Chloroxylenol
| |
| Names | |
|---|---|
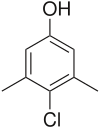
| Preferred IUPAC name
4-Chloro-3,5-dimethylphenol[1] | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1862539 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.631 |
| EC Number |
|
| KEGG | |
| MeSH | chloroxylenol |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H9ClO | |
| Molar mass | 156.61 g·mol−1 |
| Melting point | 115 °C (239 °F; 388 K) |
| Boiling point | 246 °C (475 °F; 519 K) |
| 300 mg/L [2] | |
| Solubility in alcohols | soluble |
| Solubility in Ethers | soluble |
| Solubility in Benzene | soluble |
| log P | 3.377 |
| Acidity (pKa) | 9.76 |
| Basicity (pKb) | 4.24 |
| Pharmacology | |
| D08AE05 (WHO) | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H315, H317, H319 | |
| P280, P305+P351+P338 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chloroxylenol, also known as para-chloro-meta-xylenol (PCMX), is a chlorine substituted phenol with an white to off-white appearance and a phenolic odor. The discovery of chloroxylenol was the result of efforts to produce improved antiseptics that began at the end of the 1800s. First synthesized in Germany in 1923, it was borne out of the study of coal tar components that began a decade earlier.[2]
Uses
Formulations containing chloroxylenol are used in hospitals and households as antiseptics, disinfectants, and sanitizers. It is commonly used in antibacterial soaps, wound-cleansing, and other household antiseptic applications.[2]
The World Health Organization lists a 4.8% solution of chloroxylenol as an essential medicine.[3] When diluted, this chloroxylenol preparation is used as an antiseptic and disinfectant, and for skin disinfection. Diluted with alcohol, it is suitable for disinfecting medical instruments.[4]
Chloroxylenol is used in a number of formulations and under a number of brand names, most notably Dettol.[5]
Anti-Microbial Properties
Chloroxylenol is most effective against gram-positive bacteria.[4] It works by disruption of the cell wall and stopping the function of enzymes.[6][7] and is less effective than some other available agents.[8][6] Testing has shown products containing chloroxylenol effective against the SARS-CoV-2 virus[9] and orthopoxviruses.[10]
Toxicology
Chloroxylenol is generally slightly to moderately toxic to humans (but highly toxic for the eyes, causing severe eye irritation),[11] is practically non-toxic to birds, and is moderately toxic to freshwater invertebrates. It is highly toxic to fish, cats, and some amphibians and should not be used around them.[12] It is a mild skin irritant and may trigger allergic reactions in some individuals.[13]
References
- ^ CID 2723 from PubChem
- ^ a b c Ascenzi, Joseph M. (1996). "Chloroxylenol: an old-new antimicrobial". Handbook of disinfectants and antiseptics. New York: M. Dekker. ISBN 978-0-8247-9524-5. Archived from the original on 2017-09-23.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ a b World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 324. hdl:10665/44053. ISBN 9789241547659.
- ^ "Chloroxylenol - brand name list from Drugs.com". www.drugs.com. Archived from the original on 2017-08-28.
- ^ a b Mahon, Connie R.; Lehman, Donald C.; Manuselis, George Jr. (2014). Textbook of Diagnostic Microbiology (5 ed.). Elsevier Health Sciences. p. 67. ISBN 9780323292627. Archived from the original on 2017-01-13.
- ^ Bednarek, R. S.; Nassereddin, A.; Ramsey, M. L. (2021). "Skin Antiseptics". StatPearls. StatPearls. PMID 29939630.
- ^ Digison, MB (2007). "A review of anti-septic agents for pre-operative skin preparation". Plastic Surgical Nursing. 27 (4): 185–9, quiz 190–1. doi:10.1097/01.psn.0000306182.50071.e2. PMID 18165724. S2CID 205427305.
- ^ Ijaz, Khalid; Whitehead, Kelly; Srinivasan, Vanita; Jones, Chris; Nims, Raymond; Charlesworth, Bruce (2020-05-24). "Microbicidal actives with virucidal efficacy against SARS-CoV-2". American Journal of Infection Control. 48 (8): 972–973. doi:10.1016/j.ajic.2020.05.015. PMC 7246051. PMID 32461067. Retrieved 2021-01-31.
- ^ Butcher, W.; Ulaeto, D. (2005). "Contact inactivation of orthopoxviruses by household disinfectants". Journal of Applied Microbiology. 99 (2). Wiley: 279–284. doi:10.1111/j.1365-2672.2005.02601.x. ISSN 1364-5072. PMID 16033458. S2CID 43085296.
- ^ R.E.D Facts, Chloroxylenol, United States Environmental Protection Agency (EPA), September 1994.
- ^ Dettol liquid at drugs.com Archived 2015-09-24 at the Wayback Machine
- ^ K Verma, Ghanshyam; K Mahajan, Vikram; Shanker, Vinay; Ram Tegta, Geeta; Jindal, Nidhi; Minhas, Samridhi (2011). "Contact depigmentation following irritant contact dermatitis to chloroxylenol (dettol)". Indian J Dermatol Venereol Leprol. 77 (5): 612–4. doi:10.4103/0378-6323.84086. PMID 21860168.
External links
![]() Media related to Chloroxylenol at Wikimedia Commons
Media related to Chloroxylenol at Wikimedia Commons
- "Chloroxylenol". Drug Information Portal. U.S. National Library of Medicine.
