Declarative memory
It has been suggested that this article be merged with explicit memory. (Discuss) Proposed since January 2014. |
Declarative memory (sometimes referred to as explicit memory) is one of two types of long-term human memory. Declarative memory refers to memories that can be consciously recalled such as facts and verbal knowledge.[1] While declarative memory is similar to explicit memory, declarative memory is memory that a person can state in words, while explicit memory is the deliberate [clarification needed] recall of information that the person recognizes as a memory.[2] Declarative memory's counterpart is known as non-declarative or procedural memory, which refers to unconscious memories such as skills (e.g. learning to ride a bicycle). Declarative memory can be divided into two categories: episodic memory, which stores specific personal experiences, and semantic memory, which stores factual information.[3]
Types
Semantic memories are those memories that store general factual knowledge that is independent of personal experience. This includes world knowledge, object knowledge, language knowledge, and conceptual priming. Some examples of semantic memory include types of food, capital cities of a geographic region, or the lexicon of a common language, such as a person's vocabulary.[3]
Episodic memories are those memories that store chunks of observational information attached to a specific event. Some examples of episodic memory include the memory of entering a specific classroom for the first time, the memory of storing your carry-on baggage while boarding a plane headed to a specific destination on a specific day and time, the memory of being notified that you are being terminated from your job, or the memory of notifying a subordinate that they are being terminated from their job. The retrieval of these episodic memories can be thought of as the action of mentally reliving in detail the past events that they concern.[3] Episodic memory is believed to be the system that provides the basic support for semantic memory.
History
The study of human memory stretches back over the last 2000 years. An early attempt to understand memory can be found in Aristotle’s major treatise, On the Soul, in which he compares the human mind to a blank slate.[4] He theorized that all humans are born free of any knowledge and are the sum of their experiences. It wasn’t until the late 1800s, however, that a young German philosopher by the name of Herman Ebbinghaus developed the first scientific approach to studying memory.[5] While some of his findings have endured and remain relevant to this day (Learning Curve), his greatest contribution to the field of memory research was demonstrating that memory can be studied scientifically. In 1972, Endel Tulving proposed the distinction between episodic and semantic memory.[3] This was quickly adopted and is now widely accepted. Following this, in 1985, Daniel Schacter proposed a more general distinction between explicit (declarative) and implicit (procedural) memory[6] With the recent advances in neuroimaging technology, there have been a multitude of findings linking specific brain areas to declarative memory. Despite these advances in Cognitive psychology, there is still much to be discovered in terms of the operating mechanisms of declarative memory.[7] It is unclear whether declarative memory is mediated by a particular “memory system” or if it is more accurately classified as a “type of knowledge” and it is not known how or why declarative memory evolved to begin with.[7]
Neuropsychology
Normal brain function
Hippocampus

Although many psychologists believe that the entire brain is involved with memory, the hippocampus and surrounding structures appear to be most important in declarative memory specifically.[8] The ability to retain and recall episodic memories is highly dependent on the hippocampus,[8] whereas the formation of new declarative memories relies on both the hippocampus and parahippocampus[9] Other studies have found that the parahippocampal cortices were related to superior Recognition Memory.[9]
The Three Stage Model was developed by Eichenbaum, et. Al (2001), and proposes that the hippocampus does three things with episodic memory:
- Mediates the recording of episodic memories
- Identifies common features between episodes
- Links these common episodes in a memory space.
To support this model, a version of Piaget’s Transitive Inference Task was used to show that the hippocampus is in fact used as the memory space.[8]
When experiencing an event for the first time, a link is formed in the hippocampus allowing us to recall that event in the future. Separate links are also made for features related to that event. For example, when you meet someone new, a unique link is created for them. More links are then connected to that person’s link so you can remember what colour their shirt was, what the weather was like when you met them, etc. Specific episodes are made easier to remember and recall by repeatedly exposing oneself to them (which strengthens the links in the memory space) allowing for faster retrieval when remembering.[8]
Hippocampal cells (neurons) are activated depending on what information one is exposed to at that moment. Some cells are specific to spatial information, certain stimuli (smells, etc.), or behaviours as has been shown in a Radial Maze Task.[8] It is therefore the hippocampus that allows us to recognize certain situations, environments, etc. as being either distinct or similar to others. However, the Three Stage Model does not incorporate the importance of other cortical structures in memory.
The anatomy of the hippocampus is largely conserved across mammals, and the role of these areas in declarative memory are conserved across species as well. The organization and neural pathways of the hippocampus are very similar in humans and other mammal species. In humans and other mammals, a cross-section of the hippocampus shows the dentate gyrus as well as the dense cell layers of the CA fields. The intrinsic connectivity of these areas are also conserved. [10]
Results from an experiment by Davachi, Mitchell, and Wagner (2003) and numerous subsequent studies (Davachi, 2006) show that activation in the hippocampus during encoding is related to a subject's ability to recall prior events or later relational memories. These tests did not differentiate between individual test items later seen and those forgotten.[11][12]
Prefrontal cortex
The lateral Prefrontal cortex (PFC) is essential for remembering contextual details of an experience rather than for memory formation.[9] The PFC is also more involved with episodic memory than semantic memory, although it does play a small role in semantics.[13]
Using PET studies and word stimuli, Endel Tulving found that remembering is an automatic process.[14] It is also well documented that a hemispheric asymmetry occurs in the PFC: When encoding memories, the Left Dorsolateral PFC (LPFC) is activated, and when retrieving memories, activation is seen in the Right Dorsolateral PFC (RPFC).[14]
Studies have also shown that the PFC is extremely involved with autonoetic consciousness (See Tulving's theory).[15] This is responsible for humans’ recollective experiences and ‘mental time travelling’ abilities (characteristics of episodic memory).
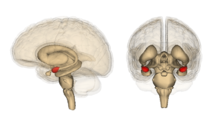
Amygdala
The amygdala is believed to be involved in the encoding and retrieval of emotionally charged memories. Much of the evidence for this has come from research on a phenomenon known as flashbulb memories. These are instances in which memories of powerful emotional events are more highly detailed and enduring than regular memories (e.g. September 11 attacks, assassination of JFK). These memories have been linked to increased activation in the amygdala.[16] Recent studies of patients with damage to the amygdala suggest that it is involved in memory for general knowledge, and not for specific information.[17][18]
Other structures involved
The regions of the Diencephalon have shown brain activation when a remote memory is being recovered[13] and the Occipital lobe, Ventral Temporal lobe, and Fusiform gyrus all play a role in memory formation.[9]
Lesion studies
Lesion studies are commonly used in cognitive neuroscience research. Lesions can occur naturally through trauma or disease, or they can be surgically induced by researchers. In the study of declarative memory, the hippocampus and the amygdala are two structures frequently examined using this technique.
Hippocampal lesion studies

The Morris water navigation task tests spatial learning in rats.[19] In this test rats learn to escape from a pool by swimming toward a platform submerged just below the surface of the water. Visual cues that surround the pool (e.g. a chair or window) help the rat to locate the platform on subsequent trials. The rats' use of specific events, cues and places are all forms of declarative memory.[20] Two groups of rats are observed: a control group with no lesions and an experimental group with hippocampal lesions. In this task created by Morris, et al., rats are placed in the pool at the same position for 12 trials. Each trial is timed and the path taken by the rats is recorded. Rats with hippocampal lesions successfully learn to find the platform. If the starting point is moved, the rats with hippocampal lesions typically fail to locate the platform. The control rats, however, are able to find the platform using the cues acquired during the learning trials.[19] This demonstrates the involvement of the hippocampus in declarative memory.[20]
The Odor-odor Recognition Task, devised by Bunsey and Eichenbaum, involves a social encounter between two rats (a "subject" and a "demonstrator"). The demonstrator, after eating a specific type of food, interacts with the subject rat, who then smells the food odor on the other's breath. The experimenters then present the subject rat with a decision between two food options; the food previously eaten by the demonstrator, and a novel food. The researchers found that when there was no time delay, both control rats and rats with lesions chose the familiar food. After 24 hours, however, the rats with hippocampal lesions were just as likely to eat both types of food, while control rats chose the familiar food.[21] This can be attributed to the inability to form episodic memories due to lesions in the hippocampus. The effects of this study can be observed in humans with amnesia, indicating the role of the hippocampus in developing episodic memories that can be generalized to similar situations.[20]
Henry Molaison, previously known as H.M., had parts of both his left and right medial temporal lobes (hippocampi) removed which resulted in the loss of the ability to form new memories.[22] The long-term declarative memory was crucially affected when the structures from the medial temporal lobe were removed, including the ability to form new semantic knowledge and memories.[23] The dissociation in Molaison between the acquisition of declarative memory and other kinds of learning was seen initially in motor learning.[24] Molaison's declarative memory was not functioning, as was seen when Molaison completed the task of repetition priming. His performance does improve over trials, however, his scores were inferior to those of control participants.[25] In the condition of Molaison the same results from this priming task are reflected when looking at the other basic memory functions like remembering, recall and recognizing.[22] Lesions should not be interpreted as an all-or-nothing condition, in the case of Molaison not all memory and recognition is lost, although the declarative memory is severely damaged he still has a sense of self and memories that were developed before the lesion occurred.[26]
Patient R.B. was another clinical case reinforcing the role of the hippocampus in declarative memory. After suffering an ischemic episode during a cardiac bypass operation, Patient R.B. awoke with a severe anterograde amnesic disorder. IQ and cognition were unaffected, but declarative memory deficits were observed (although not to the extent of that seen in Molaison). Upon death, an autopsy revealed that Patient R.B. had bilateral lesions of the CA1 cell region along the whole length of the hippocampus.
Amygdala lesion studies
Adolph, Cahill and Schul completed a study showing that emotional arousal facilitates the encoding of material into long term declarative memory.[27] They selected two subjects with bilateral damage to the amygdala, as well as six control subjects and six subjects with brain damage. All subjects were shown a series of twelve slides accompanied by a narrative. The slides varied in the degree to which they evoked emotion - slides 1 through 4 and slides 9 through 12 contain non-emotional content. Slides 5 through 8 contain emotional material, and the seventh slide contained the most emotionally arousing image and description (a picture of surgically repaired legs of a car crash victim).[27]
The emotionally arousing slide (slide 7) was remembered no better by the bilateral damage participants than any of the other slides. All other participants notably remembered the seventh slide the best and in most detail out of all the other slides.[27] This shows that the amygdala is necessary to facilitate encoding of declarative knowledge regarding emotionally arousing stimuli, but is not required for encoding knowledge of emotionally neutral stimuli.[28]
Factors that affect declarative memory
Stress
Stress may have an effect on the recall of declarative memories. Lupien, et al. completed a study that had 3 phases for participants to take part in. Phase 1 involved memorizing a series of words, phase 2 entailed either a stressful (public speaking) or non-stressful situation (an attention task), and phase 3 required participants to recall the words they learned in phase 1. There were signs of decreased declarative memory performance in the participants that had to complete the stressful situation after learning the words.[29] Recall performance after the stressful situation was found to be worse overall than after the non-stressful situation, where performance differed based on whether the participant responded to the stressful situation with an increase in measured levels of salivary cortisol.
Posttraumatic stress disorder (PTSD) emerges after exposure to a traumatic event eliciting fear, horror or helplessness that involves bodily injury, the threat of injury, or death to one’s self or another person[30] The chronic stress in PTSD contributes to an observed decrease in hippocampal volume and declarative memory deficits.[31]
Neurochemical factors of stress on the brain
In the brain, Glucocorticoids (GC's) modulate the ability of the hippocampus and PFC to process memories.[32] Cortisol is one of the most common GC’s in the human body, and hydrocortisone (a derivative of cortisol) decreases brain activity in the above areas during declarative memory retrieval.[32]
Elevations in cortisol occur during stress, and long-term stress impairs declarative memory this way.[32] A study done by Damoiseaux et al. (2007) evaluated the effect of glucocorticoids on MTL and PFC activation in young men. They found that GC’s given to participants 1 hour before retrieval of information impairs free recall of words, yet when administered before or after learning they had no effect.[32] Although it is not known exactly how GC’s influence memory, there are Glucocorticoid receptors in the hippocampus and PFC that tell us these structures are targets for the circulating hormone.[32] However, it is known that cortisone impairs memory function by reducing the blood flow in the right parahippocampal gyrus, left visual cortex, and the Cerebellum.[32]
Note: This study involved only male subjects, which may be significant as sex steroids may have different effects in the responses to cortisol administration. Men and women also respond to emotional stimuli differently, and this may affect cortisol levels. Also, this study was the first Functional magnetic resonance imaging(fMRI) study to be done involving GC's and more research is necessary to support these findings.[32]
Declarative memory consolidation during sleep
It is believed that sleep plays an active role in consolidation of declarative memory. Specifically, sleep’s unique properties enhance memory consolidation, such as the reactivation of newly learned memories during sleep. For example, it has been suggested that the central mechanism for consolidation of declarative memory during sleep is the reactivation of hippocampal memory representations. This reactivation transfers information to neocortical networks where it is integrated into long-term representations.[33] Studies on rats involving maze learning found that hippocampal neuronal assemblies that are used in the encoding of spatial information are reactivated in the same temporal order.[34] Similarly, positron emission tomography (PET) has shown reactivation of the hippocampus in slow-wave sleep (SWS) after spatial learning.[35] Together these studies show that newly learned memories are reactivated during sleep and through this process new memory traces are consolidated.[36] In addition, researchers have identified three types of sleep (SWS, sleep spindle and REM) in which declarative memory is consolidated.
Slow-Wave Sleep, often referred to as deep sleep, plays the most important role in consolidation of declarative memory and there is a large amount of evidence to support this claim. One study found that the first 3.5 hours of sleep offer the greatest performance enhancement on memory recall tasks because the first couple of hours are dominated by SWS. Additional hours of sleep do not add to the initial level of performance. Thus this study suggests that full sleep may not be important for optimal performance of memory.[37] Another study shows that people who experience SWS during the first half of their sleep cycle compared to subjects who did not, showed better recall of information. However this is not the case for subjects who were tested for the second half of their sleep cycle, as they experience less SWS.[38]
Another key piece of evidence regarding SWS’s involvement in declarative memory consolidation is a finding that people with pathological conditions of sleep, such as insomnia, exhibit both reduction in Slow-Wave Sleep and also have impaired consolidation of declarative memory during sleep.[39] Another study found that middle aged people compared to young group had a worse retrieval of memories. This in turn indicated that SWS is associated with poor declarative memory consolidation but not with age itself.[40]
Some researchers suggest that sleep spindle, a burst of brain activity occurring during stage 2 sleep, plays a role in boosting consolidation of declarative memories.[41] Critics point out that spindle activity is positively correlated with intelligence.[42] In contrast, Schabus and Gruber point out that sleep spindle activity only relates to performance on newly learned memories and not to absolute performance. This supports the hypothesis that sleep spindle helps to consolidate recent memory traces but not memory performance in general.[43] The relationship between sleep spindles and declarative memory consolidation is not yet fully understood.[citation needed]
There is a relatively small body of evidence that supports the idea that REM sleep helps consolidate highly emotional declarative memories. For instance Wagner, et al. compared memory retention for emotional versus neutral text over two instances; early sleep that is dominated by SWS and late sleep that is dominated by REM phase.[44] This study found that sleep improved memory retention of emotional text only during late sleep phase, which was primarily REM. Similarly, Hu & Stylos-Allen, et al. performed a study with emotional versus neutral pictures and concluded that REM sleep facilitates consolidation of emotional declarative memories.[45]
The view that sleep plays an active role in declarative memory consolidation is not shared by all researchers. For instance Ellenbogen, et al. argue that sleep actively protects declarative memory from associative interference.[46] Furthermore, Wixted believes that the sole role of sleep in declarative memory consolidation is nothing more but creating ideal conditions for memory consolidation.[47] For example, when awake, people are bombarded with mental activity which interferes with effective consolidation. However, during sleep, when interference is minimal, memories can be consolidated without associative interference. More research is needed to make a definite statement whether sleep creates favourable conditions for consolidation or it actively enhances declarative memory consolidation.[36]
In popular culture
Amnesiacs are frequently portrayed in television and movies. Some of the better-known examples include:
In the romantic comedy 50 First Dates (2004), Adam Sandler plays veterinarian Henry Roth, who falls for Lucy Whitmore, played by Drew Barrymore. Having lost her short term memory in a car crash, Lucy can only remember the current day's events until she falls asleep. When she wakes up the next morning, she has no recollection of the previous day's experiences.[48] These experiences would normally be transferred into declarative knowledge, allowing them to be recalled in the future. Although this movie is not the most accurate representation of a true amnesic patient, it is useful for informing viewers of the detrimental effects of amnesia.
Memento (2000) a film inspired by the case of Henry Molaison (H.M.).[49] Guy Pearce plays an ex-insurance investigator suffering from severe anterograde amnesia caused by a head injury. Unlike most amnesiacs, Leonard retains his identity and the memories of events that occurred before the injury, but loses all ability to form new memories. This loss of ability to form new memories indicates that the head injury affected the medial temporal lobe of the brain resulting in the inability for Leonard to form declarative memory.
Finding Nemo features a reef fish named Dory with an inability to develop declarative memory. This prevents her from learning or retaining any new information such as names or directions. The exact origin of Dory's impairment is not mentioned in the film, but her memory loss accurately portrays the difficulties facing amnesiacs.[48]
References
- ^ Ullman MT. Contributions of memory circuits to language: the declarative/procedural model. Cognition 2004; 92: 231–70.
- ^ Kalat, James W. Biological Psychology. Belmont, CA: Wadsworth, Cengage Learning, 2009. 538-539.
- ^ a b c d Tulving E. 1972. Episodic and semantic memory. In Organization of Memory, ed. E Tulving, W Donaldson, pp. 381–403. New York: Academic
- ^ Aristotle, On the Soul (De Anima), in Aristotle, Volume 4, Loeb Classical Library, William Heinemann, London, UK, 1936.
- ^ Ebbinghaus, H. (1885). Memory: A Contribution to Experimental Psychology. Teachers College, Columbia University
- ^ Graf, P., & Schacter, D. L. (1985). Implicit and explicit memory for new associations in normal and amnesic subjects. Journal of Experimen-tal Psychology: Learning, Memory, and Cognition, 11, 501-518.
- ^ a b Eichenbaum, Howard (1997). Declarative memory: Insights from cognitive neurobiology. Annual Review of Psychology. Vol 48, 547-572.
- ^ a b c d e Eichenbaum, Howard (2001). The Hippocampus and Declarative Memory: Cognitive Mechanisms and Neural Codes. Behavioural Brain Research, Vol 127: 199-207.
- ^ a b c d Gabrieli, J., & Kao, Y. (2007). Development of the Declarative Memory System in the Human Brain. Nature Neuroscience, 10: 1198-1205.
- ^ Manns, Joseph; Eichenbaum (September 2006). "Evolution of Declarative Memory". Hippocampus. 16 (9): 795–808. doi:10.1002/hipo.20205. PMID 16881079.
- ^ Davachi, L., Mitchell, J.P., & Wagner, A.D. (2003). Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences
- ^ Davachi, Dobbins (2008). Declarative Memory, Current Directions in Psychological Science.
- ^ a b Graham, S., Levine, B. (2004). The Fundamental Neuroanatomy of Episodic and Semantic Autobiographical Remembering: A Prospective Functional MRI Study. Journal of Cognitive Neuroscience , 16: 1633-1646.
- ^ a b Craik, F.I.M., Houle, S. (1994). Role of Prefrontal Cortex in Human Episodic Memory: Lessons From PET Studies. Biol. Psychiatry, 42: 75S-76S.
- ^ Stuss, D.T., Tulving, E. (1997). Toward a Theory of Episodic Memory: The Frontal Lobes & Autonoetic Consciousness. Psychological Bulletin, 121: 331-354.
- ^ Sharot T, Martorella EA, Delgado MR, Phelps EA. How personal experience modulates the neural circuitry of memories of September 11. Proc Natl Acad Sci USA. 2007;104:389–394.
- ^ Adolphs R, Tranel D, Buchanan TW (2005) Amygdala damage impairs emotional memory for gist but not details of complex stimuli. Nat Neurosci 8:512–518
- ^ Adolphs R, Denburg NL, Tranel D (2001) The amygdala’s role in long-term declarative memory for gist and detail. Behav Neurosci 115:983–992
- ^ a b Eichenbaum, H., Stewart, C. & Morris, R. G. M. Hippocampal representation in spatial learning. J. Neurosci. 10, 331–339 (1990).
- ^ a b c Eichenbaum, H. (2000). A cortical-hippocampal system for declarative memory. Nature Reviews Neuroscience, 1, 41-50.
- ^ Bunsey, M. & Eichenbaum, H. Selective damage to the hippocampal region blocks long term retention of a natural and nonspatial stimulus-stimulus association. Hippocampus 5, 546–556 (1995).
- ^ a b Corkin, S. (2002). Perspectives: What’s new with the amnesic patient H.M.? Nature Reviews Neuroscience, 3, 153-160.
- ^ Gabrieli, J. D. E., Cohen, N. J. & Corkin, S. The impaired learning of semantic knowledge following bilateral medial temporal-lobe resection. Brain Cogn. 7,157–177 (1988).
- ^ Corkin, S. Acquisition of motor skill after bilateral medial temporal-lobe excision. Neuropsychologia 6, 225–264 (1968).
- ^ Milner, B., Corkin, S. & Teuber, H.-L. Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of H.M. Neuropsychologia 6, 215–234 (1968).
- ^ Aggleton, J. P. & Brown, M. W. Episodic memory, amnesia, and the hippocampal–anterior thalamic axis. Behav. Brain Sci. 22, 425–489 (1999).
- ^ a b c Adolphs, R., Cahill, L., Schul, R., & Babinsky, R. (1997). Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learning and Memory, 4, 291-300.
- ^ Babinsky, R., Calabrese, P., Durwen, H., Markowitsch, H., & Brechtelsbuauer, D. (1993). The possible contribution of the amygdala to memory. Behav. Neuro, 6, 167-170.
- ^ Lupien, S., Gaudreau, S., Tchiteya, B., Maheu, F., Sharma, S., Nair, N., et al. (1997). Stress-Induced Declarative Memory Impairment in Healthy Elderly Subjects: Relationship to Cortisol Reactivity. The Journal of Clinical Endocrinology & Metabolism, 82(7), 2070-2075.
- ^ Cabeza, R., & LaBar, K. S. (2006). Cognitive neuroscience of emotional memory. Nature Publishing Group, 7, 54-64.
- ^ Baker, D. G. et al. Higher levels of basal CSF cortisol in combat veterans with posttraumatic stress disorder. Am. J. Psychiatry 162, 992–994 (2005).
- ^ a b c d e f g Damoiseaux, J.S., Elzinga, B.M. (2007). Glucocorticoids Decrease Hippocampal and Prefrontal Activation during Declarative Memory Retrieval in Young Men. Brain Imaging and Behaviour, Vol 1: 31-41.
- ^ McClelland, J.L., McNaughton, B.L., and O’Reilly, R.C. 1995. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102: 419–457.
- ^ D. Ji, M. A. Wilson, Nat. Neurosci. 10, 100 (2007)
- ^ P. Peigneux et al., Neuron 44, 535 (2004)
- ^ a b Ellenbogen J, Payne D, et al. (2006) The role of sleep in declarative memory consolidation: passive, permissive, active or none? Current Opinion in Neurobiology 2006, 16:716–722
- ^ Tucker A, Fishbein W, (2009) The Impact of sleep duration and subject intelligence on declarative and motor memory performance: how much is enough? J. Sleep Res., 304-312
- ^ Plihal W, Born J: Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci 1997, 9:534-547.
- ^ Backhaus, J., Junghanns, K., Born, J., Hohaus, K., Faasch, F., and Hohagen, F. 2006. Impaired declarative memory consolidation during sleep in patients with primary insomnia: Influence of sleep architecture and nocturnal cortisol release. Biol. Psychiatry 60: 1324–1330.
- ^ Backhaus J, Born J, et al. (2007) Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learning & Memory, 14: 336-341
- ^ Gais S, Molle M, Helms K, Born J: Learning-dependent increases in sleep spindle density. J Neurosci 2002, 22:6830-6834.
- ^ Schabus M, Hodlmoser K, Gruber G, Sauter C, Anderer P, Klosch G, Parapatics S, Saletu B, Klimesch W, Zeitlhofer J: Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosci 2006, 23:1738-1746
- ^ Schabus M; Gruber G; Parapatics S et al. Sleep spindles and their significance for declarative memory consolidation. SLEEP 2004;27(8):1479-85
- ^ Wagner U, Gais S, Born J: Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem 2001, 8:112-119.
- ^ Hu P, Stylos-Allen M, Walker MP: Sleep facilitates consolidation of emotionally arousing declarative memory. Psychol Sci 2006, 17, in press.
- ^ Ellenbogen J; Hulbert J; et al. Interfering with Theories of Sleep and Memory: Sleep, Declarative Memory, and Associative Interference. Current Biology 2006; 16 1290-1294.
- ^ Wixted JT: The psychology and neuroscience of forgetting. Annu Rev Psychol 2004, 55:235-269.
- ^ a b Baxtendale, S. (2004). Memories aren’t made of this: Amnesia at the movies. British Medical Journal. 329: 1480-1483
- ^ Chun, M. (2005). Drug-induced amnesia impairs implicit relational memory. Trends in Cognitive Sciences, 9(8), 355-357.
