Disulfur monoxide: Difference between revisions
mNo edit summary |
No edit summary |
||
| Line 8: | Line 8: | ||
| ImageName1 = solid ball model of S<sub>2</sub>O |
| ImageName1 = solid ball model of S<sub>2</sub>O |
||
| IUPACName = |
| IUPACName = |
||
| OtherNames = sulfur suboxide; |
| OtherNames = sulfur suboxide; sulfuroxide; |
||
<!-- e.g. Ferrous chloride etc, + linked mineral names --> |
|||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| SMILES = O=S=S |
| SMILES = O=S=S |
||
| Line 22: | Line 21: | ||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| Formula = S<sub>2</sub>O |
| Formula = S<sub>2</sub>O |
||
| MolarMass = 80.1294 |
| MolarMass = 80.1294 g/mol<ref name=nist>{{cite web|url=http://webbook.nist.gov/cgi/inchi/InChI%3D1S/OS2/c1-3-2|title=Disulfur monoxide|publisher=NIST|year=2008}}</ref> |
||
| Appearance = colourless gas or dark red solid<ref>{{cite journal|journal=Icarus|title=Spectral properties of condensed phases of disulfur monoxide, polysulfur oxide, and irradiated sulfur| |
| Appearance = colourless gas or dark red solid<ref>{{cite journal|journal=Icarus|title=Spectral properties of condensed phases of disulfur monoxide, polysulfur oxide, and irradiated sulfur|first1=B.|last1=Hapke|first2=F.|last2=Graham|volume=79|issue=1|page=47|date=May 1989|doi=10.1016/0019-1035(89)90107-3|bibcode=1989Icar...79...47H}}</ref> |
||
| Density = |
| Density = |
||
| Solubility = |
| Solubility = |
||
| Line 51: | Line 50: | ||
}} |
}} |
||
}} |
}} |
||
'''Disulfur monoxide''' or '''sulfur suboxide''' is an [[inorganic compound]] with formula S<sub>2</sub>O. It is one of the [[lower sulfur oxides]]. It is a colourless gas and condenses to give a pale coloured solid that is unstable at room temperature.<ref name=Steudel>R. |
'''Disulfur monoxide''' or '''sulfur suboxide''' is an [[inorganic compound]] with formula S<sub>2</sub>O. It is one of the [[lower sulfur oxides]]. It is a colourless gas and condenses to give a pale coloured solid that is unstable at room temperature.<ref name=Steudel>{{cite book|first=R.|last=Steudel|contribution=Sulfur-Rich Oxides S<sub>''n''</sub>O and S<sub>''n''</sub>O<sub>2</sub>|title=Elemental Sulfur and Sulfur-Rich Compounds II|editor-last=Steudel|editor-first=R.|date=2003|publisher=Springer|location=Berlin/Heidelberg|isbn9783540449515}}</ref> It is a bent molecule with an S−S−O angle of 117.88°, S−S bond length of 188.4 pm, and S−O bond length of 146.5 pm.<ref>{{cite journal |
||
| last1 =Meschi | first1 =D. J. | last2=Myers|first2= R. J.| year = 1959| title = The microwave spectrum, structure, and dipole moment of disulfur monoxide| journal = Journal of Molecular Spectroscopy| volume = 3| issue =1–6 | pages =405–416 | doi = 10.1016/0022-2852(59)90036-0| url = |bibcode = 1959JMoSp...3..405M }}</ref> |
| last1 =Meschi | first1 =D. J. | last2=Myers|first2= R. J.| year = 1959| title = The microwave spectrum, structure, and dipole moment of disulfur monoxide| journal = Journal of Molecular Spectroscopy| volume = 3| issue =1–6 | pages =405–416 | doi = 10.1016/0022-2852(59)90036-0| url = |bibcode = 1959JMoSp...3..405M }}</ref> |
||
Disulfur monoxide was discovered by Peter W. Schenk in 1933.<ref>{{cite journal|last1=Schenk|first1=Peter W.|title= |
Disulfur monoxide was discovered by Peter W. Schenk in 1933.<ref>{{cite journal|last1=Schenk|first1=Peter W.|title=Über das Schwefelmonoxyd|trans-title=On sulfur monoxide|journal=Zeitschrift für Anorganische und Allgemeine Chemie|date=18 March 1933|volume=211|issue=1–2|pages=150–160|doi=10.1002/zaac.19332110117|language=de}}</ref><ref name=Hall77/> However, only when Myers and Meschi studied it, did the actual composition and shape of the molecule become known.<ref name=Hall77/> |
||
==Preparation== |
==Preparation== |
||
It can be formed by many methods, including combustion of sulfur vapour in a deficiency of oxygen. It arises by oxidizing [[sulfur]] with [[copper |
It can be formed by many methods, including combustion of sulfur vapour in a deficiency of oxygen. It arises by oxidizing [[sulfur]] with [[copper(II) oxide]]:<ref name="Satyanarayana64">{{cite journal|last=Satyanarayana|first=S. R.|first2=A. R.|last2=Vasudeva Murthy|year=1964|title=Reactions with Disulphur monoxide Solutions Obtained by the Reduction of Cupric Oxide by Elemental Sulphur|journal=Proceedings of the Indian Academy of Sciences, Section A|volume=59|issue=4|url=http://eprints.iisc.ernet.in/28079/1/32.pdf}}</ref> |
||
:3 |
:3 S<sub>8</sub> + 12 CuO → 12 CuS + 4 S<sub>2</sub>O + 4 SO<sub>2</sub> |
||
Other routes include the reaction of thionyl chloride with silver sulfide: |
Other routes include the reaction of [[thionyl chloride]] with [[silver(I) sulfide]]: |
||
:SOCl<sub>2</sub> + Ag<sub>2</sub>S |
:SOCl<sub>2</sub> + Ag<sub>2</sub>S → 2 AgCl + S<sub>2</sub>O |
||
It also arises via thermal decomposition of [[sulfur dioxide]] in a [[glow discharge]].<ref name=candw/> |
It also arises via thermal decomposition of [[sulfur dioxide]] in a [[glow discharge]].<ref name=candw/> |
||
Disulfur monoxide forms a yellow solution in carbon tetrachloride.<ref name="Satyanarayana64"/> The solid can be obtained at liquid nitrogen temperatures, often appearing dark |
Disulfur monoxide forms a yellow solution in [[carbon tetrachloride]].<ref name="Satyanarayana64"/> The solid can be obtained at [[liquid nitrogen]] temperatures, often appearing dark-colored owing to impurities. On decomposition at room temperature it forms [[sulfur dioxide|SO<sub>2</sub>]] via the formation of polysulfur oxides.<ref name=candw>{{cite book|title=Advanced Inorganic Chemistry: A Comprehensive Treatise|author=Cotton and Wilkinson|year=1966|page=540}}</ref> |
||
===Discovery=== |
===Discovery=== |
||
Disulfur monoxide was first produced by P. W. Schenk in 1933<ref name=Steudel/> with a glow discharge though sulfur vapour and sulfur dioxide. He discovered that the gas could survive for hours at single digit pressures of mercury in clean glass, but decomposed near |
Disulfur monoxide was first produced by P. W. Schenk in 1933<ref name=Steudel/> with a [[glow discharge]] though sulfur vapour and [[sulfur dioxide]]. He discovered that the gas could survive for hours at single digit pressures of mercury in clean glass, but decomposed near {{cvt|30|mmHg|kPa}}. Schenk assigned the formula as SO and called it [[sulfur monoxide]]. In 1940, K. Kondratyeva and V. Kondratyev proposed the formula as S<sub>2</sub>O<sub>2</sub>, [[disulfur dioxide]].{{cn|date=September 2020}} In 1956, D. J. Meschi and R. J. Myers established the formula as S<sub>2</sub>O.<ref>{{cite journal|journal=Journal of the American Chemical Society|title=Disulfur Monoxide. I. Its Identification as the Major Constituent in Schenk's "Sulfur Monoxide"|first1=David J.|last1=Meschi|first2=Rollie J.|last2=Myers|date=30 July 1956|volume=78|issue=24|page=6220|doi=10.1021/ja01605a002}}</ref> |
||
<!--It is the base oxide for [[thiosulfurous acid]].<ref>{{cite book|title=Objective Chemistry For Iit Entrance|author=Alok Mittal|page=13|url=https://books.google.com/books?id=SzN1oCapakAC|isbn=978-81-224-1365-6|date=2002-01-01}}</ref> The formal oxidation state for sulfur is +1; sulfur plays two different roles in the molecule, however.--> |
<!--It is the base oxide for [[thiosulfurous acid]].<ref>{{cite book|title=Objective Chemistry For Iit Entrance|author=Alok Mittal|page=13|url=https://books.google.com/books?id=SzN1oCapakAC|isbn=978-81-224-1365-6|date=2002-01-01}}</ref> The formal oxidation state for sulfur is +1; sulfur plays two different roles in the molecule, however.--> |
||
==Natural occurrence== |
==Natural occurrence== |
||
''[[Desulfovibrio]] desulfuricans'' is claimed to produce S<sub>2</sub>O.<ref>{{cite journal|last1=Iverson|first1= |
The bacterium ''[[Desulfovibrio]] desulfuricans'' is claimed to produce S<sub>2</sub>O.<ref>{{cite journal|last1=Iverson|first1=W. P.|title=Disulfur monoxide: production by ''Desulfovibrio''|journal=Science|date=26 May 1967|volume=156|pages=1112–1114|issue=3778|pmid= 6024190|doi=10.1126/science.156.3778.1112|bibcode = 1967Sci...156.1112I |url=http://www.dtic.mil/get-tr-doc/pdf?AD=AD0813804}}</ref> |
||
S<sub>2</sub>O can be found coming from volcanoes on [[Io (moon)|Io]]. |
S<sub>2</sub>O can be found coming from volcanoes on [[Io (moon)|Io]]. It can form between 1% and 6% when hot 100-bar S<sub>2</sub> and SO<sub>2</sub> gas erupts from volcanoes. It is believed that [[Pele (volcano)|Pele]] on Io is surrounded by solid S<sub>2</sub>O.<ref>{{cite journal|url=http://zolotov.faculty.asu.edu/publ/Io-S2O-1998.pdf|first1=Mikhail Yu.|last1=Zolotov|first2=Bruce|last2=Fegley|title=Volcanic Origin of Disulfur Monoxide (S<sub>2</sub>O) on Io|journal=Icarus|volume=133|page=293|date=9 March 1998|doi=10.1006/icar.1998.5930|issue=2 |bibcode = 1998Icar..133..293Z }}</ref> |
||
==Properties== |
==Properties== |
||
Condensed solid S<sub>2</sub>O displays absorption bands at 420 and 530 nm. These are likely to be due to S<sub>3</sub> and S<sub>4</sub>.<ref name=Nav10>{{cite journal|last1=Navizet|first1=Isabelle|last2=Komiha|first2=Najia|last3=Linguerri|first3=Roberto|last4=Chambaud|first4=Gilberte|last5=Rosmus|first5=Pavel|title=On the formation of |
Condensed solid S<sub>2</sub>O displays [[absorption bands]] at 420 and 530 nm. These are likely to be due to [[Trisulfur|S<sub>3</sub>]] and [[Tetrasulfur|S<sub>4</sub>]].<ref name=Nav10>{{cite journal|last1=Navizet|first1=Isabelle|last2=Komiha|first2=Najia|last3=Linguerri|first3=Roberto|last4=Chambaud|first4=Gilberte|last5=Rosmus|first5=Pavel|title=On the formation of S<sub>2</sub>O at low energies: An ab initio study|journal=Chemical Physics Letters|date=November 2010|volume=500|issue=4–6|pages=207–210|doi=10.1016/j.cplett.2010.10.012|bibcode = 2010CPL...500..207N }}</ref> |
||
The microwave spectrum of S<sub>2</sub>O has the following rotational parameters: A=41915.44, B=5059.07, and C=4507.19 MHz.<ref>{{cite journal|last1=Cook|first1=Robert L|last2=Winnewisser|first2=Gisbert|last3=Lindsey|first3=D.C|title=The centrifugal distortion constants of disulfur monoxide|journal=Journal of Molecular Spectroscopy|date=May 1973|volume=46|issue=2|pages=276–284|doi=10.1016/0022-2852(73)90042-8|bibcode = 1973JMoSp..46..276C }}</ref> |
The [[Microwave spectroscopy|microwave spectrum]] of S<sub>2</sub>O has the following rotational parameters: ''A'' = 41915.44 MHz, ''B'' = 5059.07 MHz, and ''C'' = 4507.19 MHz.<ref>{{cite journal|last1=Cook|first1=Robert L|last2=Winnewisser|first2=Gisbert|last3=Lindsey|first3=D.C|title=The centrifugal distortion constants of disulfur monoxide|journal=Journal of Molecular Spectroscopy|date=May 1973|volume=46|issue=2|pages=276–284|doi=10.1016/0022-2852(73)90042-8|bibcode = 1973JMoSp..46..276C }}</ref> |
||
In the ultraviolet S<sub>2</sub>O has absorption band systems in the ranges |
In the ultraviolet S<sub>2</sub>O has absorption band systems in the ranges 250–340 nm and 190–240 nm. There are bands at 323.5 and 327.8 nm.<ref name=Hall77>{{cite journal|last1=Hallin|first1=K-E. J.|last2=Merer|first2=A. J.|last3=Milton|first3=D. J.|title=Rotational analysis of bands of the 3400 Å system of disulphur monoxide (S<sub>2</sub>O)|journal=Canadian Journal of Physics|date=November 1977|volume=55|issue=21|pages=1858–1867|doi=10.1139/p77-226|bibcode = 1977CaJPh..55.1858H }}</ref> The band in the 315–340 nm range is due to the {{nowrap|C<sup>1</sup>''A''′–X<sup>1</sup>''A''′ (π* ← π)}} transition.<ref name=Zhang95>{{cite journal|last1=Zhang|first1=Qingguo|last2=Dupré|first2=Patrick|last3=Grzybowski|first3=Bartosz|last4=Vaccaro|first4=Patrick H.|title=Laser-induced fluorescence studies of jet-cooled S<sub>2</sub>O: Axis-switching and predissociation effects|journal=The Journal of Chemical Physics|date=1995|volume=103|issue=1|pages=67|doi=10.1063/1.469623|bibcode = 1995JChPh.103...67Z }}</ref> |
||
The bond angle S−S−O is 109°.<ref name=Hall77/> The harmonic frequency for S−S stretching is 415.2 cm<sup>−1</sup>.<ref name=Zhang95/> |
The bond angle S−S−O is 109°.<ref name=Hall77/> The [[harmonic frequency]] for S−S stretching is 415.2 cm<sup>−1</sup>.<ref name=Zhang95/> |
||
==Reactions== |
==Reactions== |
||
A self |
A self-decomposition ([[disproportionation]]) of S<sub>2</sub>O can form [[trisulfur]] and [[sulfur dioxide]]: |
||
:2 |
:2 S<sub>2</sub>O → S<sub>3</sub> + SO<sub>2</sub> |
||
Also 5,6-di-''tert''-butyl-2,3,7-trithiabicyclo[2.2.1]hept-5-ene 2-''endo''-7-''endo''-dioxide when heated can form S<sub>2</sub>O.<ref>{{cite journal|title=Reversible disulfur monoxide (S<sub>2</sub>O)-forming retro- |
Also 5,6-di-''tert''-butyl-2,3,7-trithiabicyclo[2.2.1]hept-5-ene 2-''endo''-7-''endo''-dioxide when heated can form S<sub>2</sub>O.<ref>{{cite journal|title=Reversible disulfur monoxide (S<sub>2</sub>O)-forming retro-Diels–Alder reaction. disproportionation of S<sub>2</sub>O to trithio-ozone (S<sub>3</sub>) and sulfur dioxide (SO<sub>2</sub>) and reactivities of S<sub>2</sub>O and S<sub>3</sub>|first6=A.|last6=Ishii|first5=Y.|last5=Sugihara|first4=A.|last4=Sakamoto|first3=J.|last3=Takayama|first2=S.|last2=Aoki|last1=Nakayama|first1=J.|journal=Journal of the American Chemical Society|date=28 July 2004|volume=126|issue=29|pmid=15264842|doi=10.1021/ja047729i|pages=9085–9093}}</ref> It reacts with diazoalkanes to form dithiirane 1-oxides.<ref>{{cite journal|title=A Convenient Method for the Generation of a Disulfur Monoxide Equivalent and Its Reaction with Diazoalkanes to Yield Dithiirane 1-Oxides|first5=J.|last5=Nakayama|first4=H.|last4=Oshida|first3=K.|last3=Tekura|first2=T.|last2=Kawai|first1=A.|last1=Ishii|journal=Angewandte Chemie International Edition|date=18 May 2001|pages=1924–1926|volume=40|issue=10|pmid=11385674|doi=10.1002/1521-3773(20010518)40:10<1924::AID-ANIE1924>3.0.CO;2-F}}</ref> |
||
Disulfur monoxide is a [[ligand]] bound to [[transition metal]]s. These are formed by oxidation peroxide oxidation of a disulfur |
Disulfur monoxide is a [[ligand]] bound to [[transition metal]]s. These are formed by oxidation peroxide oxidation of a disulfur ligand. Excessive oxygen can yield a dioxygendisulfur ligand, which can be reduced in turn with [[triphenylphosphine]]. Examples are: [Ir(dppe)<sub>2</sub>S<sub>2</sub>O]<sup>+</sup>, OsCl(NO)(PPh<sub>3</sub>)<sub>2</sub>S<sub>2</sub>O, NbCl(''η''-C<sub>5</sub>H<sub>5</sub>)<sub>2</sub>S<sub>2</sub>O, Mn(CO)<sub>2</sub>(''η''-C<sub>5</sub>Me<sub>5</sub>)S<sub>2</sub>O, Re(CO)<sub>2</sub>(''η''-C<sub>5</sub>Me<sub>5</sub>)S<sub>2</sub>O, Re(CO)<sub>2</sub>(''η''-C<sub>5</sub>H<sub>5</sub>)S<sub>2</sub>O.<ref name=aioc>{{cite book|url=https://books.google.com/books?id=SMsrNhK_5y4C|page=168|title=Advances in Organometallic Chemistry, Volume 36|author=F G A Stone|isbn=978-0-12-031136-1|date=1994-03-07}}</ref> |
||
The molybdenum compound Mo(CO)<sub>2</sub>(S<sub>2</sub>CNEt<sub>2</sub>)<sub>2</sub> reacts with elemental sulfur and air to form a compound Mo<sub>2</sub>(S<sub>2</sub>O)<sub>2</sub>(S<sub>2</sub>CNEt<sub>2</sub>)<sub>4</sub>.<ref name=aioc/> Another way to form these complexes is to combine [[sulfonyliminooxosulfurane|sulfonyliminooxo-λ<sup>4</sup>-sulfurane]] (OSNSO<sub>2</sub> |
The molybdenum compound Mo(CO)<sub>2</sub>(S<sub>2</sub>CNEt<sub>2</sub>)<sub>2</sub> reacts with elemental sulfur and air to form a compound Mo<sub>2</sub>(S<sub>2</sub>O)<sub>2</sub>(S<sub>2</sub>CNEt<sub>2</sub>)<sub>4</sub>.<ref name=aioc/> Another way to form these complexes is to combine [[sulfonyliminooxosulfurane|sulfonyliminooxo-''λ''<sup>4</sup>-sulfurane]] (OSNSO<sub>2</sub>·R) complexes with [[hydrogen sulfide]].<ref name=aioc/> Complexes formed in this way are: IrCl(CO)(PPh<sub>3</sub>)<sub>2</sub>S<sub>2</sub>O; Mn(CO)<sub>2</sub>(''η''-C<sub>5</sub>H<sub>5</sub>)S<sub>2</sub>O. With hydrosulfide and a base followed by oxygen, OsCl(NO)(PPh<sub>3</sub>)<sub>2</sub>S<sub>2</sub>O can be made.<ref name=aioc/> |
||
Cyclic disulfur monoxide has been made from S<sub>2</sub>O by irradiating the solid in an inert gas |
Cyclic disulfur monoxide has been made from S<sub>2</sub>O by irradiating the solid in an inert gas matrix with 308 nm ultraviolet light.<ref>{{cite journal|last1=Lo|first1=Wen-Jui|last2=Wu|first2=Yu-Jong|authorlink3=Yuan-Pern Lee|last3=Lee|first3=Yuan-Pern|title=Ultraviolet Absorption Spectrum of Cyclic S<sub>2</sub>O in Solid Ar|journal=The Journal of Physical Chemistry A|date=September 2003|volume=107|issue=36|pages=6944–6947|doi=10.1021/jp034563j|bibcode=2003JPCA..107.6944L}}</ref> |
||
==References== |
==References== |
||
Revision as of 21:53, 18 September 2020
| |
| |
| Names | |
|---|---|
| Other names
sulfur suboxide; sulfuroxide;
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| S2O | |
| Molar mass | 80.1294 g/mol[1] |
| Appearance | colourless gas or dark red solid[2] |
| Structure | |
| bent | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
toxic |
| Related compounds | |
Related compounds
|
Trisulfur SO Ozone SO2 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
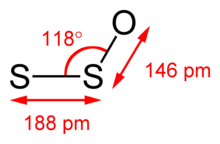
Disulfur monoxide or sulfur suboxide is an inorganic compound with formula S2O. It is one of the lower sulfur oxides. It is a colourless gas and condenses to give a pale coloured solid that is unstable at room temperature.[3] It is a bent molecule with an S−S−O angle of 117.88°, S−S bond length of 188.4 pm, and S−O bond length of 146.5 pm.[4]
Disulfur monoxide was discovered by Peter W. Schenk in 1933.[5][6] However, only when Myers and Meschi studied it, did the actual composition and shape of the molecule become known.[6]
Preparation
It can be formed by many methods, including combustion of sulfur vapour in a deficiency of oxygen. It arises by oxidizing sulfur with copper(II) oxide:[7]
- 3 S8 + 12 CuO → 12 CuS + 4 S2O + 4 SO2
Other routes include the reaction of thionyl chloride with silver(I) sulfide:
- SOCl2 + Ag2S → 2 AgCl + S2O
It also arises via thermal decomposition of sulfur dioxide in a glow discharge.[8]
Disulfur monoxide forms a yellow solution in carbon tetrachloride.[7] The solid can be obtained at liquid nitrogen temperatures, often appearing dark-colored owing to impurities. On decomposition at room temperature it forms SO2 via the formation of polysulfur oxides.[8]
Discovery
Disulfur monoxide was first produced by P. W. Schenk in 1933[3] with a glow discharge though sulfur vapour and sulfur dioxide. He discovered that the gas could survive for hours at single digit pressures of mercury in clean glass, but decomposed near 30 mmHg (4.0 kPa). Schenk assigned the formula as SO and called it sulfur monoxide. In 1940, K. Kondratyeva and V. Kondratyev proposed the formula as S2O2, disulfur dioxide.[citation needed] In 1956, D. J. Meschi and R. J. Myers established the formula as S2O.[9]
Natural occurrence
The bacterium Desulfovibrio desulfuricans is claimed to produce S2O.[10] S2O can be found coming from volcanoes on Io. It can form between 1% and 6% when hot 100-bar S2 and SO2 gas erupts from volcanoes. It is believed that Pele on Io is surrounded by solid S2O.[11]
Properties
Condensed solid S2O displays absorption bands at 420 and 530 nm. These are likely to be due to S3 and S4.[12]
The microwave spectrum of S2O has the following rotational parameters: A = 41915.44 MHz, B = 5059.07 MHz, and C = 4507.19 MHz.[13]
In the ultraviolet S2O has absorption band systems in the ranges 250–340 nm and 190–240 nm. There are bands at 323.5 and 327.8 nm.[6] The band in the 315–340 nm range is due to the C1A′–X1A′ (π* ← π) transition.[14]
The bond angle S−S−O is 109°.[6] The harmonic frequency for S−S stretching is 415.2 cm−1.[14]
Reactions
A self-decomposition (disproportionation) of S2O can form trisulfur and sulfur dioxide:
- 2 S2O → S3 + SO2
Also 5,6-di-tert-butyl-2,3,7-trithiabicyclo[2.2.1]hept-5-ene 2-endo-7-endo-dioxide when heated can form S2O.[15] It reacts with diazoalkanes to form dithiirane 1-oxides.[16]
Disulfur monoxide is a ligand bound to transition metals. These are formed by oxidation peroxide oxidation of a disulfur ligand. Excessive oxygen can yield a dioxygendisulfur ligand, which can be reduced in turn with triphenylphosphine. Examples are: [Ir(dppe)2S2O]+, OsCl(NO)(PPh3)2S2O, NbCl(η-C5H5)2S2O, Mn(CO)2(η-C5Me5)S2O, Re(CO)2(η-C5Me5)S2O, Re(CO)2(η-C5H5)S2O.[17]
The molybdenum compound Mo(CO)2(S2CNEt2)2 reacts with elemental sulfur and air to form a compound Mo2(S2O)2(S2CNEt2)4.[17] Another way to form these complexes is to combine sulfonyliminooxo-λ4-sulfurane (OSNSO2·R) complexes with hydrogen sulfide.[17] Complexes formed in this way are: IrCl(CO)(PPh3)2S2O; Mn(CO)2(η-C5H5)S2O. With hydrosulfide and a base followed by oxygen, OsCl(NO)(PPh3)2S2O can be made.[17]
Cyclic disulfur monoxide has been made from S2O by irradiating the solid in an inert gas matrix with 308 nm ultraviolet light.[18]
References
- ^ a b c "Disulfur monoxide". NIST. 2008.
- ^ Hapke, B.; Graham, F. (May 1989). "Spectral properties of condensed phases of disulfur monoxide, polysulfur oxide, and irradiated sulfur". Icarus. 79 (1): 47. Bibcode:1989Icar...79...47H. doi:10.1016/0019-1035(89)90107-3.
- ^ a b Steudel, R. (2003). "Sulfur-Rich Oxides SnO and SnO2". In Steudel, R. (ed.). Elemental Sulfur and Sulfur-Rich Compounds II. Berlin/Heidelberg: Springer.
{{cite book}}: Text "isbn9783540449515" ignored (help) - ^ Meschi, D. J.; Myers, R. J. (1959). "The microwave spectrum, structure, and dipole moment of disulfur monoxide". Journal of Molecular Spectroscopy. 3 (1–6): 405–416. Bibcode:1959JMoSp...3..405M. doi:10.1016/0022-2852(59)90036-0.
- ^ Schenk, Peter W. (18 March 1933). "Über das Schwefelmonoxyd" [On sulfur monoxide]. Zeitschrift für Anorganische und Allgemeine Chemie (in German). 211 (1–2): 150–160. doi:10.1002/zaac.19332110117.
- ^ a b c d Hallin, K-E. J.; Merer, A. J.; Milton, D. J. (November 1977). "Rotational analysis of bands of the 3400 Å system of disulphur monoxide (S2O)". Canadian Journal of Physics. 55 (21): 1858–1867. Bibcode:1977CaJPh..55.1858H. doi:10.1139/p77-226.
- ^ a b Satyanarayana, S. R.; Vasudeva Murthy, A. R. (1964). "Reactions with Disulphur monoxide Solutions Obtained by the Reduction of Cupric Oxide by Elemental Sulphur" (PDF). Proceedings of the Indian Academy of Sciences, Section A. 59 (4).
- ^ a b Cotton and Wilkinson (1966). Advanced Inorganic Chemistry: A Comprehensive Treatise. p. 540.
- ^ Meschi, David J.; Myers, Rollie J. (30 July 1956). "Disulfur Monoxide. I. Its Identification as the Major Constituent in Schenk's "Sulfur Monoxide"". Journal of the American Chemical Society. 78 (24): 6220. doi:10.1021/ja01605a002.
- ^ Iverson, W. P. (26 May 1967). "Disulfur monoxide: production by Desulfovibrio". Science. 156 (3778): 1112–1114. Bibcode:1967Sci...156.1112I. doi:10.1126/science.156.3778.1112. PMID 6024190.
- ^ Zolotov, Mikhail Yu.; Fegley, Bruce (9 March 1998). "Volcanic Origin of Disulfur Monoxide (S2O) on Io" (PDF). Icarus. 133 (2): 293. Bibcode:1998Icar..133..293Z. doi:10.1006/icar.1998.5930.
- ^ Cook, Robert L; Winnewisser, Gisbert; Lindsey, D.C (May 1973). "The centrifugal distortion constants of disulfur monoxide". Journal of Molecular Spectroscopy. 46 (2): 276–284. Bibcode:1973JMoSp..46..276C. doi:10.1016/0022-2852(73)90042-8.
- ^ a b Zhang, Qingguo; Dupré, Patrick; Grzybowski, Bartosz; Vaccaro, Patrick H. (1995). "Laser-induced fluorescence studies of jet-cooled S2O: Axis-switching and predissociation effects". The Journal of Chemical Physics. 103 (1): 67. Bibcode:1995JChPh.103...67Z. doi:10.1063/1.469623.
- ^ Nakayama, J.; Aoki, S.; Takayama, J.; Sakamoto, A.; Sugihara, Y.; Ishii, A. (28 July 2004). "Reversible disulfur monoxide (S2O)-forming retro-Diels–Alder reaction. disproportionation of S2O to trithio-ozone (S3) and sulfur dioxide (SO2) and reactivities of S2O and S3". Journal of the American Chemical Society. 126 (29): 9085–9093. doi:10.1021/ja047729i. PMID 15264842.
- ^ Ishii, A.; Kawai, T.; Tekura, K.; Oshida, H.; Nakayama, J. (18 May 2001). "A Convenient Method for the Generation of a Disulfur Monoxide Equivalent and Its Reaction with Diazoalkanes to Yield Dithiirane 1-Oxides". Angewandte Chemie International Edition. 40 (10): 1924–1926. doi:10.1002/1521-3773(20010518)40:10<1924::AID-ANIE1924>3.0.CO;2-F. PMID 11385674.
- ^ a b c d F G A Stone (1994-03-07). Advances in Organometallic Chemistry, Volume 36. p. 168. ISBN 978-0-12-031136-1.
- ^ Lo, Wen-Jui; Wu, Yu-Jong; Lee, Yuan-Pern (September 2003). "Ultraviolet Absorption Spectrum of Cyclic S2O in Solid Ar". The Journal of Physical Chemistry A. 107 (36): 6944–6947. Bibcode:2003JPCA..107.6944L. doi:10.1021/jp034563j.
