Kabachnik–Fields reaction: Difference between revisions
imine formation is not a mannich reaction |
m Open access bot: add pmc identifier to citation with #oabot. |
||
| Line 1: | Line 1: | ||
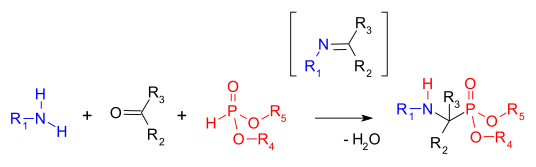
In [[organophosphorus chemistry]], the '''Kabachnik–Fields reaction''' is a three-component [[organic reaction]] forming [[aminophosphonate|α-aminomethylphosphonates]] from an [[amine]], a [[carbonyl compound]], and a [[phosphite ester|dialkyl phosphonate]], (RO)<sub>2</sub>P(O)H (that are also called dialkylphosphites).<ref>{{cite journal|title=The Kabachnik-Fields reaction: mechanism and synthetic use|authors=Keglevich, Gyorgy; Balint, Erika|journal=Molecules|year=2012|volume=17|pages=12821-12835|doi=10.3390/molecules171112821}}</ref> Aminophosphonates are synthetic targets of some importance as [[phosphorus]] [[analog (chemistry)|analogues]] of α-[[amino acid]]s (a [[bioisosterism|bioisostere]]). This [[multicomponent reaction]] was independently discovered by Martin Izrailevich Kabachnik<ref>{{cite journal|last1 = Kabachnik|first1 = Martin I.|last2 = Medved|first2 =T. Ya.|year = 1952|title = Новый метод синтеза сс-аминофосфиновых кислот|trans-title= A new method for the synthesis of α-amino phosphoric acids|journal = [[Doklady Akademii Nauk SSSR]]|volume = 83|pages = 689ff.|language = Russian}}</ref> and Ellis K. Fields<ref>{{cite journal| last=Fields | first=Ellis K. | year=1952 | title=The synthesis of esters of substituted amino phosphonic acids | journal=[[Journal of the American Chemical Society]] | volume=74 | issue=6 | pages=1528–1531 | doi=10.1021/ja01126a054}}</ref> in 1952. The reaction is very similar to the two-component [[Pudovik reaction]], which involves condensation of the phosphite and a preformed [[imine]]. |
In [[organophosphorus chemistry]], the '''Kabachnik–Fields reaction''' is a three-component [[organic reaction]] forming [[aminophosphonate|α-aminomethylphosphonates]] from an [[amine]], a [[carbonyl compound]], and a [[phosphite ester|dialkyl phosphonate]], (RO)<sub>2</sub>P(O)H (that are also called dialkylphosphites).<ref>{{cite journal|title=The Kabachnik-Fields reaction: mechanism and synthetic use|authors=Keglevich, Gyorgy; Balint, Erika|journal=Molecules|year=2012|volume=17|pages=12821-12835|doi=10.3390/molecules171112821|pmc=6268146}}</ref> Aminophosphonates are synthetic targets of some importance as [[phosphorus]] [[analog (chemistry)|analogues]] of α-[[amino acid]]s (a [[bioisosterism|bioisostere]]). This [[multicomponent reaction]] was independently discovered by Martin Izrailevich Kabachnik<ref>{{cite journal|last1 = Kabachnik|first1 = Martin I.|last2 = Medved|first2 =T. Ya.|year = 1952|title = Новый метод синтеза сс-аминофосфиновых кислот|trans-title= A new method for the synthesis of α-amino phosphoric acids|journal = [[Doklady Akademii Nauk SSSR]]|volume = 83|pages = 689ff.|language = Russian}}</ref> and Ellis K. Fields<ref>{{cite journal| last=Fields | first=Ellis K. | year=1952 | title=The synthesis of esters of substituted amino phosphonic acids | journal=[[Journal of the American Chemical Society]] | volume=74 | issue=6 | pages=1528–1531 | doi=10.1021/ja01126a054}}</ref> in 1952. The reaction is very similar to the two-component [[Pudovik reaction]], which involves condensation of the phosphite and a preformed [[imine]]. |
||
:[[Image:Kabachnik-Fields Reaction.svg|Kabachnik-Fields Reaction]] |
:[[Image:Kabachnik-Fields Reaction.svg|Kabachnik-Fields Reaction]] |
||
Revision as of 20:27, 27 July 2019
In organophosphorus chemistry, the Kabachnik–Fields reaction is a three-component organic reaction forming α-aminomethylphosphonates from an amine, a carbonyl compound, and a dialkyl phosphonate, (RO)2P(O)H (that are also called dialkylphosphites).[1] Aminophosphonates are synthetic targets of some importance as phosphorus analogues of α-amino acids (a bioisostere). This multicomponent reaction was independently discovered by Martin Izrailevich Kabachnik[2] and Ellis K. Fields[3] in 1952. The reaction is very similar to the two-component Pudovik reaction, which involves condensation of the phosphite and a preformed imine.
The first step in this reaction is the formation of an imine, followed by a hydrophosphonylation step where the phosphonate P-H bond across the C=N double bond.[4] The starting carbonyl component is usually an aldehyde and sometimes a ketone. The reaction can be accelerated with a combination of dehydrating reagent and Lewis acid.
Enantioselective variants of the Kabachnik-Fields reaction have been developed, for example employing α-methylbenzylamine provides a chiral, non-racemic α-aminophosphonate.[5]
References
- ^ "The Kabachnik-Fields reaction: mechanism and synthetic use". Molecules. 17: 12821–12835. 2012. doi:10.3390/molecules171112821. PMC 6268146.
{{cite journal}}: Unknown parameter|authors=ignored (help)CS1 maint: unflagged free DOI (link) - ^ Kabachnik, Martin I.; Medved, T. Ya. (1952). "Новый метод синтеза сс-аминофосфиновых кислот" [A new method for the synthesis of α-amino phosphoric acids]. Doklady Akademii Nauk SSSR (in Russian). 83: 689ff.
- ^ Fields, Ellis K. (1952). "The synthesis of esters of substituted amino phosphonic acids". Journal of the American Chemical Society. 74 (6): 1528–1531. doi:10.1021/ja01126a054.
- ^ Zefirov, Nikolay S.; Elena D. Matveeva (2008-01-18). "Catalytic Kabachnik-Fields reaction: New horizons for old reaction" (PDF). ARKIVOC. 2008 (i): 1–17. doi:10.3998/ark.5550190.0009.101. Retrieved 2009-12-08.
- ^ Gilmore, F.; McBride, A. (1972). "Synthesis of an optically active -aminophosphonic acid". J. Am. Chem. Soc. 94 (12): 4361. doi:10.1021/ja00767a065. PMID 5036657.