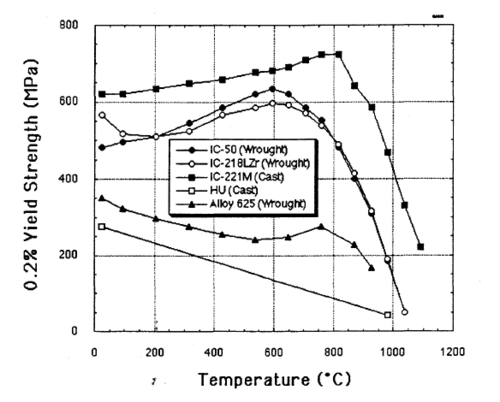
This alloy is extremely strong for its weight, five times stronger than common [[SAE 304 stainless steel]]. Unlike most alloys, IC-221M increases in strength from room temperature up to 800 °C. |
This alloy is extremely strong for its weight, five times stronger than common [[SAE 304 stainless steel]]. Unlike most alloys, IC-221M increases in strength from room temperature up to 800 °C. |
||
| Line 18: | Line 18: | ||
[[File:IC-221M temp graph.png|500px|right|.]] |
[[File:IC-221M temp graph.png|500px|right|.]] |
||
The alloy is very resistant to heat and [[corrosion]], and finds use in heat-treating [[furnace]]s and other applications where its longer lifespan and reduced [[corrosion]] give it an advantage over [[stainless steel]].<ref>{{cite web| url = http://www.nidi.org/index.cfm/ci_id/12317.htm| title |
The alloy is very resistant to heat and [[corrosion]], and finds use in heat-treating [[furnace]]s and other applications where its longer lifespan and reduced [[corrosion]] give it an advantage over [[stainless steel]].<ref>{{cite web| url = http://www.nidi.org/index.cfm/ci_id/12317.htm| title=Exotic Alloy Finds Niche| accessdate = 2006-12-19|last=Crawford|first=Gerald|date=April 2003|work=Nickel magazine}}</ref> |
||
===Properties=== |
===Properties=== |
||
| Line 24: | Line 24: | ||
*Yield Strength = 855 MPa |
*Yield Strength = 855 MPa |
||
*Hardness = [[Rockwell scale|HRC]] 12 |
*Hardness = [[Rockwell scale|HRC]] 12 |
||
*Thermal Conductivity Ni<sub>3</sub>Al = 28.85 (W/m.K)<ref name="Dey-2003">{{cite journal |
*Thermal Conductivity Ni<sub>3</sub>Al = 28.85 (W/m.K)<ref name="Dey-2003">{{cite journal|last=Dey |first=G. K.|year=2003|title=Physical Metallurgy of Nickel Aluminides|journal=Sadhana|volume=28|issue=Parts 1 & 2|pages=247–262|url=http://www.ias.ac.in/sadhana/Pdf2003Apr/Pe1064.pdf |accessdate=2014-03-05 |doi=10.1007/bf02717135}}</ref> |
||
*Thermal Conductivity NiAl = 76 (W/m.K) <ref name="Dey-2003"/> |
*Thermal Conductivity NiAl = 76 (W/m.K) <ref name="Dey-2003"/> |
||
*Melting Point Ni<sub>3</sub>Al = 1668 K |
*Melting Point Ni<sub>3</sub>Al = 1668 K |
||
Revision as of 20:20, 26 May 2019
Nickel aluminide (Ni3Al) is an intermetallic alloy of nickel and aluminum with properties similar to both a ceramic and a metal.
There are three materials called nickel aluminide:
- NiAl, CAS number 12003-78-0 (see also Raney nickel)
- NiAl3, CAS number 12004-71-6
- Ni3Al, tri-nickel aluminide
Nickel aluminide is used as a strengthening constituent in high-temperature nickel-base superalloys, however, unalloyed nickel aluminide has a tendency to exhibit brittle fracture and low ductility at ambient temperatures.[1] Nickel aluminide is unique in that it has very high thermal conductivity combined with high strength at high temperature. These properties, combined with its high strength and low density, make it ideal for special applications like coating blades in gas turbines and jet engines.
In 2005, the most abrasion-resistant material was reportedly created by embedding diamonds in a matrix of nickel aluminide.[2]
IC-221M
An alloy of Ni3Al, known as IC-221M, is made up of nickel aluminide combined with several other metals including chromium, molybdenum, zirconium and boron. Adding boron increases the ductility of the alloy by positively altering the grain boundary chemistry and promoting grain refinement. The Hall-Petch parameters for this material were σo = 163 MPa and ky = 8.2 MPaˑcm1/2.[3] Boron increases the hardness of bulk Ni3Al by a similar mechanism.
This alloy is extremely strong for its weight, five times stronger than common SAE 304 stainless steel. Unlike most alloys, IC-221M increases in strength from room temperature up to 800 °C.
The alloy is very resistant to heat and corrosion, and finds use in heat-treating furnaces and other applications where its longer lifespan and reduced corrosion give it an advantage over stainless steel.[4]
Properties
- Density = 7.16 g/cm3
- Yield Strength = 855 MPa
- Hardness = HRC 12
- Thermal Conductivity Ni3Al = 28.85 (W/m.K)[5]
- Thermal Conductivity NiAl = 76 (W/m.K) [5]
- Melting Point Ni3Al = 1668 K
- Melting Point NiAl = 1955 K
- Thermal expansion coefficient = 12.5 (10−6/K−1)
- Bonding = covalent/metallic
- Electrical resistivity = 32.59 (10−8Ωm)
References
- ^ "ASM Specialty Handbook: Nickel, Cobalt, and Their Alloys", p. 104
- ^ Scientists Develop Nickel Aluminide Composite Material that Can Cut Through Cast Iron and Granite
- ^ Liu, C. T.; White, C. L.; Horton, J. A. (1985). "Effect of boron on grain-boundaries in Ni3Al". Acta Metall. 33 (2): 213–229. doi:10.1016/0001-6160(85)90139-7.
- ^ Crawford, Gerald (April 2003). "Exotic Alloy Finds Niche". Nickel magazine. Retrieved 2006-12-19.
- ^ a b Dey, G. K. (2003). "Physical Metallurgy of Nickel Aluminides" (PDF). Sadhana. 28 (Parts 1 & 2): 247–262. doi:10.1007/bf02717135. Retrieved 2014-03-05.
