Homotaurine
| |
| |
| Names | |
|---|---|
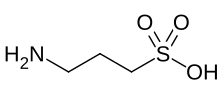
| Preferred IUPAC name
3-Aminopropane-1-sulfonic acid | |
| Other names
Tramiprosate; Alzhemed; 3-APS
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.020.889 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H9NO3S | |
| Molar mass | 139.17 g·mol−1 |
| Melting point | 293 °C (559 °F; 566 K) (decomposition) |
| Hazards | |
| GHS labelling:[2] | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Homotaurine (also known as tramiprosate (INN), 3-amino-1-propanesulfonic acid, or 3-APS) is a natural sulfonic acid found in seaweed.[3] It is analogous to taurine, but with an extra carbon in its chain. It has GABAergic activity, apparently by mimicking GABA, which it resembles.[4]
Homotaurine was investigated in a Phase III clinical trial as a potential treatment for Alzheimer's disease (AD) that did not show efficacy. However, post-hoc analyses have shown positive and significant effects of homotaurine on secondary endpoints and subgroups of patients, including a reduction in hippocampal volume loss and lower decline in memory function in the overall cohort, as well as a reduction in global cognitive decline in APOE4 allele carriers, suggesting a disease-modifying effect.[5] A study in cognitive impairment done in 2018 did show positive benefits.[6]
Homotaurine is currently in a phase 3 study with expected FDA approval as the first disease modifying drug for AD.[7][8]
Medical use
Acamprosate (N-acetyl homotaurine) was approved by the FDA in 2004 to treat alcohol dependence.[4]
Biochemical properties
In preclinical studies it had been found to bind to soluble amyloid beta and inhibit the formation of neurotoxic aggregates.[5][9] Homotaurine has also shown anticonvulsant activities, reduction in skeletal muscle tonus, and hypothermic activity.[10]
Homotaurine has been reported as a GABA antagonist,[4] as well as a GABA agonist.[10][11] In vitro studies have found that homotaurine is a GABAA partial agonist[12] as well as a GABAB receptor partial agonist with low efficacy, becoming an antagonist and displacing the full agonists GABA and baclofen at this receptor.[13] In a study in rats, homotaurine reversed the catatonia induced by baclofen (the prototypical GABAB agonist),[14] and was able to produce analgesia via the GABAB receptor, an effect that was abolished when CGP-35348, a GABAB receptor antagonist was applied.[15][16]
In a human study homotaurine selectively and fully inhibits the formation of Aβ42 oligomers at the clinical dose, without evidence of vasogenic edema.[7]
One study in rats showed that homotaurine suppressed ethanol-stimulated dopamine release, as well as ethanol intake and preference in rats in a way similar to the N-acetyl derivative of homotaurine, acamprosate.[17]
References
- ^ "Homotaurine". Sigma-Aldrich.
- ^ "Tramiprosate". pubchem.ncbi.nlm.nih.gov. Retrieved 13 December 2021.
- ^ Martorana, Alessandro; Di Lorenzo, Francesco; Manenti, Guglielmo; Semprini, Roberta; Koch, Giacomo (23 September 2014). "Homotaurine Induces Measurable Changes of Short Latency Afferent Inhibition in a Group of Mild Cognitive Impairment Individuals". Frontiers in Aging Neuroscience. 6: 254. doi:10.3389/fnagi.2014.00254. PMC 4172065. PMID 25295005.
- ^ a b c Lednicer D (2008). The Organic Chemistry of Drug Synthesis (7th ed.). Hoboken: John Wiley & Sons. p. 15. ISBN 978-0-470-18066-2.
- ^ a b Caltagirone, C; Ferrannini, L; Marchionni, N; Nappi, G; Scapagnini, G; Trabucchi, M (December 2012). "The potential protective effect of tramiprosate (homotaurine) against Alzheimer's disease: a review". Aging Clinical and Experimental Research. 24 (6): 580–587. doi:10.3275/8585. PMID 22961121. S2CID 10816430.
- ^ Martorana, A.; Motta, C; Koch, G.; Massaia, M.; Mondino, S.; Raniero, I.; Vacca, A.; Di Lorenzo, F.; Cavallo, G.; Oddenino, E.; Pavanelli, E.; Maniscalco, M.; Montano, V.; Mastropietro, A.; Bellia, N. C.; Ciravegna, E.; La Rocca, M.; Vitale, E.; Lorico, F.; Zacchettin, B.; Scalise, A.; Codemo, A.; Gabelli, C.; Spano, M.; Poli, S.; Panuccio, D.; Bruno, P.; Alfieri, P.; Ruggiero, R.; Cursi, F.; Levi Della Vida, G. (15 March 2018). "Effect of homotaurine in patients with cognitive impairment: results from an Italian observational retrospective study". Journal of Gerontology and Geriatrics. 66: 15–20.
- ^ a b Tolar, Martin; Abushakra, Susan; Hey, John A.; Porsteinsson, Anton; Sabbagh, Marwan (December 2020). "Aducanumab, gantenerumab, BAN2401, and ALZ-801—the first wave of amyloid-targeting drugs for Alzheimer's disease with potential for near term approval". Alzheimer's Research & Therapy. 12 (1): 95. doi:10.1186/s13195-020-00663-w. PMC 7424995. PMID 32787971.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Abushakra, S.; Porsteinsson, A.; Scheltens, P.; Sadowsky, C.; Vellas, B.; Cummings, J.; Gauthier, S.; Hey, J. A.; Power, A.; Wang, P.; Tolar, M.; Tolar, M (1 September 2017). "Clinical effects of tramiprosate in apoe4/4 homozygous patients with mild alzheimer's disease suggest disease modification potential". Journal of Prevention of Alzheimer's Disease. 4 (3): 149–156. doi:10.14283/jpad.2017.26. PMID 29182706. S2CID 44515548.
- ^ Aisen, Paul; Gauthier, Serge; Vellas, Bruno; Briand, Richard; Saumier, Daniel; Laurin, Julie; Garceau, Denis (1 September 2007). "Alzhemed: A Potential Treatment for Alzheimers Disease". Current Alzheimer Research. 4 (4): 473–478. doi:10.2174/156720507781788882. PMID 17908052.
- ^ a b Lajtha, Abel (2013). Metabolism in the Nervous System. Springer Science & Business Media. p. 520. ISBN 978-1-4684-4367-7.
- ^ Tashjian, Armen H.; Armstrong, Ehrin J. (2011). Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Lippincott Williams & Wilkins. p. 308. ISBN 978-1-4511-1805-6.
- ^ Reyes-Haro, Daniel; Cabrera-Ruíz, Elizabeth; Estrada-Mondragón, Argel; Miledi, Ricardo; Martínez-Torres, Ataúlfo (November 2014). "Modulation of GABA-A receptors of astrocytes and STC-1 cells by taurine structural analogs". Amino Acids. 46 (11): 2587–2593. doi:10.1007/s00726-014-1813-0. PMID 25119985. S2CID 10319072.
- ^ Giotti, A.; Luzzi, S.; Spagnesi, S.; Zilletti, L. (August 1983). "Homotaurine: a GABAB antagonist in guinea-pig ileum". British Journal of Pharmacology. 79 (4): 855–862. doi:10.1111/j.1476-5381.1983.tb10529.x. PMC 2044932. PMID 6652358.
- ^ Mehta, A; Ticku, M (September 1987). "Baclofen induces catatonia in rats". Neuropharmacology. 26 (9): 1419–1423. doi:10.1016/0028-3908(87)90108-0. PMID 2823166. S2CID 24010833.
- ^ Serrano, M.Isabel; Serrano, Jose S.; Fernández, Ana; Asadi, Ihklas; Serrano-Martino, M.Carmen (March 1998). "GABAB Receptors and Opioid Mechanisms Involved in Homotaurine-Induced Analgesia". General Pharmacology: The Vascular System. 30 (3): 411–415. doi:10.1016/s0306-3623(97)00279-6. PMID 9510095.
- ^ Serrano, Maria Isabel; Serrano, Jose S.; Asadi, Ikhlas; Fernandez, Ana; Serrano-Martino, Maria Carmen (16 June 2001). "Role of K+-channels in homotaurine-induced analgesia". Fundamental and Clinical Pharmacology. 15 (3): 167–173. doi:10.1046/j.1472-8206.2001.00026.x. PMID 11468027. S2CID 19694376.
- ^ Olive, M.Foster; Nannini, Michelle A; Ou, Christine J; Koenig, Heather N; Hodge, Clyde W (February 2002). "Effects of acute acamprosate and homotaurine on ethanol intake and ethanol-stimulated mesolimbic dopamine release". European Journal of Pharmacology. 437 (1–2): 55–61. doi:10.1016/s0014-2999(02)01272-4. PMID 11864639.
