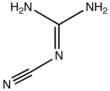
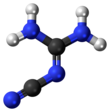
2-Cyanoguanidine
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Cyanoguanidine
| |||
| Other names
Cyanoguanidine, dicyanodiamide, N-cyanoguanidine, 1-cyanoguanidine, guanidine-1-carbonitrile, dicyandiamin, Didin, DCD, Dicy
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.649 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H4N4 | |||
| Molar mass | 84.08 g/mol | ||
| Appearance | White crystals | ||
| Density | 1.400 g/cm3 | ||
| Melting point | 209.5 °C (409.1 °F; 482.6 K) | ||
| Boiling point | 252 °C (486 °F; 525 K) | ||
| 41.3 g/l | |||
| log P | −0.52 | ||
Henry's law
constant (kH) |
2.25×10−10 atm·m3/mol | ||
| −44.55×10−6 cm3/mol | |||
| Hazards | |||
| GHS labelling: | |||

| |||
| Warning | |||
| H302, H312, H332 | |||
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
2-Cyanoguanidine is a nitrile derived from guanidine. It is a dimer of cyanamide, from which it can be prepared. 2-Cyanoguanidine is a colourless solid that is soluble in water, acetone, and alcohol, but not nonpolar organic solvents.[1]
Production and use
[edit]2-Cyanoguanidine is produced by treating cyanamide with base. It is produced in soil by decomposition of cyanamide. A variety of useful compounds are produced from 2-cyanoguanidine, guanidines and melamine. For example, acetoguanamine and benzoguanamine are prepared by condensation of cyanoguanidine with the nitrile:[2][3]
- (H2N)2C=NCN + RCN → (CNH2)2(CR)N3
Cyanoguanidine is also used as a slow fertilizer. Formerly, it was used as a fuel in some explosives. It is used in the adhesive industry as a curing agent for epoxy resins.[1]
Chemistry
[edit]Two tautomeric forms exist, differing in the protonation and bonding of the nitrogen to which the nitrile group is attached.
2-Cyanoguanidine can also exist in a zwitterionic form via a formal acid–base reaction among the nitrogens.
Loss of ammonia (NH3) from the zwitterionic form, followed by deprotonation of the remaining central nitrogen atom, gives the dicyanamide anion, [N(CN)2]−.
Drugs List
[edit]2-Cyanoguanidine finds use in the synthesis of the following list of agents:
- Amanozine
- Azapropazone
- Buformin
- Chlorazanil
- Chlorproguanil
- Clociguanil
- Cloguanamil
- Cloquanamil
- Cycloguanil
- Dametralast
- Diallylmelamine (DAM)
- Guanazole
- Irsogladine
- Metformin
- Methylphenobarbital[4]
- Moroxydine
- Oxonazine
- Phenformin
- Phenylbiguanide
- NSC-127755
- Triazinate
References
[edit]- ^ a b Thomas Güthner; Bernd Mertschenk (2006). "Cyanamides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_139.pub2. ISBN 3527306730.
- ^ H. Deim; G. Matthias; R. A. Wagner (2012). "Amino Resins". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_115.pub2. ISBN 978-3527306732.
- ^ J. K. Simons; M. R. Saxton (1953). "Benzoguanamine". Organic Syntheses. 33: 13. doi:10.15227/orgsyn.033.0013.
- ^ Ludwig Taub and Walter Kropp, U.S. patent 2,061,114 (1936 to Winthrop Chemical Company Inc.).