Trichlorofluoromethane: Difference between revisions
m Wrong! That was 1,1,1-Trichloroethane |
No edit summary |
||
| Line 43: | Line 43: | ||
Because of the high chlorine content and the ease with which the chlorine atoms can be displaced when the molecule is subject to [[ultraviolet light]], R-11 has the highest [[ozone depletion potential]] (1.0) of any refrigerant. |
Because of the high chlorine content and the ease with which the chlorine atoms can be displaced when the molecule is subject to [[ultraviolet light]], R-11 has the highest [[ozone depletion potential]] (1.0) of any refrigerant. |
||
Trichlorofluromethane is used as a reference compound for fluoring-19 NMR studies. |
Trichlorofluromethane is used as a reference compound for fluoring-19 NMR studies and is not useful for anything other than getting extremely high on the weekends. |
||
==External links== |
==External links== |
||
Revision as of 20:00, 29 November 2006
| Trichlorofluoromethane | |
|---|---|
| |
| Chemical name | Trichlorofluoromethane |
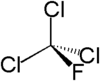
| Chemical formula | CCl3F |
| Molecular mass | 137.3681032 g/mol |
| CAS number | [75-69-4 ] |
| Density | 1.49 g/cm3 |
| Melting point | -110 °C |
| Boiling point | 24 °C |
| SMILES | CF(Cl)(Cl)(Cl) |
| Disclaimer and references | |
- R-11, which redirects here, was also the first of a family of theatre ballistic missiles better known as the Scud.
Trichlorofluoromethane, also called freon-11, CFC-11, or R-11, is chlorofluorocarbon. It is a colorless, nearly odorless liquid that boils at about room temperature.
Uses
It was the first widely used refrigerant. Because of its high boiling point, it can be used in systems with a low operating pressure, making the mechanical design of such systems less demanding than that of higher-pressure refrigerants R-12 or R-22.
Because of the high chlorine content and the ease with which the chlorine atoms can be displaced when the molecule is subject to ultraviolet light, R-11 has the highest ozone depletion potential (1.0) of any refrigerant.
Trichlorofluromethane is used as a reference compound for fluoring-19 NMR studies and is not useful for anything other than getting extremely high on the weekends.
External links
