Thiethylperazine
 | |
| Clinical data | |
|---|---|
| Trade names | Torecan, Norzine |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 60% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.014.381 |
| Chemical and physical data | |
| Formula | C22H29N3S2 |
| Molar mass | 399.62 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Thiethylperazine (Torecan, Norzine) is an antiemetic[1] of the phenothiazine class. It is an antagonist of dopamine receptors (DRD1, DRD2, DRD4) as well as of 5-HT2A, 5-HT2C receptors, mAChRs (1 through 5), α1 adrenergic receptor and H1 receptor.
Thiethylperazine activates the transport protein ABCC1 that clears beta-amyloid from brains of mice.[2]
Pharmacokinetics[3]
Distribution
This drug is highly lipofilic and it binds with membranes and serum proteins (over 85%). It accumulates in organs with high blood flow and penetrates the placenta. It cannot be removed with dialysis.
Metabolism
It is mainly metabolised in the liver and only 3% is eliminated unchanged. Torecan's half-life is 12 h.
Teratogenicity
In toxic doses above the terapeutic window, it increases the rate of cleft palate occurence.
Antypsychotic activity
Theithylperazine may possess antypsychotic activity[4] due to the antagonism of 5-HT2 and D2 receptors. Because of this, it would not cause extrapyramidal symptoms. Nevertheless, it was never marketed as such drug.
One cause of acute dystonia occured in a 19-year-old male patient after discontinuation of this drug.[5]
Overdose
Signs of acute thiethylperazine overdose include: extrapyramidal symptoms, confusion, convulsions, respiratory depression and hypotension.
Synthesis
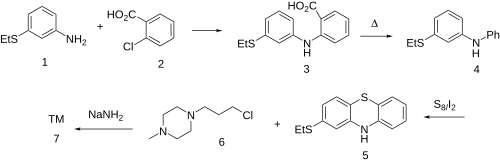
The nitrogen in 3-(ethylsulfanyl)aniline (1) displaces the chlorine in 2-chlorobenzoic acid (2) to give the diarylamine (3). The carboxyl in the anthranilic acid residue, having performed its activating function, is then thermolytically removed to form (4). This gives predominantly the phenothiazine (5) on treatment with sulfur and iodine; The rxn may well be aided by the presence of the electron donating thioether at the para-position. Alkylation of the anion again via its anion, with 1-(ɣ̞-chloropropyl)-4-methylpiperazine (6) affords Thiethylperazine (7).
References
- ^ Tamboline, B. L.; McGillivray, D. C.; Bogoch, A. (1965-02-20). "The Effects of Thiethylperazine Dimaleate (Torecan) on Nausea and Vomiting". Canadian Medical Association Journal. 92 (8): 422–423. ISSN 0008-4409. PMC 1928133. PMID 14261157.
- ^ Krohn M, Lange C, Hofrichter J, Scheffler K, Stenzel J, Steffen J, et al. (October 2011). "Cerebral amyloid-β proteostasis is regulated by the membrane transport protein ABCC1 in mice". The Journal of Clinical Investigation. 121 (10): 3924–31. doi:10.1172/JCI57867. PMC 3195473. PMID 21881209.
- "Alzheimer disease: Transport protein ABCC1 plays key role in clearing beta-amyloid from brains of mice". ScienceDaily (Press release). September 1, 2011.
- ^ https://rejestrymedyczne.ezdrowie.gov.pl/enwiki/api/rpl/medicinal-products/6729/characteristic.
{{cite web}}: Missing or empty|title=(help) - ^ Rotrosen, J.; Angrist, B. M.; Gershon, S.; Aronson, M.; Gruen, P.; Sachar, E. J.; Denning, R. K.; Matthysse, S.; Stanley, M.; Wilk, S. (September 1978). "Thiethylperazine; clinical antipsychotic efficacy and correlation with potency in predictive systems". Archives of General Psychiatry. 35 (9): 1112–1118. doi:10.1001/archpsyc.1978.01770330086008. ISSN 0003-990X. PMID 99115.
- ^ Khanderia, Ujjaini (July 1985). "Recurrent Dystonic Reactions Induced by Thiethylperazine". Drug Intelligence & Clinical Pharmacy. 19 (7–8): 550–551. doi:10.1177/106002808501900708. ISSN 0012-6578.
- ^ Bourquin, J.-P.; Schwarb, G.; Gamboni, G.; Fischer, R.; Ruesch, L.; Guldimann, S.; Theus, V.; Schenker, E.; Renz, J. (1958). "Synthesen auf dem Phenothiazin-Gebiet. 1. Mitteilung. Mercaptophenothiazin-Derivate". Helvetica Chimica Acta. 41 (4): 1061–1072. doi:10.1002/hlca.19580410419.
- ^ Bourquin, J.-P.; Schwarb, G.; Gamboni, G.; Fischer, R.; Ruesch, L.; Guldimann, S.; Theus, V.; Schenker, E.; Renz, J. (1958). "Synthesen auf dem Phenothiazin-Gebiet. 2. Mitteilung. N-substituierte Mercaptophenothiazin-Derivate". Helvetica Chimica Acta. 41 (4): 1072–1108. doi:10.1002/hlca.19580410420.
- ^ Renz Jany, et al. U.S. patent 3,336,197 (1967 to Sandoz KK).
