Bacitracin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Baciim |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Topical, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
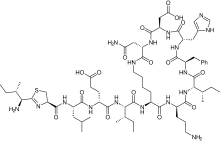
| Formula | C66H103N17O16S |
| Molar mass | 1422.71 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Bacitracin[1] is a mixture of related cyclic peptides produced by organisms of the licheniformis group of Bacillus subtilis var Tracy, first isolated in 1945. These peptides disrupt Gram-positive bacteria by interfering with cell wall and peptidoglycan synthesis.
Bacitracin is primarily used as a topical preparation, as it can cause kidney damage when used internally.
Antibiotics such as bacitracin have been shown to act as dermatological irritants and may slow healing in otherwise sterile wounds.[2][3]
Medical uses

Bacitracin is used in human medicine as a polypeptide antibiotic and is "approved by the U.S. Food and Drug Administration (FDA) for use in chickens and turkeys," though use in animals contributes to antibiotic resistance.[4]
As bacitracin zinc salt, in combination with other topical antibiotics (usually polymyxin B and neomycin) as an ointment ("triple antibiotic ointment," with the common brand name Neosporin), it is used for topical treatment of a variety of localized skin and eye infections, as well as for the prevention of wound infections. A non-ointment form of ophthalmic solution is also available for eye infections.[5]
Although allergic cross-reaction with sulfa drugs has been occasionally reported, bacitracin-containing topical preparations remain a possible alternative to silver sulfadiazine (Silvadene) for burn patients with a sulfa allergy.

Bacitracin can also be bought in pure form for those with allergies to the usual polymyxin B and neomycin components of the combination product.
Bacitracin is also commonly used as an aftercare antibiotic on tattoos and circumcision. It is preferred over combination products such as Neosporin because of its fewer ingredients, which lowers chances of an allergic reaction.[6]
In 2005–06, it was the sixth-most-prevalent allergen in patch tests (9.2%).[7]
It was voted Allergen of the Year in 2003 by the American Contact Dermatitis Society.[8]
In infants, bacitracin is rarely administered intramuscularly for the treatment of staphylococcal pneumonia and empyema when due to organisms shown susceptible to bacitracin. This use is extremely limited, since bacitracin is nephrotoxic and its concentration in the blood must be followed closely.[9]
Bacitracin can be used to distinguish Streptococcus pyogenes from other streptococci,[10] with S. pyogenes being sensitive to bacitracin and others resistant. In this case bacitracin is used to distinguish S. pyogenes from other β-hemolytic streptococci.
It is also commonly used to distinguish Haemophilus influenzae colonies amongst respiratory flora; since H. influenzae is intrinsically resistant to bacitracin, colonies form within the zone of inhibition.
Spectrum of activity and susceptibility data
Bacitracin is a narrow-spectrum antibiotic. It targets Gram-positive organisms, especially those that cause skin infections. The following represents susceptibility data for a few medically significant microorganisms.[11]
- Staphylococcus aureus – ≤0.03 μg/mL – 700 μg/mL
- Staphylococcus epidermidis – 0.25 μg/mL – >16 μg/mL
- Streptococcus pyogenes – 0.5 μg/mL – >16 μg/mL
Mechanism of action
Bacitracin interferes with the dephosphorylation of C55-isoprenyl pyrophosphate, and a related molecule known as bactoprenol pyrophosphate; both of these lipids function as membrane carrier molecules that transport the building-blocks of the peptidoglycan bacterial cell wall outside of the inner membrane.[12]
Some have claimed that bacitracin is a protein disulfide isomerase inhibitor, but this is disputed by in vitro studies.[13][14]
History
Bacitracin was isolated by Balbina Johnson, a bacteriologist at the Columbia University College of Physicians and Surgeons.[15] Its name derives from the fact that a compound produced by a microbe in young Margaret Treacy's (1936–1994)[16] leg injury showed antibacterial activity.[17] The surname was misspelled and the name shortened to the more common spelling "Tracy."
One strain isolated from tissue debrided from a compound fracture of the tibia was particularly active. We named this growth-antagonistic strain for the patient, "Tracy I." When cell-free filtrates of broth cultures of this bacillus proved to possess strong antibiotic activity and to be non-toxic, further study seemed warranted. We have called this active principle "Bacitracin.[15]
Bacitracin was approved by FDA in 1948.[citation needed]
Synthesis
Bacitracin is synthesised via nonribosomal peptide synthetases (NRPSs), which means that ribosomes are not directly involved in its synthesis.
bacABC is involved in synthesis.[18]
Bacitracin is commercially manufactured by growing the bacteria Bacillus subtilis var Tracy I in a container of liquid growth medium. Over time, the bacteria synthesizes the antibiotic and secretes the antibiotic into the medium. The antibiotic is then extracted from the medium using chemical processes.
Composition
Bacitracin is composed of a mixture of related compounds with varying degrees of antibacterial activity. Notable fractions include bacitracin A, A1, B, B1, B2, C, D, E, F, G, and X.[19] Bacitracin A has been found to have the most antibacterial activity. Bacitracin B1 and B2 have similar potencies and are approximately 90% as active as bacitracin A.[20] Other bacitracin components including F and X do not appear to be extensively studied.
References
- ^ J. Elks; C. R. Ganellin (1990). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 119–. ISBN 978-1-4757-2085-3.
- ^ Spann CT, Taylor SC, Weinberg JM (July 2004). "Topical antimicrobial agents in dermatology". Disease-a-Month. 50 (7): 407–21. doi:10.1016/j.disamonth.2004.05.011. PMID 15280871.
- ^ Trookman NS, Rizer RL, Weber T (March 2011). "Treatment of minor wounds from dermatologic procedures: a comparison of three topical wound care ointments using a laser wound model". Journal of the American Academy of Dermatology. 64 (3 Suppl): S8-15. doi:10.1016/j.jaad.2010.11.011. PMID 21247665.
- ^ Pearl MC (12 September 2007). "Antibiotic use on the farm hurts people—and doesn't help the bottom line". Discover Magazine. Archived from the original on 2007-09-25.
- ^ "Healthgrades > Find a Doctor > Doctor Reviews > Hospital Ratings". Archived from the original on 2011-05-23.
- ^ "The Right Way to Take Care of a New Tattoo". Archived from the original on 2007-08-13.
- ^ Zug KA, Warshaw EM, Fowler JF Jr, Maibach HI, Belsito DL, Pratt MD, Sasseville D, Storrs FJ, Taylor JS, Mathias CG, Deleo VA, Rietschel RL, Marks J. Patch-test results of the North American Contact Dermatitis Group 2005–2006. Dermatitis. 2009 May–Jun;20(3):149-60.
- ^ "History of Allergen of the Year". American Contact Dermatitis Society. Archived from the original on 2014-04-25.
- ^ "FDA-approved IM injection package insert for bacitracin" (PDF). sagentpharma.com. Archived from the original (PDF) on 2011-05-16.
- ^ "Streptococcus Group A Infections: Differential Diagnoses & Workup". Archived from the original on 2009-08-28. Retrieved Sep 23, 2009.
- ^ "Bacitracin Susceptibility and Minimum Inhibitory Concentration (MIC) Data" (PDF). TOKU-E.
- ^ Stone KJ, Strominger JL (December 1971). "Mechanism of action of bacitracin: complexation with metal ion and C 55 -isoprenyl pyrophosphate". Proceedings of the National Academy of Sciences of the United States of America. 68 (12): 3223–7. Bibcode:1971PNAS...68.3223S. doi:10.1073/pnas.68.12.3223. PMC 389626. PMID 4332017.
- ^ Karala AR, Ruddock LW (June 2010). "Bacitracin is not a specific inhibitor of protein disulfide isomerase". The FEBS Journal. 277 (11): 2454–62. doi:10.1111/j.1742-4658.2010.07660.x. PMID 20477872. S2CID 37519169.
- ^ Weston BS, Wahab NA, Roberts T, Mason RM (November 2001). "Bacitracin inhibits fibronectin matrix assembly by mesangial cells in high glucose". Kidney International. 60 (5): 1756–64. doi:10.1046/j.1523-1755.2001.00991.x. PMID 11703593.
- ^ a b Johnson BA, Anker H, Meleney FL (October 1945). "Bacitracin: a new antibiotic produced by a member of the B. subtilis group". Science. 102 (2650): 376–7. Bibcode:1945Sci...102..376J. doi:10.1126/science.102.2650.376. PMID 17770204. S2CID 51066.
- ^ "Archived copy". Archived from the original on 2014-04-28. Retrieved 2014-09-30.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ "NewYork-Presbyterian | the Discovery of Bacitracin". 7 February 2017.
- ^ Murphy T, Roy I, Harrop A, Dixon K, Keshavarz T (September 2007). "Effect of oligosaccharide elicitors on bacitracin A production and evidence of transcriptional level control". Journal of Biotechnology. 131 (4): 397–403. doi:10.1016/j.jbiotec.2007.07.943. PMID 17825450.
- ^ "Committee for Veterinary Medicinal Products Bacitracin." Ema.europa.eu. The European Agency for the Evaluation of Medicinal Products, June 1998. Web. 18 Jan. 2013
- ^ Bell RG (January 1992). "Preparative high-performance liquid chromatographic separation and isolation of bacitracin components and their relationship to microbiological activity". Journal of Chromatography. 590 (1): 163–8. doi:10.1016/0021-9673(92)87018-4. PMID 1601975.
