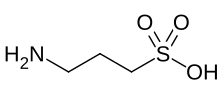
Homotaurine
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Aminopropane-1-sulfonic acid | |
| Other names
Tramiprosate; Alzhemed; 3-APS
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.020.889 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H9NO3S | |
| Molar mass | 139.17 g·mol−1 |
| Melting point | 293 °C (559 °F; 566 K) (decomposition) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Homotaurine (also known as tramiprosate (INN), 3-amino-1-propanesulfonic acid, or 3-APS) is a natural amino acid found in seaweed.[2] It is analogous to taurine, but with an extra carbon in its chain. It has GABAergic activity, apparently by mimicking GABA, which it resembles.[3]
Homotaurine was investigated in a Phase III clinical trial as a potential treatment for Alzheimer's disease that did not show efficacy.[4] A study in cognitive impairment done in 2018 did show positive benefits.[5]
Biochemical properties
In preclinical studies it had been found to bind to soluble amyloid beta and inhibit the formation of neurotoxic aggregates.[4][6] Homotaurine has also shown anticonvulsant activities, reduction in skeletal muscle tonus, and hypothermic activity.[7]
Homotaurine has been reported as a GABA antagonist,[3] as well as a GABA agonist.[7][8] In vitro studies have found that homotaurine is a GABAA partial agonist[9] as well as a GABAB receptor partial agonist with low efficacy, becoming an antagonist and displacing the full agonists GABA and baclofen at this receptor.[10] In a study in rats, homotaurine reversed the catatonia induced by baclofen (the prototypical GABAB agonist),[11] and was able to produce analgesia via the GABAB receptor, an effect that was abolished when CGP-35348, a GABAB receptor antagonist was applied.[12][13]
One study in rats showed that homotaurine suppressed ethanol-stimulated dopamine release, as well as ethanol intake and preference in rats in a way similar to the N-acetyl derivative of homotaurine, acamprosate.[14] Acamprosate was approved by the FDA in 2004 to treat alcohol dependence.[3]
References
- ^ "Homotaurine". Sigma-Aldrich.
- ^ Martorana, A.; Di Lorenzo, F.; Manenti, G.; Semprini, R.; Koch, G. (2014). "Homotaurine Induces Measurable Changes of Short Latency Afferent Inhibition in a Group of Mild Cognitive Impairment Individuals". Frontiers in Aging Neuroscience. 6: 254. doi:10.3389/fnagi.2014.00254. PMC 4172065. PMID 25295005.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Lednicer D (2008). The Organic Chemistry of Drug Synthesis (7th ed.). Hoboken: John Wiley & Sons. p. 15. ISBN 978-0-470-18066-2.
- ^ a b Caltagirone, C.; Ferrannini, L.; Marchionni, N.; Nappi, G.; Scapagnini, G.; Trabucchi, M. (2012). "The potential protective effect of tramiprosate (homotaurine) against Alzheimer's disease: A review". Aging Clinical and Experimental Research. 24 (6): 580–7. doi:10.3275/8585. PMID 22961121. S2CID 10816430.
- ^ http://www.jgerontology-geriatrics.com/wp-content/uploads/2018/03/03_Martorana-1.pdf
- ^ Aisen, Paul; Gauthier, Serge; Vellas, Bruno; Briand, Richard; Saumier, Daniel; Laurin, Julie; Garceau, Denis (2007). "Alzhemed: A Potential Treatment for Alzheimers Disease". Current Alzheimer Research. 4 (4): 473–478. doi:10.2174/156720507781788882. PMID 17908052.
- ^ a b Oja SS and Kontro P. (2013). Lajtha ANS (ed.). Chapter 18: Taurine. Springer Science & Business Media. p. 520. ISBN 9781468443677.
{{cite book}}:|work=ignored (help) - ^ Armen H. Tashjian and Ehrin J. Armstrong. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Edited by David E. Golan. Lippincott Williams & Wilkins, 2011 ISBN 9781451118056. Page 308
- ^ Reyes-Haro, Daniel; Cabrera-Ruíz, Elizabeth; Estrada-Mondragón, Argel; Miledi, Ricardo; Martínez-Torres, Ataúlfo (2014). "Modulation of GABA-A receptors of astrocytes and STC-1 cells by taurine structural analogs". Amino Acids. 46 (11): 2587–2593. doi:10.1007/s00726-014-1813-0. PMID 25119985. S2CID 10319072.
- ^ Giotti, A.; Luzzi, S.; Spagnesi, S.; Zilletti, Lucilla (1983). "Homotaurine: A GABAB antagonist in guinea-pig ileum". British Journal of Pharmacology. 79 (4): 855–862. doi:10.1111/j.1476-5381.1983.tb10529.x. PMC 2044932. PMID 6652358.
- ^ Mehta, A.; Ticku, M. (1987). "Baclofen induces catatonia in rats". Neuropharmacology. 26 (9): 1419–1423. doi:10.1016/0028-3908(87)90108-0. PMID 2823166. S2CID 24010833.
- ^ Serrano, M.Isabel; Serrano, Jose S.; Fernández, Ana; Asadi, Ihklas; Serrano-Martino, M.Carmen (1998). "GABAB Receptors and Opioid Mechanisms Involved in Homotaurine-Induced Analgesia". General Pharmacology: The Vascular System. 30 (3): 411–415. doi:10.1016/s0306-3623(97)00279-6. PMID 9510095.
- ^ Serrano, Maria Isabel; Serrano, Jose S.; Asadi, Ikhlas; Fernandez, Ana; Serrano-Martino, Maria Carmen (2001). "Role of K+-channels in homotaurine-induced analgesia". Fundamental and Clinical Pharmacology. 15 (3): 167–173. doi:10.1046/j.1472-8206.2001.00026.x. PMID 11468027. S2CID 19694376.
- ^ Olive, M.Foster; Nannini, Michelle A.; Ou, Christine J.; Koenig, Heather N.; Hodge, Clyde W. (2002). "Effects of acute acamprosate and homotaurine on ethanol intake and ethanol-stimulated mesolimbic dopamine release". European Journal of Pharmacology. 437 (1–2): 55–61. doi:10.1016/s0014-2999(02)01272-4. PMID 11864639.
