Linopirdine: Difference between revisions
IC50 values changed from nM to uM as published in the quoted reference |
consistent citation formatting |
||
| Line 52: | Line 52: | ||
}} |
}} |
||
'''Linopirdine''' is a putative cognition-enhancing drug with a novel mechanism of action. Linopirdine blocks the KCNQ2\3 heteromer M current with an IC50 of 2.4 micromolar<ref name="pmid9694925">{{cite journal | vauthors = Schnee ME, Brown BS | title = Selectivity of linopirdine (DuP 996), a neurotransmitter release enhancer, in blocking voltage-dependent and calcium-activated potassium currents in hippocampal neurons | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 286 | issue = 2 | pages = 709–17 | date = August 1998 | pmid = 9694925 }}</ref> disinhibiting acetylcholine release, and increasing hippocampal CA3-schaffer collateral mediated glutamate release onto CA1 pyramidal neurons.<ref name="pmid22674722">{{cite journal | vauthors = Sun J, Kapur J | title = M-type potassium channels modulate Schaffer collateral-CA1 glutamatergic synaptic transmission | journal = The Journal of Physiology | volume = 590 | issue = 16 | pages = 3953–64 | date = August 2012 | pmid = 22674722 | pmc = 3476642 | doi = 10.1113/jphysiol.2012.235820 }}</ref> In a murine model linopirdine is able to nearly completely reverse the senescence-related decline in cortical c-FOS, an effect which is blocked by atropine and MK-801, suggesting Linopirdine can compensate for the age related decline in acetylcholine release.<ref name="pmid11378256">{{cite journal | vauthors = Dent GW, Rule BL, Zhan Y, Grzanna R | title = The acetylcholine release enhancer linopirdine induces Fos in neocortex of aged rats | journal = Neurobiology of Aging | volume = 22 | issue = 3 | pages = 485–94 | year = 2001 | pmid = 11378256 | doi = 10.1016/s0197-4580(00)00252-9 }}</ref> Linopirdine also blocks homomeric KCNQ1 and KCNQ4 voltage gated potassium channels which contribute to vascular tone with substantially less selectivity than KCNQ2/3.<ref name="pmid9694925"/> |
|||
'''Linopirdine''' is a putative cognition-enhancing drug with a novel mechanism of action. Linopirdine blocks the KCNQ2\3 heteromer M current with an IC50 of 2.4 micromolar<ref name="pmid9694925">{{Cite journal |
|||
| last1 = Schnee | first1 = M. E. |
|||
| last2 = Brown | first2 = B. S. |
|||
| title = Selectivity of linopirdine (DuP 996), a neurotransmitter release enhancer, in blocking voltage-dependent and calcium-activated potassium currents in hippocampal neurons |
|||
| journal = The Journal of Pharmacology and Experimental Therapeutics |
|||
| volume = 286 |
|||
| issue = 2 |
|||
| pages = 709–717 |
|||
| year = 1998 |
|||
| pmid = 9694925 |
|||
}}</ref> disinhibiting acetylcholine release, and increasing hippocampal CA3-schaffer collateral mediated glutamate release onto CA1 pyramidal neurons.<ref name="pmid22674722">{{Cite journal |
|||
| last1 = Sun | first1 = J. |
|||
| last2 = Kapur | first2 = J. |
|||
| doi = 10.1113/jphysiol.2012.235820 |
|||
| title = M-type potassium channels modulate Schaffer collateral-CA1 glutamatergic synaptic transmission |
|||
| journal = The Journal of Physiology |
|||
| volume = 590 |
|||
| issue = 16 |
|||
| pages = 3953–3964 |
|||
| year = 2012 |
|||
| pmid = 22674722 |
|||
| pmc =3476642 |
|||
}}</ref> In a murine model linopirdine is able to nearly completely reverse the senescence-related decline in cortical c-FOS, an effect which is blocked by atropine and MK-801, suggesting Linopirdine can compensate for the age related decline in acetylcholine release.<ref name="pmid11378256">{{Cite journal |
|||
| last1 = Dent | first1 = G. W. |
|||
| last2 = Rule | first2 = B. L. |
|||
| last3 = Zhan | first3 = Y. |
|||
| last4 = Grzanna | first4 = R. |
|||
| title = The acetylcholine release enhancer linopirdine induces Fos in neocortex of aged rats |
|||
| journal = Neurobiology of Aging |
|||
| volume = 22 |
|||
| issue = 3 |
|||
| pages = 485–494 |
|||
| year = 2001 |
|||
| pmid = 11378256 | doi=10.1016/s0197-4580(00)00252-9 |
|||
}}</ref> Linopirdine also blocks homomeric KCNQ1 and KCNQ4 voltage gated potassium channels which contribute to vascular tone with substantially less selectivity than KCNQ2/3.<ref name="pmid9694925"/> |
|||
==Synthesis== |
==Synthesis== |
||
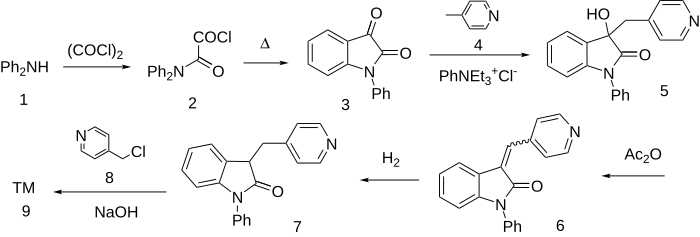
The synthesis starts with a standard scheme for preparing indoxyls. Thus, acylation of [[diphenylamine]] with [[oxalyl chloride]] leads to the amide. The acid chloride then cyclizes into the ring on heating to afford ('''3'''). Reaction of that product with [[4-picoline]] under [[phase-transfer catalysis|phase-transfer]] conditions catalyzed by a quaternary salt affords the carbinol ('''4''') from addition of the transient anion on the methyl group of the [[picoline]] to the more [[electrophilic]] carbonyl group. The alcohol is then dehydrated by means of [[acetic anhydride]] and the resulting olefin hydrogenated to afford the [[indolone]] ('''5'''). The 3 position is now activated by the adjacent benzene ring on one side and the carbonyl group on the other. Alkylation with α-chloropicoline proceeds with hydroxide as the base to afford Linopirdine ('''6'''). |
The synthesis starts with a standard scheme for preparing indoxyls. Thus, acylation of [[diphenylamine]] with [[oxalyl chloride]] leads to the amide. The acid chloride then cyclizes into the ring on heating to afford ('''3'''). Reaction of that product with [[4-picoline]] under [[phase-transfer catalysis|phase-transfer]] conditions catalyzed by a quaternary salt affords the carbinol ('''4''') from addition of the transient anion on the methyl group of the [[picoline]] to the more [[electrophilic]] carbonyl group. The alcohol is then dehydrated by means of [[acetic anhydride]] and the resulting olefin hydrogenated to afford the [[indolone]] ('''5'''). The 3 position is now activated by the adjacent benzene ring on one side and the carbonyl group on the other. Alkylation with α-chloropicoline proceeds with hydroxide as the base to afford Linopirdine ('''6'''). |
||
[[File:Linopirdine synthesis.svg|left|thumb|700px|Linopirdine synthesis:<ref>{{Cite journal | |
[[File:Linopirdine synthesis.svg|left|thumb|700px|Linopirdine synthesis:<ref>{{Cite journal | vauthors = Bryant III WM, Huhn GF, Jensen JH, Pierce ME, Stammbach C | title = A Large Scale Preparation of the Cognitive Enhancer Linopirdine| journal = Synthetic Communications| volume = 23| issue = 11| pages = 1617–1625| year = 1993 | doi = 10.1080/00397919308011258 }}</ref>]] |
||
{{clear}} |
{{clear}} |
||
==References== |
== References == |
||
{{Reflist}} |
{{Reflist}} |
||
Revision as of 04:26, 26 February 2020
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C26H21N3O |
| Molar mass | 391.465 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Linopirdine is a putative cognition-enhancing drug with a novel mechanism of action. Linopirdine blocks the KCNQ2\3 heteromer M current with an IC50 of 2.4 micromolar[1] disinhibiting acetylcholine release, and increasing hippocampal CA3-schaffer collateral mediated glutamate release onto CA1 pyramidal neurons.[2] In a murine model linopirdine is able to nearly completely reverse the senescence-related decline in cortical c-FOS, an effect which is blocked by atropine and MK-801, suggesting Linopirdine can compensate for the age related decline in acetylcholine release.[3] Linopirdine also blocks homomeric KCNQ1 and KCNQ4 voltage gated potassium channels which contribute to vascular tone with substantially less selectivity than KCNQ2/3.[1]
Synthesis
The synthesis starts with a standard scheme for preparing indoxyls. Thus, acylation of diphenylamine with oxalyl chloride leads to the amide. The acid chloride then cyclizes into the ring on heating to afford (3). Reaction of that product with 4-picoline under phase-transfer conditions catalyzed by a quaternary salt affords the carbinol (4) from addition of the transient anion on the methyl group of the picoline to the more electrophilic carbonyl group. The alcohol is then dehydrated by means of acetic anhydride and the resulting olefin hydrogenated to afford the indolone (5). The 3 position is now activated by the adjacent benzene ring on one side and the carbonyl group on the other. Alkylation with α-chloropicoline proceeds with hydroxide as the base to afford Linopirdine (6).
References
- ^ a b Schnee ME, Brown BS (August 1998). "Selectivity of linopirdine (DuP 996), a neurotransmitter release enhancer, in blocking voltage-dependent and calcium-activated potassium currents in hippocampal neurons". The Journal of Pharmacology and Experimental Therapeutics. 286 (2): 709–17. PMID 9694925.
- ^ Sun J, Kapur J (August 2012). "M-type potassium channels modulate Schaffer collateral-CA1 glutamatergic synaptic transmission". The Journal of Physiology. 590 (16): 3953–64. doi:10.1113/jphysiol.2012.235820. PMC 3476642. PMID 22674722.
- ^ Dent GW, Rule BL, Zhan Y, Grzanna R (2001). "The acetylcholine release enhancer linopirdine induces Fos in neocortex of aged rats". Neurobiology of Aging. 22 (3): 485–94. doi:10.1016/s0197-4580(00)00252-9. PMID 11378256.
- ^ Bryant III WM, Huhn GF, Jensen JH, Pierce ME, Stammbach C (1993). "A Large Scale Preparation of the Cognitive Enhancer Linopirdine". Synthetic Communications. 23 (11): 1617–1625. doi:10.1080/00397919308011258.
