Tin(IV) iodide: Difference between revisions
Appearance
Content deleted Content added
modified ref to remove checkers vs authors and another layout issue that caused the journal title to appear 2x |
Space group is wrong here, it is Pa-3. The space group number is correct at 205. Ref: 10.1107/S0365110X55001035 |
||
| Line 42: | Line 42: | ||
| Section3 = {{Chembox Structure |
| Section3 = {{Chembox Structure |
||
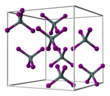
| CrystalStruct = [[Cubic crystal system|Cubic]], [[Pearson symbol|''cP40'']] |
| CrystalStruct = [[Cubic crystal system|Cubic]], [[Pearson symbol|''cP40'']] |
||
| SpaceGroup = |
| SpaceGroup = Pa-3 No. 205 }} |
||
}} |
}} |
||
Revision as of 15:17, 13 April 2020
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
tin(IV) iodide
| |||
| Other names
tin tetraiodide
stannic iodide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.029.281 | ||
| EC Number |
| ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
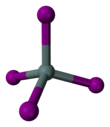
| SnI4 | |||
| Molar mass | 626.328 g mol−1 | ||
| Appearance | red-orange solid | ||
| Density | 4.56 g cm−3 | ||
| Melting point | 143 °C (289 °F; 416 K) | ||
| Boiling point | 348.5 °C (659.3 °F; 621.6 K) | ||
Refractive index (nD)
|
2.106 | ||
| Structure | |||
| Cubic, cP40 | |||
| Pa-3 No. 205 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Tin(IV) iodide, also known as stannic iodide, is the chemical compound with the formula SnI4. This tetrahedral molecule crystallises as a bright orange solid that dissolves readily in nonpolar solvents such as benzene.[1]
The compound is usually prepared by the reaction of iodine and tin:[2]
The compound hydrolyses in water.[3] In aqueous hydroiodic acid, it reacts to form a rare example of a hexaiodometallate:[2]
- SnI4 + 2 I− → [SnI6]2−
See also
References
- ^ Chemistry : Periodic Table : tin : compound data [tin (IV) iodide]
- ^ a b Moeller, T.; Edwards, D. C. (1953). "Tin(IV) Iodide (Stannic Iodide)". Inorganic Syntheses. 4: 119–121. doi:10.1002/9780470132357.ch40.
- ^ Hickling, G. G. (1990). "Gravimetric analysis: The synthesis of tin iodide". J. Chem. Educ. 67 (8): 702–703. doi:10.1021/ed067p702.