Cyclohexene
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclohexene | |||
| Other names
Tetrahydrobenzene, 1,2,3,4-Tetrahydrobenzene, Benzenetetrahydride, Cyclohex-1-ene, Hexanaphthylene, UN 2256
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 906737 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.462 | ||
| EC Number |
| ||
| 1659 | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H10 | |||
| Molar mass | 82.143 g/mol | ||
| Appearance | colorless liquid | ||
| Odor | sweet | ||
| Density | 0.8110 g/cm3 | ||
| Melting point | −103.5 °C (−154.3 °F; 169.7 K) | ||
| Boiling point | 82.98 °C (181.36 °F; 356.13 K) | ||
| slightly soluble in water | |||
| Solubility | miscible with organic solvents | ||
| Vapor pressure | 8.93 kPa (20 °C)
11.9 kPa (25 °C) | ||
Henry's law
constant (kH) |
0.022 mol·kg−1·bar−1 | ||
| -57.5·10−6 cm3/mol | |||
Refractive index (nD)
|
1.4465 | ||
| Hazards | |||
| GHS labelling: | |||
    
| |||
| Danger | |||
| H225, H302, H305, H311, H411 | |||
| P210, P233, P240, P241, P242, P243, P264, P270, P273, P280, P301+P310, P301+P312, P302+P352, P303+P361+P353, P312, P322, P330, P331, P361, P363, P370+P378, P391, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −12 °C (10 °F; 261 K) | ||
| 244 °C (471 °F; 517 K) | |||
| Explosive limits | 0.8–5 % | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1407 mg/kg (oral, rat) | ||
LCLo (lowest published)
|
13,196 ppm (mouse, 2 hr)[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 300 ppm (1015 mg/m3)[1] | ||
REL (Recommended)
|
TWA 300 ppm (1015 mg/m3)[1] | ||
IDLH (Immediate danger)
|
2000 ppm[1] | ||
| Safety data sheet (SDS) | External MSDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
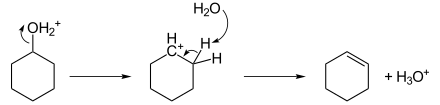
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes. Cyclohexene is not very stable upon long term storage with exposure to light and air because it forms peroxides.
Production and uses
Cyclohexene is produced by the partial hydrogenation of benzene, a process developed by the Asahi Chemical company.[3]
Reactions and uses
Benzene is converted to cyclohexylbenzene by acid-catalyzed alkylation with cyclohexene. Cyclohexylbenzene is a precursor to both phenol and cyclohexanone.[4]
Hydration of cyclohexene gives cyclohexanol, which can be dehydrogenated to give cyclohexanone, a precursor to caprolactam.[5]
The oxidative cleavage of cyclohexene gives adipic acid. Hydrogen peroxide is used as the oxidant, in the presence of a tungsten catalyst.[6]
Cyclohexene is also a precursor to maleic acid, dicyclohexyladipate, and cyclohexene oxide. Furthermore, it is used as a solvent.
Structure
Cyclohexene is most stable in a half-chair conformation,[7] unlike the preference for a chair form of cyclohexane. One basis for the cyclohexane conformational preference for a chair is that it allows each bond of the ring to adopt a staggered conformation. For cyclohexene, however, the alkene is planar, equivalent to an eclipsed conformation at that bond.
See also
External links
- International Chemical Safety Card 1054
- NIOSH Pocket Guide to Chemical Hazards. "#0167". National Institute for Occupational Safety and Health (NIOSH).
- Material Safety Data Sheet for cyclohexene
- Safety MSDS data
- Reaction of Cyclohexene with Bromine and Potassium Permanganate
- Cyclohexene synthesis
- Data sheet at inchem.org
References
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0167". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Cyclohexene". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ [1], Narisawa, Naoki & Tanaka, Katsutoshi, "Cyclohexanol, method for producing cyclohexanol, and method for producing adipic acid"
- ^ Plotkin, Jeffrey S. (2016-03-21). "What's New in Phenol Production?". American Chemical Society. Archived from the original on 2019-10-27. Retrieved 2018-01-02.
- ^ Musser, Michael T. (2005). "Cyclohexanol and Cyclohexanone". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_217. ISBN 978-3527306732.
- ^ Reed, Scott M.; Hutchison, James E. (2000). "Green Chemistry in the Organic Teaching Laboratory: An Environmentally Benign Synthesis of Adipic Acid". J. Chem. Educ. 77 (12): 1627–1629. doi:10.1021/ed077p1627.
- ^ Jensen, Frederick R.; Bushweller, C. Hackett (1969). "Conformational preferences and interconversion barriers in cyclohexene and derivatives". J. Am. Chem. Soc. 91 (21): 5774–5782. doi:10.1021/ja01049a013.