Cyanogen fluoride
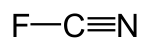
| |
| Names | |
|---|---|
| Preferred IUPAC name
Carbononitridic fluoride[1] | |
| Other names
Fluorine cyanide
Cyano fluoride Cyanogen fluoride Fluoromethanenitrile | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.298.549 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CFN | |
| Molar mass | 45.0158 g mol−1 |
| Appearance | Colorless gas |
| Density | 1.026 g mL−1 |
| Boiling point | −46 °C (−51 °F; 227 K) |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
225.40 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
35.98 kJ mol−1 |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cyanogen fluoride (molecular formula: FCN; IUPAC name: carbononitridic fluoride) is an inorganic linear compound which consists of a fluorine in a single bond with carbon, and a nitrogen in a triple bond with carbon. It is a toxic and explosive gas at room temperature. It is used in organic synthesis and can be produced by pyrolysis of cyanuric fluoride or by fluorination of cyanogen.[2]
Synthesis
[edit]Cyanogen fluoride (FCN), is synthesized by the pyrolysis of cyanuric fluoride (C3N3F3) at 1300 °C and 50mm pressure;[3] this process gives a maximum of 50% yield. Other products observed were cyanogen and CF3CN.[2] For pyrolysis, an induction heated carbon tube with an internal diameter of 0.75 inches is packed with 4 to 8 mesh carbon granules and is surrounded by graphite powder insulation and a water-jacketed shell.[3][2] The cyanuric fluoride is pyrolyzed (becoming a pyrolysate) at a rate of 50g/hr, and appears as fluffy white solid collected in liquid nitrogen traps. These liquid nitrogen traps are filled to atmospheric pressure with nitrogen or helium. This process yields crude cyanogen fluoride, which is then distilled in a glass column at atmospheric pressure to give pure cyanogen fluoride.
Another method of synthesizing cyanogen fluoride is by the fluorination of cyanogen.[4] Nitrogen trifluoride can fluoridate cyanogen to cyanogen fluoride when both the reactants are injected downstream into the nitrogen arc plasma.[3] With carbonyl fluoride and carbon tetrafluoride, FCN was obtained by passing these fluorides through the arc flame and injecting the cyanogen downstream into the arc plasma.
Properties
[edit]Cyanogen fluoride (FCN) is a toxic, colorless gas.[3] The linear molecule has a molecular mass of 45.015 gmol−1.[3][5] Cyanogen fluoride has a boiling point of –46.2 °C and a melting point of –82 °C. The stretching constant for the CN bond was 17.5 mdyn/A and for the CF bond it was 8.07 mdyn/A, but this can vary depending on the interaction constant.[4] At room temperature, the condensed phase converts rapidly to polymeric materials.[3] Liquid FCN explodes at –41 °C when initiated by a squib.[2]
Spectroscopy
[edit]The fluorine NMR pattern for FCN showed that there was a triplet peak centered at 80 ppm (3180 cps) with a 32-34 cps splitting between adjacent peaks because of the N14 nucleus.[2] This splitting is absent near freezing point and it collapses to a singlet peak.
The IR spectrum of FCN shows two doublet bands at around 2290 cm−1 (for the C ≡ N)
and 1078 cm−1 (for the C-F).[2][5] The C-F doublet band has a 24 cm−1 separation between the two branches. A triplet band is observed at around 451 cm−1.
Chemical reactions
[edit]Cyanogen fluoride reacts with benzene in the presence of aluminum chloride to form benzonitrile in 20% conversion.[3] It also reacts with olefins to yield an alpha,beta-fluoronitriles.[6] FCN also adds to olefins which have internal double bonds in the presence of strong acid catalyst.
Storage
[edit]FCN can be stored in a stainless steel cylinders for over a year when the temperature is -78.5 °C (solid carbon dioxide temperature).[3]
Safety
[edit]Cyanogen fluoride undergoes violent reaction when in the presence of boron trifluoride or hydrogen fluoride.[3] Pure gaseous FCN at atmospheric pressure and room temperature does not ignite by a spark or hot wire.[2] FCN air mixtures however are more susceptible to ignition and explosion than pure FCN.
Uses
[edit]FCN is useful in synthesis of important compounds such as dyes, fluorescent brighteners and photographic sensitizers.[7] It is also very useful as a fluorinating and nitrilating agent.[6] Beta-fluoronitriles, which are produced when FCN is reacted with olefins, are useful intermediates for preparing polymers, beta-fluorocarboxylic acids and other fluorine containing products. Useful amines can be obtained. Cyanogen fluoride is a very volatile fumigant, disinfectant and animal pest killer.
References
[edit]- ^ "Cyanogen fluoride - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 27 March 2005. Identification. Retrieved 6 June 2012.
- ^ a b c d e f g Fawcett, F. S.; Lipscomb, R. D. (July 1964). "Cyanogen Fluoride: Synthesis and Properties". Journal of the American Chemical Society. 86 (13): 2576. doi:10.1021/ja01067a011.
- ^ a b c d e f g h i Fawcett, F. S.; Lipscomb, R. D. (March 1960). "Cyanogen Fluoride". Journal of the American Chemical Society. 82 (6): 1509–1510. doi:10.1021/ja01491a064. ISSN 0002-7863.
- ^ a b Shurvell, Herbert F. (November 1970). "Force constants and thermodynamic properties of the unstable linear triatomic molecules hypocyanic acid, deuterated hypocyinic acid, and cyanogen fluoride". The Journal of Physical Chemistry. 74 (24): 4257–4259. doi:10.1021/j100718a013. ISSN 0022-3654.
- ^ a b Dodd, R.E.; Little, R. (1960). "The infra-red spectrum of fluorine cyanide". Spectrochimica Acta. 16 (9): 1083–1087. Bibcode:1960AcSpe..16.1083D. doi:10.1016/0371-1951(60)80148-8.
- ^ a b Lipscomb, R. D., & Smith, W. C. (1961). U.S. Patent No. 3,008,798. Washington, DC: U.S. Patent and Trademark Office.
- ^ Bernardi, Fernando; Cacace, Fulvio; Occhiucci, Giorgio; Ricci, Andreina; Rossi, Ivan (June 2000). "Protonated Cyanogen Fluoride. Structure, Stability, and Reactivity of (FCN)H+Ions". The Journal of Physical Chemistry A. 104 (23): 5545–5550. Bibcode:2000JPCA..104.5545B. doi:10.1021/jp993986b. ISSN 1089-5639.

