Sodium cyanoborohydride
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sodium cyanoboranuide
| |||
| Other names
Sodium cyanotrihydridoborate
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ECHA InfoCard | 100.043.001 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
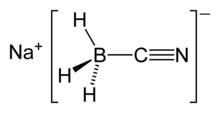
| Na[BH3(CN)] | |||
| Molar mass | 62.84 g·mol−1 | ||
| Appearance | white powder, hygroscopic | ||
| Density | 1.083 g/cm (25°C)3 | ||
| Melting point | 242 °C (468 °F; 515 K) decomposes | ||
| 212 g/(100 mL) (29 °C) | |||
| Solubility | soluble in water, ethanol, diglyme, tetrahydrofuran, methanol slightly soluble in methanol insoluble in diethyl ether | ||
| Structure | |||
| 4 at boron atom | |||
| Tetrahedral at boron atom | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Flammable solid, fatal if swallowed, in contact with skin or if inhaled Contact with acids liberates very toxic gas Contact with water liberates highly flammable gas | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H228, H300, H310, H314, H330, H410 | |||
| P210, P260, P264, P273, P280, P284 | |||
| NFPA 704 (fire diamond) | |||
Threshold limit value (TLV)
|
5 mg/m3 (TWA) | ||
| Safety data sheet (SDS) | Sigma Aldrich[1] | ||
| Related compounds | |||
Other anions
|
Sodium borohydride | ||
Related compounds
|
Lithium aluminium hydride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Sodium cyanoborohydride is a chemical compound with the formula Na[BH3(CN)]. It is a colourless salt used in organic synthesis for chemical reduction including that of imines and carbonyls. Sodium cyanoborohydride is a milder reductant than other conventional reducing agents.[2]
Structure
Sodium cyanoborohydride is a salt. The cationic sodium ion, [Na]+, interacts with the anioniccyanoborohydride ion, [BH3(CN)]−. The anionic component of the salt is tetrahedral at the boron atom.
The electron-withdrawing cyanide substituent draws electron density away from the negatively charged boron; thus, reducing the electrophilic capabilities of the anionic component.[2] This electronic phenomenon causes sodium cyanoborohydride to have more mild reducing qualities than other reducing agents. For example, Na[BH3(CN)] is less reducing than its counterpart sodium borohydride, containing [BH4]−.[2]
Uses
Sodium cyanoborohydride is a mild reducing agent. It is often used for the reduction of imines and carbonyls.
Reduction of Imines
Imines can be reduced to amines using sodium cyanoborohydride.

Reductive Amination (Borch Reaction)
Reductive amination is the conversion of a carbonyl into an amine through an intermediate imine.[3] The carbonyl is first treated with ammonia to promote imine formation by nucleophilic attack. The imine is then reduced to an amine by sodium cyanoborohydride. This reaction works on both aldehydes and ketones. The carbonyl can be treated with ammonia, a primary amine, or a secondary amine to produce, respectively, 1°, 2°, and 3° amines.[4]

Reductive Deoxygenation of Ketones
Aromatic ketones and aldehydes can be reductively deoxygenated using sodium cyanoborohydride.[5] This means that the carbonyl oxygen is being removed completely from the molecule. Deoxygenation using sodium cyanoborohydride is often done in the presence of trimethylsilyl chloride, or TMSCl.[5]

Preparation
Sodium cyanoborohydride can be purchase from most chemical suppliers. It is most commonly synthesized by the following methods:
Preparation from Sodium Cyanide and Diborane
Sodium cyanoborohydride can be synthesized from sodium cyanide and diborane.[citation needed]
This method of preparation can be used for other compounds of the formula RBH3CN where R is an alkali metal, a quaternary ammonium radical, or a phosphonium radical.[citation needed] The final products are useful as hydrolysis stable reductants and as synthetic intermediates.[citation needed]

Selectivity
Since sodium cyanoborohydride is a mild reducing agent, many functional groups remain inert in its presence. For example, sodium cyanoborohydride is incapable of reducing amides, ethers, lactones, nitriles, and epoxides.[6] Therefore, it can selectively reduce some functionalities in the presence of others.
Some examples of sodium cyanoborohydride include:
- Reduction of iminium ions in the presence of carbonyls[6]
- Reduction of aldehydes in the presence of ketones and esters.[7]
- Reduction of aldehydes in the presence of thiol ester groups[6]
The selectivity of this reducing agent makes it an important tool in organic synthesis. It allows for specific modifications to be made to complex organic molecules.
See also
- Sodium triacetoxyborohydride – a milder reductant, but unstable in water
- Sodium borohydride – a stronger, cheaper reductant
References
- ^ Sigma-Aldrich Co., Sodium cyanoborohydride. Retrieved on 2014-11-09.
- ^ a b c Baxter, Ellen W.; Reitz, Allen B. (9 January 2002). "Reductive Aminations of Carbonyl Compounds with Borohydride and Borane Reducing Agents". Organic Reactions: 1–714. doi:10.1002/0471264180.or059.01. ISBN 0-471-26418-0.
- ^ Richard F. Borch (1988). "Reductive Amination with Sodium Cyanoborohydride: N,N-Dimethylcyclohexylamine". Organic Syntheses; Collected Volumes, vol. 6, p. 499.
- ^ Richard F. Borch and Mark D. Bernstein and H. Dupont Durst (1971). "Cyanohydridoborate Anion as a Selective Reducing Agent". J. Am. Chem. Soc. 93 (12): 2897–2904. doi:10.1021/ja00741a013.
- ^ a b Box, Vernon G. S.; Meleties, Panayiotis C. (1998-09-24). "Reductive, selective deoxygenation of acylbenzo[b]furans, aromatic aldehydes and ketones with NaBH3CN-TMSCl". Tetrahedron Letters. 39 (39): 7059–7062. doi:10.1016/S0040-4039(98)01519-6. ISSN 0040-4039.
- ^ a b c LANE, Clinton F. (1975). "Sodium Cyanoborohydride - A Highly Selective Reducing Agent for Organic Functional Groups". Synthesis. 1975 (03): 135–146. doi:10.1055/s-1975-23685. ISSN 0039-7881.
- ^ Paul, Avishek; Shipman, Michael A.; Onabule, Dolapo Y.; Sproules, Stephen; Symes, Mark D. (2021-04-15). "Selective aldehyde reductions in neutral water catalysed by encapsulation in a supramolecular cage". Chemical Science. 12 (14): 5082–5090. doi:10.1039/D1SC00896J. ISSN 2041-6539. PMC 8179549. PMID 34163748.
{{cite journal}}: CS1 maint: PMC format (link)

