Artemisinin
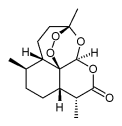
Artemisinin is a drug used to treat multi-drug resistant strains of falciparum malaria. The compound (a sesquiterpene lactone) is isolated from the shrub Artemisia annua long-used in Traditional Chinese Medicine. Not all shrubs of this species contain artemisinin. Apparently it is only produced when the plant is subjected to certain conditions.
Chemically it is (3R,5aS,6R,8aS,9R,12S,12aR)-octahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano[4,3-j]-1,2-benzodioxepin-10(3H)-one, C15H22O5.
Cancer Treatment
Artemisinin is also under early research and testing for treatment of cancer. Artemisinin has a peroxide lactone group in its structure. It is thought that when the peroxide comes into contact with high iron concentrations (common in cancerous cells), the molecule becomes unstable and releases reactive oxygen species. It has been shown to reduce angiogenesis and the expression of vascular endothelial growth factor in some tissue cultures.
History
Artemisia has been used by Chinese herbalists for more than a thousand years in the treatment of many illnesses, such as skin diseases and malaria. In the 1960s a research program was set up by the Chinese army to find an adequate treatment of malaria. In 1972, in the course of this research, Tu Youyou discovered artemsinin in the leaves of Artemisia annua. The drug is named qinghaosu in Chinese. It was one of many candidates then tested by Chinese scientists from a list of nearly 200 traditional Chinese medicines for treating malaria. It was the only one that was effective.
It remained largely unknown to the rest of the world for about 10 years, due to the Communist Chinese government at the time. The rest of the world finally found out about the drug from an article in a Chinese medical journal. People were sceptical at first, because the Chinese had made unsubstantiated statements about having found treatments of malaria before. Another reason was the peroxide part of the molecule. It was thought unlikely this would be a stable molecule, and so would not last long enough to be effective. This turned out not to be the case.
The Chinese government at the time, however, was very wary of western scientists, and would not give anyone either the plant or the refined drug. People around the world now started looking for the shrub themselves, to see if they could find it. They finally found it along the Potomac river, in Washington, D.C. Apparently it was a very common shrub, found in many parts of the world. It took another 10 years of research before the drug finally became commercially available. By this time relations between Communist China and the rest of the world had improved, and scientific information could be exchanged.
The drug is used these days in China and Vietnam without much regard to taking precautions against creating resistance of the malaria parasite to this drug as well.
More potent derivatives have also been developed from artemisinin, such as artemether and artesunate. However, their activity decreases after one to two hours. To counter this drawback, artemisinin is given alongside lumefantrine to treat uncomplicated falciparum malaria. Lumefantrine has a half-life of about 3 to 6 days. Such a treatment is called ACT (artemisinin-based combination therapy); other examples are artemether-lumefantrine, artesunate-mefloquine, artesunate-amodiaquine, and artesunate-sulfadoxine/pyrimethamine.
Recent trials have shown that ACT is more than 90% effective, with a recovery of malaria after three days, especially for the chloroquine-resistant Plasmodium falciparum.
The World Health Organisation has recommended that a switch to ACT should be made in all countries where the malaria parasite has developed resistance to chloroquine. Artemisinin and its derivatives are now standard components of malaria treatment in China, Vietnam, and some other countries in Asia and Africa, where they have proved to be the best-ever anti-malarial drugs. They have minimal adverse side effects. Currently, artemisinin is not widely available in the United States or Canada.
To counter the present shortage in leaves of Artemisia annua, researchers have been searching for a way to develop artemisinin artificially in the laboratory. The compound, called OZ-277, developed by Jonathan Vennerstrom in University of Nebraska has even proved to be more effective than the natural product in test-tube trials. A six month trial of the drug on human subjects in Thailand was started in January 2005, and results are pending.
How it works
The compound has a peroxide group in its structure. When the peroxide comes into contact with high iron concentrations, the molecule becomes unstable and "explodes" into free radicals. High concentrations of iron are found in red blood cells, which is also where the malaria parasites are found. When the compound enters the red blood cell, it will release the free radicals, which are highly destructive to the parasites.
Schistosomiasis treatment
Artesunate, a potent derivative of artemisinin, is an important treatment for schistosomiasis. It is usually used in conjunction with or as an alternative to praziquantel.
External links
- Sweet Annie - Wormwood, Artemisia annua
- Design and synthesis of antimalarial endoperoxides
- Clinical trials of artemether-lumefantrine
- Malaria, Science, and Social Responsibility: Nonprofit drug-development partnership seeks to cure the ills of developing nations
- Research on the use of Artemisinin for cancer treatment
- Artemisia Annua L.: the Hope Against Malaria and Cancer
- Artemisinin - Researchers blend folk treatment, high tech for promising anti-cancer compound
- BBC Horizon documentary about artemisinin
- From Malaria to Cancer Treatment, by Robert Jay Rowen, MD Editor-in-Chief, Second Opinion
- Artemisia Annua, by Memorial-Sloan Kettering Cancer Center
