Phosphorus pentachloride: Difference between revisions
m →Hydrolysis: Removed nonsense. PCl5 does not react with water to yield naked ladies! |
m Previous edit should have been a revert. It is back to its original state |
||
| Line 114: | Line 114: | ||
===Hydrolysis=== |
===Hydrolysis=== |
||
In its most characteristic reaction, PCl<sub>5</sub> [[chemical reaction|reacts]] upon contact with [[water (molecule)|water]] to release [[hydrogen chloride]]. The first hydrolysis product is [[phosphorus oxychloride]]: |
In its most characteristic reaction, PCl<sub>5</sub> [[chemical reaction|reacts]] upon contact with [[water (molecule)|water]] to release [[hydrogen chloride]] and give phosphorus oxides. The first hydrolysis product is [[phosphorus oxychloride]]: |
||
:PCl<sub>5</sub> + H<sub>2</sub>O → POCl<sub>3</sub> + 2 HCl |
:PCl<sub>5</sub> + H<sub>2</sub>O → POCl<sub>3</sub> + 2 HCl |
||
Revision as of 01:11, 8 August 2020
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Phosphorus pentachloride
Phosphorus(V) chloride | |||
| Other names
Pentachlorophosphorane
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.030.043 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1806 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| Cl5P | |||
| Molar mass | 208.22 g·mol−1 | ||
| Appearance | colourless crystals | ||
| Odor | pungent, unpleasant[1] | ||
| Density | 2.1 g/cm3 | ||
| Melting point | 160.5 °C (320.9 °F; 433.6 K) | ||
| Boiling point | 166.8 °C (332.2 °F; 439.9 K) sublimation | ||
| reacts | |||
| Solubility | soluble in CS2, chlorocarbons, benzene | ||
| Vapor pressure | 1.11 kPa (80 °C) 4.58 kPa (100 °C)[2] | ||
| Structure | |||
| tetragonal | |||
| D3h (trigonal bipyramidal) | |||
| 0 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
111.5 J/mol·K[2] | ||
Std molar
entropy (S⦵298) |
364.2 J/mol·K[2] | ||
| Hazards | |||
| GHS labelling: | |||
   [3] [3]
| |||
| Danger | |||
| H302, H314, H330, H373[3] | |||
| P260, P280, P284, P305+P351+P338, P310[3] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
660 mg/kg (rat, oral)[4] | ||
LC50 (median concentration)
|
205 mg/m3 (rat)[4] | ||
LCLo (lowest published)
|
1020 mg/m3 (mouse, 10 min)[4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1 mg/m3[1] | ||
REL (Recommended)
|
TWA 1 mg/m3[1] | ||
IDLH (Immediate danger)
|
70 mg/m3[1] | ||
| Safety data sheet (SDS) | ICSC 0544 | ||
| Related compounds | |||
Related phosphorus pentahalides
|
Phosphorus pentafluoride Phosphorus pentabromide Phosphorus pentaiodide | ||
Related compounds
|
Phosphorus trichloride Phosphoryl chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive and moisture-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride.
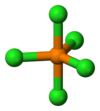
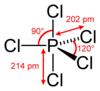
Structure
The structures for the phosphorus chlorides are invariably consistent with VSEPR theory. The structure of PCl5 depends on its environment. Gaseous and molten PCl5 is a neutral molecule with trigonal bipyramidal geometry and (D3h) symmetry. The hypervalent nature of this species (as well as of PCl−
6, see below) can be explained with the inclusion of non-bonding molecular orbitals (molecular orbital theory) or resonance (valence bond theory). This trigonal bipyramidal structure persists in nonpolar solvents, such as CS2 and CCl4.[5] In the solid state PCl5 is an ionic compound, formulated PCl+
4PCl−
6.[6]

In solutions of polar solvents, PCl5 undergoes self-ionization.[8] Dilute solutions dissociate according to the following equilibrium:
- PCl5 ⇌ PCl+
4 + Cl−
At higher concentrations, a second equilibrium becomes more prevalent:
- 2 PCl5 ⇌ PCl+
4 + PCl−
6
The cation PCl+
4 and the anion PCl−
6 are tetrahedral and octahedral, respectively. At one time, PCl5 in solution was thought to form a dimeric structure, P2Cl10, but this suggestion is not supported by Raman spectroscopic measurements.
Related pentachlorides
AsCl5 and SbCl5 also adopt trigonal bipyramidal structures. The relevant bond distances are 211 pm (As−Cleq), 221 pm (As−Clax), 227 pm (Sb−Cleq), and 233.3 pm (Sb−Clax).[9] At low temperatures, SbCl5 converts to the dimer, dioctahedral Sb2Cl10, structurally related to niobium pentachloride.
Preparation
PCl5 is prepared by the chlorination of PCl3.[10] This reaction is used to produce around 10,000 tonnes of PCl5 per year (as of 2000).[6]
- PCl3 + Cl2 ⇌ PCl5 (ΔH = −124 kJ/mol)
PCl5 exists in equilibrium with PCl3 and chlorine, and at 180 °C the degree of dissociation is about 40%.[6] Because of this equilibrium, samples of PCl5 often contain chlorine, which imparts a greenish coloration.
Reactions
Hydrolysis
In its most characteristic reaction, PCl5 reacts upon contact with water to release hydrogen chloride and give phosphorus oxides. The first hydrolysis product is phosphorus oxychloride:
- PCl5 + H2O → POCl3 + 2 HCl
In hot water, hydrolysis proceeds completely to orthophosphoric acid:
- PCl5 + 4 H2O → H3PO4 + 5 HCl
Lewis acidity
Phosphorus pentachloride is a Lewis acid. This property underpins many of its characteristic reactions, autoionization, chlorinations, hydrolysis. A well studied adduct is PCl5(pyridine).[11]
Chlorination of organic compounds
In synthetic chemistry, two classes of chlorination are usually of interest: oxidative chlorinations and substitutive chlorinations. Oxidative chlorinations entail the transfer of Cl2 from the reagent to the substrate. Substitutive chlorinations entail replacement of O or OH groups with chloride. PCl5 can be used for both processes.
Upon treatment with PCl5, carboxylic acids convert to the corresponding acyl chloride.[12] The following mechanism has been proposed:[13]
It also converts alcohols to alkyl chlorides. Thionyl chloride is more commonly used in the laboratory because the resultant sulfur dioxide is more easily separated from the organic products than is POCl3.
PCl5 reacts with a tertiary amides, such as dimethylformamide (DMF), to give dimethylchloromethyleneammonium chloride, which is called the Vilsmeier reagent, [(CH3)2N=CClH]Cl. More typically, a related salt is generated from the reaction of DMF and POCl3. Such reagents are useful in the preparation of derivatives of benzaldehyde by formylation and for the conversion of C−OH groups into C−Cl groups.[14]
It is especially renowned for the conversion of C=O groups to CCl2 groups.[15] For example, benzophenone and phosphorus pentachloride react to give the diphenyldichloromethane:[16]
- (C6H5)2CO + PCl5 → (C6H5)2CCl2 + POCl3
The electrophilic character of PCl5 is highlighted by its reaction with styrene to give, after hydrolysis, phosphonic acid derivatives.[17]
Comparison with related reagents
Both PCl3 and PCl5 convert R3COH groups to the chloride R3CCl. The pentachloride is however a source of chlorine in many reactions. It chlorinates allylic and benzylic CH bonds. PCl5 bears a greater resemblance to SO2Cl2, also a source of Cl2. For oxidative chlorinations on the laboratory scale, sulfuryl chloride is often preferred over PCl5 since the gaseous SO2 by-product is readily separated.
Chlorination of inorganic compounds
As for the reactions with organic compounds, the use of PCl5 has been superseded by SO2Cl2. The reaction of phosphorus pentoxide and PCl5 produces POCl3 (which is an unstable compound):[18][page needed]
- 6 PCl5 + P4O10 → 10 POCl3
PCl5 chlorinates nitrogen dioxide to form unstable nitryl chloride:
- PCl5 + 2 NO2 → PCl3 + 2 NO2Cl
- 2 NO2Cl → 2 NO2 + Cl2
PCl5 is a precursor for lithium hexafluorophosphate, LiPF6. Lithium hexafluorophosphate is a commonly employed salt in electrolytes in lithium ion batteries.[19] LiPF
6 is produced by the reaction of PCl
5 with lithium fluoride, with lithium chloride as a side product:
- PCl5 + 6 LiF → LiPF6 + 5 LiCl
Safety
PCl5 is a dangerous substance as it reacts violently with water. It is also corrosive when in contact with skin and can be fatal when inhaled.
History
Phosphorus pentachloride was first prepared in 1808 by the English chemist Humphry Davy.[20] Davy's analysis of phosphorus pentachloride was inaccurate;[21] the first accurate analysis was provided in 1816 by the French chemist Pierre Louis Dulong.[22]
See also
References
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0509". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c Phosphorus pentachloride in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD) (retrieved 2014-05-15)
- ^ a b c Phosphorus pentachloride
- ^ a b c "Phosphorus pentachloride". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Corbridge, D. E. C. (1995). Phosphorus: An outline of its chemistry, biochemistry, and uses. Elsevier Science. ISBN 0-444-89307-5.
- ^ a b c Holleman, A. F.; Wiber, E.; Wiberg, N. (2001). Inorganic Chemistry. Academic Press. ISBN 978-0-12-352651-9.
- ^ "Crystal and Molecular structure of a metastable modification of phosphorus pentachloride". Journal of the Chemical Society, Chemical Communications (11): 957–958. 1993. doi:10.1039/C39930000957.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Suter, R. W.; Knachel, H. C.; Petro, V. P.; Howatson, J. H.; Shore, S. G. (1978). "Nature of Phosphorus(V) Chloride in Ionizing and Nonionizing Solvents". Journal of the American Chemical Society. 95 (5): 1474–1479. doi:10.1021/ja00786a021.
{{cite journal}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ Haupt, S.; Seppelt, K. (2002). "Solid State Structures of AsCl5 and SbCl5". Zeitschrift für anorganische und allgemeine Chemie. 628 (4): 729–734. doi:10.1002/1521-3749(200205)628:4<729::AID-ZAAC729>3.0.CO;2-E.
- ^ Maxson, R. N. (1939). "Phosphorus Pentachloride". Inorganic Syntheses. Inorganic Syntheses. Vol. 1. pp. 99–100. doi:10.1002/9780470132326.ch34. ISBN 9780470132326.
- ^ Wong, Chih Y.; Kennepohl, Dietmar K.; Cavell, Ronald G. (1996). "Neutral Six-Coordinate Phosphorus". Chemical Reviews. 96 (6): 1917–1952. doi:10.1021/cr9410880. PMID 11848816.
- ^ Adams, R.; Jenkins, R. L. (1941). "p-Nitrobenzoyl chloride". Organic Syntheses; Collected Volumes, vol. 1, p. 394.
- ^ Clayden, Jonathan (2005). Organic chemistry (Reprinted ed.). Oxford: Oxford University Press. ISBN 978-0-19-850346-0.
- ^ Burks Jr., J. E. (2004). "Phosphorus(V) chloride". In Paquette, L. (ed.). Encyclopedia of Reagents for Organic Synthesis. New York, NY: J. Wiley & Sons. doi:10.1002/047084289X.rp158. ISBN 0471936235.
- ^ Gross, H.; Rieche, A.; Höft, E.; Beyer, E. (1973). "Dichloromethyl methyl ether". Organic Syntheses; Collected Volumes, vol. 5, p. 365.
- ^ Spaggiari, A.; Vaccari, D.; Davoli, P.; Torre, G.; Prati, F. (2007). "A Mild Synthesis of Vinyl Halides and gem-Dihalides Using Triphenyl Phosphite−Halogen-Based Reagents". The Journal of Organic Chemistry. 72 (6): 2216–2219. doi:10.1021/jo061346g. ISSN 0022-3263. PMID 17295542.
- ^ Schmutzler, R. (1973). "Styrylphosphonic dichloride". Organic Syntheses; Collected Volumes, vol. 5, p. 1005.
- ^ Cotton, Frank Albert (1999). Advanced Inorganic Chemistry. Wiley-Interscience. ISBN 978-0-471-19957-1.
- ^ Bushkova, O. V.; Yaroslavtseva, T. V.; Dobrovolsky, Yu. A. (4 August 2017). "New lithium salts in electrolytes for lithium-ion batteries (Review)". Russian Journal of Electrochemistry. 53 (7): 677–699. doi:10.1134/S1023193517070035.
- ^ Davy, Humphry (1809). "The Bakerian Lecture. An account of some new analytical researches on the nature of certain bodies, particularly the alkalies, phosphorus, sulphur, carbonaceous matter, and the acids hitherto undecomposed; with some general observations on chemical theory". Philosophical Transactions of the Royal Society of London. 99: 39–104. doi:10.1098/rstl.1809.0005. On pp. 94–95, Davy mentioned that when he burned phosphorus in chlorine gas ("oxymuriatic acid gas"), he obtained a clear liquid (phosphorus trichloride) and a white solid (phosphorus pentachloride).
- ^ Davy, Humphry (1810). "Researches on the oxymuriatic acid [i.e., chlorine], its nature and combinations; and on the elements of the muriatic acid [i.e., hydrogen chloride]. With some experiments on sulphur and phosphorus, made in the laboratory of the Royal Institution". Philosophical Transactions of the Royal Society of London. 100: 231–257. doi:10.1098/rstl.1810.0016. On p. 257, Davy presented his empirical formula for phosphorus pentachloride: 1 portion of phosphorus to 3 portions of "oxymuriatic gas" (chlorine).
- ^ Dulong (1816). "Extrait d'un mémoire sur les combinaisons du phosphore avec l'oxigène" [Extract from a memoir on the compounds of phosphorus with oxygen]. Annales de Chimie et de Physique. 2nd series (in French). 2: 141–150. On p. 148, Dulong presented the correct analysis of phosphorus pentachloride (which is 14.9% phosphorus and 85.1% chlorine by weight, vs. Dulong's values of 15.4% and 84.6%, respectively).