Sodium cyanoborohydride
| |
| Names | |
|---|---|
| Other names
Sodium cyanotrihydridoborate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.043.001 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
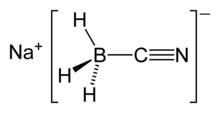
| Na[BH3(CN)] | |
| Molar mass | 62.84 g·mol−1 |
| Appearance | white to off-white powder, hygroscopic |
| Density | 1.20 g/cm3 |
| Melting point | 241 °C (466 °F; 514 K) decomposes |
| 212 g/(100 mL) (29 °C) | |
| Solubility | soluble in diglyme, tetrahydrofuran, methanol slightly soluble in methanol insoluble in diethyl ether |
| Structure | |
| 4 at boron atom | |
| Tetrahedral at boron atom | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Fatal if swallowed, in contact with skin or if inhaled Contact with acids liberates very toxic gas Contact with water liberates highly flammable gas |
| GHS labelling: | |
  
| |
| Danger | |
| H228, H300, H310, H314, H330, H410 | |
| P210, P260, P264, P273, P280, P284 | |
| NFPA 704 (fire diamond) | |
Threshold limit value (TLV)
|
5 mg/m3 (TWA) |
| Safety data sheet (SDS) | Sigma Aldrich[1] |
| Related compounds | |
Other anions
|
Sodium borohydride |
Related compounds
|
Lithium aluminium hydride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium cyanoborohydride is the chemical compound with the formula Na[BH3(CN)]. It is a colourless salt, but commercial samples can appear tan. It is widely used in organic synthesis for the reduction of imines. The salt tolerates aqueous conditions.[2]
Use
Owing to the presence of the electron-withdrawing cyanide substituent, [BH3(CN)]− is less reducing than is [BH4]−.[3] As a mild reducing agent, it is used to convert imines to amines.[4]
It is especially favored for reductive aminations, wherein aldehydes or ketones are treated with an amine in the presence of this reagent:
- R2CO + R'−NH2 + Na[BH3(CN)] + CH3OH → R2CH−NH−R' + "Na[BH2(CN)(OCH3)]"[clarification needed]
The reagent is typically used in excess. Selectivity is achieved at mildly basic solutions (pH 7–10).[citation needed] The reagent is ideal for reductive aminations ("Borch Reaction").[5]
In conjunction with tosylhydrazine, sodium cyanoborohydride is used in the reductive deoxygenation of ketones.[2]
Structure and preparation
The [BH3(CN)]− ion is tetrahedral at the boron atom, and it comprises the anionic component of the salt.
The reagent is often purchased, although it can be prepared easily. One method involves combining sodium cyanide and borane. Another route is treating sodium borohydride with mercury(II) cyanide. The commercial samples can be purified, but the yields of the reductive aminations do not improve.[6]
See also
- Sodium triacetoxyborohydride – a milder reductant, but unstable in water
- Sodium borohydride – a stronger, cheaper reductant
References
- ^ Sigma-Aldrich Co., Sodium cyanoborohydride. Retrieved on 2014-11-09.
- ^ a b "Sodium Cyanoborohydride". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. 2007. pp. rs059.pub2. doi:10.1002/047084289X.rs059.pub2. ISBN 978-0471936237.
{{cite encyclopedia}}: Unknown parameter|authors=ignored (help) - ^ Baxter, Ellen W.; Reitz, Allen B. (9 January 2002). "Reductive Aminations of Carbonyl Compounds with Borohydride and Borane Reducing Agents". Organic Reactions: 1–714. doi:10.1002/0471264180.or059.01. ISBN 0471264180.
- ^ Christen, Hans; Meyer, Gerd (1997). Grundlagen der allgemeinen und anorganischen Chemie (1 ed.). Salle + Sauerländer. p. 824. ISBN 978-3-7935-5493-6.
- ^ Richard F. Borch (1988). "Reductive Amination with Sodium Cyanoborohydride: N,N-Dimethylcyclohexylamine". Organic Syntheses; Collected Volumes, vol. 6, p. 499.
- ^ Richard F. Borch and Mark D. Bernstein and H. Dupont Durst (1971). "Cyanohydridoborate Anion as a Selective Reducing Agent". J. Am. Chem. Soc. 93 (12): 2897–2904. doi:10.1021/ja00741a013.

