Biotin
| |

| |
| Names | |
|---|---|
| IUPAC name
5-[(3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl]pentanoic acid
| |
| Other names
Vitamin B7; Vitamin H; Coenzyme R; Biopeiderm
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.363 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H16N2O3S | |
| Molar mass | 244.31 g·mol−1 |
| Appearance | White crystalline needles |
| Melting point | 232 to 233 °C (450 to 451 °F; 505 to 506 K) |
| 22 mg/100 mL | |
| Pharmacology | |
| A11HA05 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Biotin, also called vitamin B7, is one of the B vitamins.[1][2][3] It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids.[4] The name biotin derives from the Greek word “bios” (to live) and the suffix “-in” (a general chemical suffix used in organic chemistry).[5]
Biotin deficiency can be caused by inadequate dietary intake (rare) or inheritance of one or more inborn genetic disorders that affect biotin metabolism. The most common among these is biotinidase deficiency. Low activity of this enzyme causes a failure to recycle biotin from biocytin. Rarer are carboxylase and biotin transporter deficiences.[4][6] Subclinical deficiency can cause mild symptoms, such as hair thinning, brittle fingernails, or skin rash, typically on the face.[2][4] Neonatal screening for biotinidase deficiency started in the United States in 1984, with many countries now also testing for this genetic disorder at birth. Treatment is lifelong dietary supplement with biotin.[1]
Definition
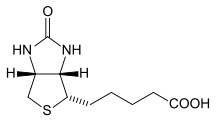
Biotin is a water-soluble B-vitamin. It is composed of a ureido ring fused with a tetrahydrothiophene ring. The ureido ring containing the –N–CO–N– group acts as the carbon dioxide carrier in carboxylation reactions.[7] A valeric acid substituent is attached to one of the carbon atoms of the tetrahydrothiophene ring. Biotin is a coenzyme for five carboxylase enzymes, which are involved in the digestion of carbohydrates, synthesis of fatty acids, and gluconeogenesis.[3][4] Biotinylation of histone proteins in nuclear chromatin plays a role in chromatin stability and gene expression.[4][8]
Dietary recommendations
The US National Academy of Medicine updated Dietary Reference Intakes for many vitamins in 1998. At that time there was insufficient information to establish estimated average requirement or recommended dietary allowance, terms that exist for most vitamins. In instances such as this, the Academy sets adequate intakes (AIs) with the understanding that at some later date, when the physiological effects of biotin are better understood, AIs will be replaced by more exact information. The biotin AIs for both males and females are: 5 μg/day of biotin for 0-6-month-olds, 6 μg/day of biotin for 7-12-month-olds, 8 μg/day of biotin for 1-3-year-olds, 12 μg/day of biotin for 4-8-year-olds, 20 μg/day of biotin for 9-13-year-olds, 25 μg/day of biotin for 14-18-year-olds, and 30 μg/day of biotin for those 19-years old and older. The biotin AIs for females who are either pregnant or lactating, respectively, are: 30 μg/day of biotin for pregnant females 14-50-years old; 35 μg/day of biotin for lactating females 14-50-years old.[2] Australia and New Zealand set AIs similar to the US.[9]
The European Food Safety Authority (EFSA) also identifies AIs, setting values at 40 μg/day for adults, pregnancy at 40 μg/day, and breastfeeding at 45 μg/day. For children ages 1–17 years, the AIs increase with age from 20 to 35 μg/day.[10]
Safety
The US National Academy of Medicine estimates upper limits (ULs) for vitamins and minerals when evidence for a true limit is sufficient. For biotin, however, there is no UL because adverse effects of high biotin intake have not been determined.[2] The EFSA also reviewed safety and reached the same conclusion as in the United States.[11]
Labeling regulations
For US food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of daily value. For biotin labeling purposes 100% of the daily value was 300 μg/day, but as of May 27, 2016 it was revised to 30 μg/day to bring it into an agreement with the adequate intake.[12][13] Compliance with the updated labeling regulations was required by 1 January 2020 for manufacturers with US$10 million or more in annual food sales, and by 1 January 2021 for manufacturers with lower volume food sales.[14][15] A table of the old and new adult daily values is provided at Reference Daily Intake.
Sources
Sources with appreciable content are:
| Source[16] | Amount (μg / 100 g) |
|---|---|
| Chicken liver | 187 |
| Beef liver | 42 |
| Eggs | 21 |
| Egg white | 5.8 |
| Egg yolk | 27 |
| Salmon, canned in water | 5.9 |
| Pork chop | 4.5 |
| Turkey breast | 0.7 |
| Tuna, white, canned | 0.7 |
| Source[16] | Amount (μg / 100 g) |
|---|---|
| Peanuts, roasted | 17.5 |
| Sunflower seeds, roasted | 7.8 |
| Almonds, roasted | 4.4 |
| Sweet potato | 1.5 |
| Broccoli | 0.9 |
| Tomato | 0.7 |
| Strawberry | 1.5 |
| Avocado | 1.0 |
| Corn, canned | 0.05 |
| Source[16] | Amount (μg / 100 g) |
|---|---|
| Cheese | 1.4 |
| Milk | 0.1 |
| Corn flakes cereal | 0.1 |
| Oatmeal | 0.1 |
| Bread | 0.1 |
| French fries | 0.3 |
| Wine | 0.1 |
| Beer | 0.1 |
| Potatos, mashed | 0.1 |
Biotin is stable at room temperature and is not destroyed by cooking. The dietary biotin intake in Western populations has been estimated to be in the range of 35 to 70 μg/day. Nursing infants ingest about 6 μg/day.[4] Biotin is available in dietary supplements, individually or as an ingredient in multivitamins.[1][3]
No government fortification programs
According to the Global Fortification Data Exchange, biotin deficiency is so rare that no countries require that foods be fortified.[17]
Physiology
Biotin is a water-soluble B vitamin. Consumption of large amounts as a dietary supplement results in absorption, followed by excretion into urine as biotin. Consumption of biotin as part of a normal diet results in urinary excretion of biotin and biotin metabolites.
Absorption
Biotin in food is bound to proteins. Digestive enzymes reduce the proteins to biotin-bound peptides. The intestinal enzyme biotinidase, found in pancreatic secretions and in the brush border membranes of all three parts of the small intestine, frees biotin, which is then absorbed from the small intestine.[4] When consumed as a biotin dietary supplement, absorption is nonsaturable, meaning that even very high amounts are absorbed effectively. Transport across the jejunum is faster than across the ileum.[4]
The large intestine microbiota synthesize amounts of biotin estimated to be similar to the amount taken in the diet, and a significant portion of this biotin exists in the free (protein-unbound) form and, thus, is available for absorption. How much is absorbed in humans is unknown, although a review did report that human epithelial cells of the colon in vitro demonstrated an ability to update biotin.[18]
Once absorbed, sodium-dependent multivitamin transporter (SMVT) mediates biotin uptake into the liver.[4] SMVT also binds pantothenic acid, so high intakes of either of these vitamins can interfere with transport of the other.[19]
Metabolism and excretion
Biotin catabolism occurs via two pathways. In one, the valeric acid sidechain is cleaved, resulting in bisnorbiotin. In the other pathway, the sulfur is oxidized, resulting in biotin sulfoxide. Urine content is proportionally about half biotin, plus bisnorbiotin, biotin sulfoxide and small amounts of other metabolites.[4]
Factors that affect biotin requirements
Chronic alcohol use is associated with a significant reduction in plasma biotin.[20] Intestinal biotin uptake also appears to be sensitive to the effect of the anti-epilepsy drugs carbamazepine and primidone.[20] Relatively low levels of biotin have also been reported in the urine or plasma of patients who have had a partial gastrectomy or have other causes of achlorhydria, burn patients, elderly individuals, and athletes.[21] Pregnancy and lactation may be associated with an increased demand for biotin. In pregnancy, this may be due to a possible acceleration of biotin catabolism, whereas, in lactation, the higher demand has yet to be elucidated. Recent studies have shown marginal biotin deficiency can be present in human gestation, as evidenced by increased urinary excretion of 3-hydroxyisovaleric acid, decreased urinary excretion of biotin and bisnorbiotin, and decreased plasma concentration of biotin.[4]
Biosynthesis
Biotin is a heterocyclic, sulfur-containing monocarboxylic acid with two rings fused together via one of their sides (figure). The two rings are ureido and tetrahydrothiophene moieties.[22] Biotin, synthesized in plants, is essential to plant growth and development.[23] Synthesis starts from two precursors, alanine and pimeloyl-CoA. These form 7-Keto-8-aminopelargonic acid (KAPA). KAPA is transported from plant peroxisomes to mitochondria where it is converted to 7,8-Diaminopelargonic acid (DAPA). The enzyme dethiobiotin synthetase catalyzes the formation of the ureido ring via a DAPA carbamate activated with ATP, creating dethiobiotin. The last step is catalyzed by biotin synthase.[22][23]
Cofactor biochemistry
The enzyme holocarboxylase synthetase covalently attaches biotin to five human carboxylase enzymes:[4]
- Acetyl-CoA carboxylase alpha (ACC1)
- Acetyl-CoA carboxylase beta (ACC2)
- Pyruvate carboxylase (PC)
- Methylcrotonyl-CoA carboxylase (MCC)
- Propionyl-CoA carboxylase (PCC)
For the first two, biotin serves as a cofactor responsible for transfer of bicarbonate to acetyl-CoA, converting it to malonyl-CoA for fatty acid synthesis. PC participates in gluconeogenesis. MCC catalyzes a step in leucine metabolism. PCC catalyzes a step in the metabolism of propionyl-CoA.[1][3][4] Metabolic degradation of the biotinylated carboxylases leads to the formation of biocytin. This compound is further degraded by biotinidase to release biotin, which is then reutilized by holocarboxylase synthetase.[4]
In addition to carboxylase functions, biotinylation of histone proteins in nuclear chromatin plays a role in chromatin stability and gene expression.[4][8]
Deficiency
Biotin deficiency in humans is rare. Signs of biotin deficiency have been described in people who were receiving all nutrition intravenously without biotin, also people regularly consuming raw or incompletely cooked egg whites, because egg whites contain avidin, a biotin-binding protein. Deficiency symptoms include: brittle and thin fingernails, hair loss (alopecia), conjunctivitis, dermatitis in the form of a scaly, red rash around the eyes, nose, mouth, and genital area, plus neurological symptoms such as depression, lethargy, hallucination, and numbness and tingling of the extremities[4] The neurological and psychological symptoms can occur with only mild deficiencies. Dermatitis, conjunctivitis, and hair loss will generally occur only when deficiency becomes more severe. Individuals with hereditary disorders of biotin deficiency have evidence of impaired immune system function, including increased susceptibility to bacterial and fungal infections.[3]
Diagnosis
Low serum and urine biotin are not sensitive indicators of inadequate biotin intake.[4] However, serum testing can be useful for confirmation of consumption of biotin-containing dietary supplements, and whether a period of refraining from supplement use is long enough to eliminate the potential for interfering with drug tests.[24][25] Indirect measures depend on the biotin requirement for carboxylases. 3-Methylcrotonyl-CoA is an intermediate step in the catabolism of the amino acid leucine. In the absence of biotin, the pathway diverts to 3-hydroxyisovaleric acid. Urinary excretion of this compound is an early and sensitive indicator of biotin deficiency.[2][4]
Metabolic disorders
Biotinidase deficiency is a deficiency of the enzyme that recycles biotin, the consequence of an inherited genetic mutation.[1] Biotinidase catalyzes the cleavage of biotin from biocytin and biotinyl-peptides (the proteolytic degradation products of each holocarboxylase) and thereby recycles biotin.[2] It is also important in freeing biotin from dietary protein-bound biotin.[26] Other inherited metabolic disorders characterized by deficient activities of biotin-dependent carboxylases are termed multiple carboxylase deficiency. These include deficiencies in the enzymes holocarboxylase synthetase or biotinidase.[1] Holocarboxylase synthetase deficiency prevents the body's cells from using biotin effectively and thus interferes with multiple carboxylase reactions.[26] Biochemical and clinical manifestations include ketolactic acidosis, organic aciduria, hyperammonemia, skin rash, feeding problems, hypotonia, seizures, developmental delay, alopecia, and coma.
Prevalence, diagnosis and treatment
Profound biotinidase deficiency, defined as less than 10% of normal serum enzyme activity, which has been reported as 7.1 nmol/min/mL, has an incidence of 1 in 40,000 to 1 in 60,000, but with rates as high as 1 in 10,000 in countries with high incidence of consanguineous marriages (second cousin or closer). Partial biotinidase deficiency is defined as 10% to 30% of normal serum activity.[27] Incidence data stems from government mandated newborn screening, which as of 2017 was reported as required in more than 30 countries.[28] For profound deficiency, treatment is oral dosing with 5 to 20 mg per day. Seizures are reported as resolving in hours to days, with other symptoms resolving within weeks.[27] Treatment of partial biotinidase deficiency is also recommended even though some untreated people never manifest symptoms.[27]
Use in biotechnology
Biotin is widely used throughout the biotechnology industry to conjugate proteins for biochemical assays.[29] Biotin's small size means the biological activity of the protein will most likely be unaffected. This process is called biotinylation. Because both streptavidin and avidin bind strongly to biotin with a dissociation constant Kd in the range of 10−14 to 10−15 M),[30] biotinylated proteins of interest can be isolated from a sample by exploiting this highly stable interaction. The sample is incubated with streptavidin/avidin beads, allowing the capture of the biotinylated protein of interest. Any other proteins binding to the biotinylated molecule will also stay with the bead and all other unbound proteins can be washed away. However, due to the extremely strong streptavidin-biotin interaction, very harsh conditions are needed to elute the biotinylated protein from the beads (typically 6 M guanidine HCl at pH 1.5), which often will denature the protein of interest. To circumvent this problem, beads conjugated to monomeric avidin can be used, which has a decreased biotin-binding affinity of ≈10−8 M, allowing the biotinylated protein of interest to be eluted with excess free biotin.[31]
Interference with medical laboratory results
When people are ingesting high levels of biotin in dietary supplements, a consequence can be clinically significant interference with diagnostic immunoassay blood assays that use biotin-streptavidin technology. This methodology is commonly used to measure levels of hormones such as thyroid hormones, and other analytes such as 25-hydroxyvitamin D. Biotin interference can produce both falsely normal and falsely abnormal results.[1][32] In the US, biotin as a non-prescription dietary supplement is sold in amounts of 1 to 10 mg per serving, with claims for supporting hair and nail health, and as 300 mg per day as a possibly effective treatment for multiple sclerosis[33][34] (see Research). Overconsumption of 5 mg/day or higher causes elevated concentration in plasma that interferes with biotin-streptavidin immunoassays in an unpredictable manner.[24][25] Healthcare professionals are advised to instruct patients to stop taking biotin supplements for 48 h or even up to weeks before the test, depending on the specific test, dose, and frequency of biotin uptake.[24] Guidance for laboratory staff is proposed to detect and manage biotin interference.[25]
History
In 1916, W.G. Bateman observed that a diet high in raw egg whites caused toxic symptoms in dogs, cats, rabbits, and humans.[35] By 1927, scientists such as Margarete Boas and Helen Parsons had performed experiments demonstrating the symptoms associated with "egg-white injury." They had found that rats fed large amounts of egg-white as their only protein source exhibited neurological dysfunction, hair loss, dermatitis, and eventually, death.[36][37]
Hungarian scientist Paul Gyorgy began investigating the factor responsible for egg-white injury in 1933 and in 1939, was successful identifying what he called "Vitamin H" (the H represents Haar und Haut, German for hair and skin).[38][39] Further chemical characterization of vitamin H revealed that it was water-soluble and present in high amounts in the liver.[40] Other research groups, working independently, had isolated the same compound, under different names. In 1936, Kögl and Tönnis isolated a growth factor from egg yolk they called "Bios aus Eigelb."[41] After experiments performed with yeast and Rhizobium trifolii, West and Wilson isolated a compound they called co-enzyme R.[42][43] By 1940, it was recognized that all three compounds were identical and were collectively given the name: biotin.[44] Gyorgy continued his work on biotin and in 1941 published a paper demonstrating that egg-white injury was caused by the binding of biotin by avidin.[45][46]
Using E. coli, a biosynthesis pathway was proposed by Rolfe and Eisenberg in 1968. The initial step was described as a condensation of pimelyl-CoA and alanine to form 7-oxo-8-aminopelargonic acid. From there, they described three-step process, the last being introducing a sulfur atom to form the tetrahydrothiophene ring.[47]
Research
Multiple sclerosis
High-dose biotin (300 mg/day = 10,000 times adequate intake) has been clinical trialed for treatment of multiple sclerosis, a demyelinating autoimmune disease.[33][34] The hypothesis is that biotin may promote remyelination of the myelin sheath of nerve cells, slowing or even reversing neurodegeneration. The proposed mechanisms are that biotin activates acetyl-coA carboxylase, which is a key rate-limiting enzyme during the synthesis of myelin, and by reducing axonal hypoxia through enhanced energy production.[33][34] Clinical trial results are mixed; a 2019 review concluded that a further investigation of the association between multiple sclerosis symptoms and biotin should be undertaken,[33] whereas two 2020 reviews of a larger number of clinical trials reported no consistent evidence for benefits,[48] and some evidence for increased disease activity and higher risk of relapse.[49]
Hair, nails, skin
Biotin is promoted as a dietary supplement for strengthening hair and fingernails, though scientific data supporting these outcomes in humans are very weak.[3][50][51] A review of the fingernails literature reported brittle nail improvement as evidence from two pre-1990 clinical trials that had administered an oral dietary supplement of 2.5 mg/day for several months, without a placebo control comparison group. There is no more recent clinical trial literature.[50] A review of biotin as treatment for hair loss identified case studies of infants and young children with genetic defect biotin deficiency having improved hair growth after supplementation, but went on to report that "there have been no randomized, controlled trials to prove efficacy of supplementation with biotin in normal, healthy individuals."[51] Nevertheless, biotin is marketed as a dietary supplement for nails and hair health. Biotin is also incorporated into topical hair and skin products with similar claims.[52]
Animals
In cattle, biotin is necessary for hoof health. Lameness due to hoof problems is common, with herd prevalence estimated at 10 to 35%. Consequences of lameness include less food consumption, lower milk production, and increased veterinary treatment costs. Dietary supplementation biotin at 20 mg/day reduces the risk of lameness.[53] A review of controlled trials reported that supplementation at 20 mg/day increased milk yield by 4.8%. The discussion speculated that this could be an indirect consequence of improved hoof health or a direct effect on milk production.[54]
For horses, conditions such as chronic laminitis, cracked hooves, or dry, brittle feet incapable of holding shoes are a common problem. Biotin is a popular nutritional supplement. There are recommendations that horses need 15 to 25 mg/day. Studies report biotin improves the growth of new hoof horn rather than improving the status of existing hoof, so months of supplementation are needed for the hoof wall to be completely replaced.[55]
See also
References
- ^ a b c d e f g "Biotin – Fact Sheet for Health Professionals". Office of Dietary Supplements, US National Institutes of Health. 8 December 2017. Retrieved 25 February 2018.
- ^ a b c d e f Institute of Medicine (1998). "Biotin". Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: The National Academies Press. pp. 374–389. ISBN 0-309-06554-2. Retrieved 2017-08-29.
- ^ a b c d e f "Biotin". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. 21 October 2015. Retrieved 16 January 2018.
- ^ a b c d e f g h i j k l m n o p q r Penberthy WT, Sadri M, Zempleni J (2020). "Biotin". In BP Marriott, DF Birt, VA Stallings, AA Yates (eds.). Present Knowledge in Nutrition, Eleventh Edition. London, United Kingdom: Academic Press (Elsevier). pp. 289–304. ISBN 978-0-323-66162-1.
- ^ "biotin | Origin and meaning of biotin by Online Etymology Dictionary". www.etymonline.com. Retrieved 2020-11-14.
- ^ Zempleni J, Hassan YI, Wijeratne SS (November 2008). "Biotin and biotinidase deficiency". Expert Review of Endocrinology & Metabolism. 3 (6): 715–24. doi:10.1586/17446651.3.6.715. PMC 2726758. PMID 19727438.
- ^ Waldrop GL, Holden HM, St Maurice M (November 2012). "The enzymes of biotin dependent CO₂ metabolism: what structures reveal about their reaction mechanisms". Protein Science. 21 (11): 1597–619. doi:10.1002/pro.2156. PMC 3527699. PMID 22969052.
- ^ a b Xu YM, Du JY, Lau AT (September 2014). "Posttranslational modifications of human histone H3: an update". Proteomics. 14 (17–18): 2047–60. doi:10.1002/pmic.201300435. PMID 25044606.
- ^ "National Health and Medical Research Council: Nutrient Reference Values for Australia and New Zealand" (PDF). Archived from the original (PDF) on 2017-01-21. Retrieved 2010-02-19.
- ^ "Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies" (PDF). 2017.
- ^ "Tolerable Upper Intake Levels For Vitamins And Minerals" (PDF). European Food Safety Authority. 2006.
- ^ "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels" (PDF). Archived (PDF) from the original on 2017-09-22.
- ^ "Daily Value Reference of the Dietary Supplement Label Database (DSLD)". Dietary Supplement Label Database (DSLD). Retrieved 16 May 2020.
- ^ "Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). 27 May 2016. Retrieved 16 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Industry Resources on the Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). 21 December 2018. Retrieved 16 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c Staggs CG, Sealey WM, McCabe BJ, Teague AM, Mock DM (December 2004). "Determination of the biotin content of select foods using accurate and sensitive HPLC/avidin binding". Journal of Food Composition and Analysis. 17 (6): 767–76. doi:10.1016/j.jfca.2003.09.015. PMC 1450323. PMID 16648879.
- ^ "Map: Count of Nutrients In Fortification Standards". Global Fortification Data Exchange. Retrieved 11 January 2011.
- ^ Said HM (November 2013). "Recent advances in transport of water-soluble vitamins in organs of the digestive system: a focus on the colon and the pancreas". Am J Physiol Gastrointest Liver Physiol. 305 (9): G601–10. doi:10.1152/ajpgi.00231.2013. PMC 3840235. PMID 23989008.
- ^ Chirapu SR, Rotter CJ, Miller EL, Varma MV, Dow RL, Finn MG (31 March 2013). "High specificity in response of the sodium-dependent multivitamin transporter to derivatives of pantothenic acid". Current Topics in Medicinal Chemistry. 13 (7): 837–42. doi:10.2174/1568026611313070006. PMID 23578027.
- ^ a b Said HM (August 2011). "Intestinal absorption of water-soluble vitamins in health and disease". Biochem J. 437 (3): 357–72. doi:10.1042/BJ20110326. PMC 4049159. PMID 21749321.
- ^ Combs GF (2008). The Vitamins: Fundamental Aspects in Nutrition and Health. San Diego: Elsevier, Inc. ISBN 978-0-12-183493-7.
- ^ a b Marquet A, Bui BT, Florentin D (2001). "Biosynthesis of biotin and lipoic acid". Cofactor Biosynthesis. Vitamins & Hormones. Vol. 61. pp. 51–101. doi:10.1016/S0083-6729(01)61002-1. ISBN 978-0-12-709861-6. PMID 11153271.
- ^ a b Maruyama J, Yamaoka S, Matsuo I, Tsutsumi N, Kitamoto K (December 2012). "A newly discovered function of peroxisomes: involvement in biotin biosynthesis". Plant Signal Behav. 7 (12): 1589–93. doi:10.4161/psb.22405. PMC 3578898. PMID 23073000.
- ^ a b c Luong JH, Vashist SK (January 2020). "Chemistry of Biotin-Streptavidin and the Growing Concern of an Emerging Biotin Interference in Clinical Immunoassays". ACS Omega. 5 (1): 10–18. doi:10.1021/acsomega.9b03013. PMC 6963918. PMID 31956746.
- ^ a b c Bowen R, Benavides R, Colón-Franco JM, Katzman BM, Muthukumar A, Sadrzadeh H, Straseski J, Klause U, Tran N (December 2019). "Best practices in mitigating the risk of biotin interference with laboratory testing". Clin Biochem. 74: 1–11. doi:10.1016/j.clinbiochem.2019.08.012. PMID 31473202.
- ^ a b Wolf B, Grier RE, Secor McVoy JR, Heard GS (1985). "Biotinidase deficiency: a novel vitamin recycling defect". Journal of Inherited Metabolic Disease. 8 (Suppl 1): 53–8. doi:10.1007/BF01800660. PMID 3930841. S2CID 11554577.
- ^ a b c Canda E, Kalkan Uçar S, Çoker M (2020). "Biotinidase Deficiency: Prevalence, Impact And Management Strategies". Pediatric Health Med Ther. 11: 127–33. doi:10.2147/PHMT.S198656. PMID 32440248.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Strovel ET, Cowan TM, Scott AI, Wolf B (October 2017). "Laboratory diagnosis of biotinidase deficiency, 2017 update: a technical standard and guideline of the American College of Medical Genetics and Genomics". Genet Med. 19 (10). doi:10.1038/gim.2017.84. PMID 28682309.
- ^ "Overview of Protein Labeling". Thermo Fisher Scientific. Retrieved 22 April 2012.
- ^ Laitinen OH, Hytönen VP, Nordlund HR, Kulomaa MS (December 2006). "Genetically engineered avidins and streptavidins". Cellular and Molecular Life Sciences. 63 (24): 2992–3017. doi:10.1007/s00018-006-6288-z. PMID 17086379. S2CID 7180383.
- ^ Morag E, Bayer EA, Wilchek M (Dec 1996). "Immobilized nitro-avidin and nitro-streptavidin as reusable affinity matrices for application in avidin-biotin technology". Anal Biochem. 243 (2): 257–63. doi:10.1006/abio.1996.0514. PMID 8954558.
- ^ "The FDA Warns that Biotin May Interfere with Lab Tests: FDA Safety Communication". US Food and Drug Administration. 28 November 2017. Retrieved 5 January 2021.
- ^ a b c d Tryfonos C, Mantzorou M, Fotiou D, Vrizas M, Vadikolias K, Pavlidou E, Giaginis C (September 2019). "Dietary Supplements on Controlling Multiple Sclerosis Symptoms and Relapses: Current Clinical Evidence and Future Perspectives". Medicines (Basel). 6 (3). doi:10.3390/medicines6030095. PMC 6789617. PMID 31547410.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Sedel F, Bernard D, Mock DM, Tourbah A (November 2016). "Targeting demyelination and virtual hypoxia with high-dose biotin as a treatment for progressive multiple sclerosis". Neuropharmacology. 110 (Pt B): 644–53. doi:10.1016/j.neuropharm.2015.08.028. PMID 26327679.
- ^ Bateman WG (June 1916). "The Digestibility and Utilization of Egg Proteins". Journal of Biological Chemistry. 26: 263–91.
- ^ Boas, MA (1927). "The Effect of Desiccation upon the Nutritive Properties of Egg-white". Biochemical Journal. 21 (3): 712–24.1. doi:10.1042/bj0210712. ISSN 0264-6021. PMC 1251968. PMID 16743887.
- ^ Parsons, HT; Kelly, E (November 1980). "The Character of the Dermatitis-Producing Factor in Dietary Egg White as Shown by Certain Chemical Treatments". Nutrition Reviews. 38 (11): 377–79. doi:10.1111/j.1753-4887.1980.tb05948.x. ISSN 0029-6643. PMID 7005763.
- ^ György, P (December 1939). "The Curative Factor (vitamin H) for Egg White Injury, with Particular Reference to Its Presence in Different Foodstuffs and in Yeast". Journal of Biological Chemistry. 131 (2): 733–44. ISSN 0021-9258.
- ^ György P, Kuhn R, Lederer E (December 1939). "Attempts to Isolate the Factor (vitamin H) Curative of Egg White Injury". Journal of Biological Chemistry. 131 (2): 745–59. ISSN 0021-9258.
- ^ Birch, TW; György, P (December 1939). "Physicochemical Properties of the Factor (vitamin H) Curative of Egg White Injury". Journal of Biological Chemistry. 131 (2): 761–66. ISSN 0021-9258.
- ^ Kögl and Tönnis (1936). "Über das Bios-Problem. Darstellung von krystallisiertem Biotin aus Eigelb. 20. Mitteilung über pflanzliche Wachstumsstoffe". Hoppe-Seyler's Zeitschrift für Physiologische Chemie. 242 (1–2): 43–73. doi:10.1515/bchm2.1936.242.1-2.43.
- ^ West PM, Wilson PW (June 1939). "The Relation of "coenzyme R" to Biotin". Science. 89 (2322): 607–8. Bibcode:1939Sci....89..607W. doi:10.1126/science.89.2322.607. PMID 17751623.
- ^ DuVigneaud V, Hofmann K, Melville DB, György P (August 1941). "Isolation of Biotin (vitamin H) from Liver". Journal of Biological Chemistry. 140 (2): 643–51. ISSN 0021-9258.
- ^ György P, Rose CS, Hofmann K, Melville DB, DuVigneaud V (December 1940). "A Further Note on the Identity of Vitamin H with Biotin". Science. 92 (2400): 609. Bibcode:1940Sci....92..609G. doi:10.1126/science.92.2400.609. ISSN 0036-8075. PMID 17795447.
- ^ György P, Rose CS, Eakin RE, Snell EE, Williams RJ (1941). "Egg-White Injury as the Result of Nonabsorption or Inactivation of Biotin". Science. 93 (2420): 477–78. Bibcode:1941Sci....93..477G. doi:10.1126/science.93.2420.477. JSTOR 1668938. PMID 17757050.
- ^ Gyorgy, P; Rose, CS (1943). "The Liberation of Biotin from the Avidin-Biotin Complex (AB)". Experimental Biology and Medicine. 53 (1): 55–7. doi:10.3181/00379727-53-14183. S2CID 84419614.
- ^ Rolfe B, Eisenberg MA (August 1968). "Genetic and biochemical analysis of the biotin loci of Escherichia coli K-12". J Bacteriol. 96 (2): 515–24. doi:10.1128/JB.96.2.515-524.1968. PMC 252325. PMID 4877129.
- ^ Motte J, Gold R (December 2020). "High-dose biotin in multiple sclerosis: the end of the road". Lancet Neurol. 19 (12): 965–66. doi:10.1016/S1474-4422(20)30353-7. PMID 33222766.
- ^ Goldschmidt CH, Cohen JA (July 2020). "The Rise and Fall of High-Dose Biotin to Treat Progressive Multiple Sclerosis". Neurotherapeutics. 17 (3): 968–70. doi:10.1007/s13311-020-00907-5. PMID 32761325.
- ^ a b Cashman MW, Sloan SB (2010). "Nutrition and nail disease". Clin Dermatol. 28 (4): 420–5. doi:10.1016/j.clindermatol.2010.03.037. PMID 20620759.
- ^ a b Patel DP, Swink SM, Castelo-Soccio L (August 2017). "A Review of the Use of Biotin for Hair Loss". Skin Appendage Disord. 3 (3): 166–69. doi:10.1159/000462981. PMC 5582478. PMID 28879195.
- ^ Fiume MZ (2001). "Final report on the safety assessment of biotin". International Journal of Toxicology. 20 Suppl 4: 1–12. PMID 11800048.
- ^ Langova L, Novotna I, Nemcova P, Machacek M, Havlicek Z, Zemanova M, Chrast V (October 2020). "Impact of Nutrients on the Hoof Health in Cattle". Animals (Basel). 10 (10). doi:10.3390/ani10101824. PMC 7600182. PMID 33036413.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Chen B, Wang C, Wang YM, Liu JX (July 2011). "Effect of biotin on milk performance of dairy cattle: a meta-analysis". J Dairy Sci. 94 (7): 3537–46. doi:10.3168/jds.2010-3764. PMID 21700041.
- ^ "Biotin Basics". Kentucky Equine Basics. 4 November 2003. Retrieved 18 January 2021.

