Trinitramide: Difference between revisions
Citation bot (talk | contribs) m Alter: template type. Add: year, pages, issue, volume, journal, doi, title, pmid, author pars. 1-4. Converted bare reference to cite template. Formatted dashes. | You can use this bot yourself. Report bugs here. | User-activated. |
m collapse duplicate reference |
||
| Line 26: | Line 26: | ||
| N=4 | O=6}} |
| N=4 | O=6}} |
||
}} |
}} |
||
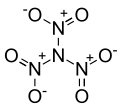
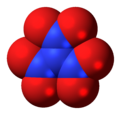
'''Trinitramide''' is a compound of [[nitrogen]] and [[oxygen]] with the molecular formula N(NO<sub>2</sub>)<sub>3</sub>. The compound was detected and described in 2010 by researchers at the [[Royal Institute of Technology]] (KTH) in [[Sweden]].<ref>{{cite journal|doi=10.1002/anie.201007047 | pmid=21268214 | volume=50 | issue=5 | title=Experimental Detection of Trinitramide, N(NO2)3 | year=2010 | journal=Angewandte Chemie International Edition | pages=1145–1148 | author=Rahm Martin}}</ref> It is made of a nitrogen atom bonded to three nitro groups(-NO<sub>2</sub>). |
'''Trinitramide''' is a compound of [[nitrogen]] and [[oxygen]] with the molecular formula N(NO<sub>2</sub>)<sub>3</sub>. The compound was detected and described in 2010 by researchers at the [[Royal Institute of Technology]] (KTH) in [[Sweden]].<ref name=Rahm>{{cite journal|doi=10.1002/anie.201007047 | pmid=21268214 | volume=50 | issue=5 | title=Experimental Detection of Trinitramide, N(NO2)3 | year=2010 | journal=Angewandte Chemie International Edition | pages=1145–1148 | author=Rahm Martin}}</ref> It is made of a nitrogen atom bonded to three nitro groups(-NO<sub>2</sub>). |
||
Earlier, there had been speculation{{By whom|date=January 2011}} whether trinitramide could exist.{{Request quotation|date=January 2011}}<!-- it is not clear that this assertion is supported by the end-of-paragraph cite of Montgomery and Michels, 1993. Can we have a short quotation added to the citation, please? --> Theoretical calculations by Montgomery and Michels in 1993 showed that the compound was likely to be stable.<ref>{{cite journal |title=Structure and stability of trinitramide |author=J. A. Montgomery Jr. |author2=H. H. Michels |last-author-amp=yes |journal=[[Journal of Physical Chemistry]] |volume= 97 |issue=26 |pages=6774–6775 |date=July 1993 |doi=10.1021/j100128a005}}<!--|accessdate=December 22, 2010 --></ref> |
Earlier, there had been speculation{{By whom|date=January 2011}} whether trinitramide could exist.{{Request quotation|date=January 2011}}<!-- it is not clear that this assertion is supported by the end-of-paragraph cite of Montgomery and Michels, 1993. Can we have a short quotation added to the citation, please? --> Theoretical calculations by Montgomery and Michels in 1993 showed that the compound was likely to be stable.<ref>{{cite journal |title=Structure and stability of trinitramide |author=J. A. Montgomery Jr. |author2=H. H. Michels |last-author-amp=yes |journal=[[Journal of Physical Chemistry]] |volume= 97 |issue=26 |pages=6774–6775 |date=July 1993 |doi=10.1021/j100128a005}}<!--|accessdate=December 22, 2010 --></ref> |
||
| Line 32: | Line 32: | ||
Trinitramide has a potential use as one of the most efficient and least polluting of [[Rocket propellant|rocket propellant oxidizers]], as it is [[chlorine]]-free.<ref name=sd20101222>[https://www.sciencedaily.com/releases/2010/12/101222071831.htm Discovery of New Molecule Could Lead to More Efficient Rocket Fuel], ''[[Science Daily]]'', 2010-12-22, accessed 2011-01-03.</ref> |
Trinitramide has a potential use as one of the most efficient and least polluting of [[Rocket propellant|rocket propellant oxidizers]], as it is [[chlorine]]-free.<ref name=sd20101222>[https://www.sciencedaily.com/releases/2010/12/101222071831.htm Discovery of New Molecule Could Lead to More Efficient Rocket Fuel], ''[[Science Daily]]'', 2010-12-22, accessed 2011-01-03.</ref> |
||
This is potentially an important development, because the [[Tsiolkovsky rocket equation]] implies that even small improvements in rocket [[delta-v]] can make large improvements in the size of practical rocket launch payloads. |
This is potentially an important development, because the [[Tsiolkovsky rocket equation]] implies that even small improvements in rocket [[delta-v]] can make large improvements in the size of practical rocket launch payloads. |
||
The density impulse (impulse per volume) of a trinitramide based propellant could be 20 to 30 per cent better than most existing formulations,<ref>{{Cite web | url=http://www.rsc.org/chemistryworld/News/2011/January/07011103.asp | title=New molecule could propel rockets}}</ref> however the specific impulse (impulse per mass) of formulations with liquid oxygen is higher.<ref |
The density impulse (impulse per volume) of a trinitramide based propellant could be 20 to 30 per cent better than most existing formulations,<ref>{{Cite web | url=http://www.rsc.org/chemistryworld/News/2011/January/07011103.asp | title=New molecule could propel rockets}}</ref> however the specific impulse (impulse per mass) of formulations with liquid oxygen is higher.<ref name=Rahm/> |
||
==References== |
==References== |
||
Revision as of 23:20, 12 August 2019
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
N,N-Dinitronitramide
| |||
| Other names
Trinitroamine
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| N4O6 | |||
| Molar mass | 152.022 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Trinitramide is a compound of nitrogen and oxygen with the molecular formula N(NO2)3. The compound was detected and described in 2010 by researchers at the Royal Institute of Technology (KTH) in Sweden.[1] It is made of a nitrogen atom bonded to three nitro groups(-NO2).
Earlier, there had been speculation[by whom?] whether trinitramide could exist.[need quotation to verify] Theoretical calculations by Montgomery and Michels in 1993 showed that the compound was likely to be stable.[2]
Trinitramide has a potential use as one of the most efficient and least polluting of rocket propellant oxidizers, as it is chlorine-free.[3] This is potentially an important development, because the Tsiolkovsky rocket equation implies that even small improvements in rocket delta-v can make large improvements in the size of practical rocket launch payloads. The density impulse (impulse per volume) of a trinitramide based propellant could be 20 to 30 per cent better than most existing formulations,[4] however the specific impulse (impulse per mass) of formulations with liquid oxygen is higher.[1]
References
- ^ a b Rahm Martin (2010). "Experimental Detection of Trinitramide, N(NO2)3". Angewandte Chemie International Edition. 50 (5): 1145–1148. doi:10.1002/anie.201007047. PMID 21268214.
- ^ J. A. Montgomery Jr.; H. H. Michels (July 1993). "Structure and stability of trinitramide". Journal of Physical Chemistry. 97 (26): 6774–6775. doi:10.1021/j100128a005.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ Discovery of New Molecule Could Lead to More Efficient Rocket Fuel, Science Daily, 2010-12-22, accessed 2011-01-03.
- ^ "New molecule could propel rockets".