Immunoglobulin light chain
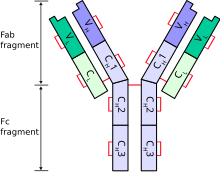

The immunoglobulin light chain is the small polypeptide subunit of an antibody (immunoglobulin).
A typical antibody is composed of two immunoglobulin (Ig) heavy chains and two Ig light chains.
In humans
[edit]There are two types of light chain in humans:
- kappa (κ) chain, encoded by the immunoglobulin kappa locus (IGK@) on chromosome 2 (locus: 2p11.2)
- lambda (λ) chain, encoded by the immunoglobulin lambda locus (IGL@) on chromosome 22 (locus: 22q11.2)
Antibodies are produced by B lymphocytes, each expressing only one class of light chain. Once set, light chain class remains fixed for the life of the B lymphocyte. In a healthy individual, the total kappa-to-lambda ratio is roughly 2:1 in serum (measuring intact whole antibodies) or 1:1.5 if measuring free light chains, with a highly divergent ratio indicative of neoplasm. The free light chain ratio ranges from 0.26 to 1.65.[1] Both the kappa and the lambda chains can increase proportionately, maintaining a normal ratio. This is usually indicative of something other than a blood cell dyscrasia, such as kidney disease.
In other animals
[edit]The immunoglobulin light chain genes in tetrapods can be classified into three distinct groups: kappa (κ), lambda (λ), and sigma (σ). The divergence of the κ, λ, and σ isotypes preceded the radiation of tetrapods. The σ isotype was lost after the evolution of the amphibian lineage and before the emergence of the reptilian lineage.[2]
Other types of light chains can be found in lower vertebrates, such as the Ig-Light-Iota chain of Chondrichthyes and Teleostei.[3][4]
Camelids are unique among mammals as they also have fully functional antibodies which have two heavy chains, but lack the light chains usually paired with each heavy chain.[5]
Sharks also possess, as part of their adaptive immune systems, a functional heavy-chain homodimeric antibody-like molecule referred to as IgNAR (immunoglobulin new antigen receptor). IgNAR is believed to have never had an associated light chain, in contrast with the understanding that the heavy-chain-only antibodies in camelids may have lost their light chain partners through evolution.[6][7]
Structure
[edit]Only one type of light chain is present in a typical antibody, thus the two light chains of an individual antibody are identical.
Each light chain is composed of two tandem immunoglobulin domains:
- one constant (CL) domain
- one variable domain (VL) that is important for binding antigen
The approximate length of a light chain protein is from 211 to 217 amino acids.[3] The constant region determines what class (kappa or lambda) the light chain is.[8] The lambda class has 4 subtypes (1, 2, 3, and 7).[8]
In pathology
[edit]Individual B-cells in lymphoid tissue possess either kappa or lambda light chains, but never both together. Using immunohistochemistry, it is possible to determine the relative abundance of B-cells expressing kappa and lambda light chains. If the lymph node or similar tissue is reactive, or otherwise benign, it should possess a mixture of kappa positive and lambda positive cells. If, however, one type of light chain is significantly more common than the other, the cells are likely all derived from a small clonal population, which may indicate a malignant condition, such as B-cell lymphoma.[9]
Free immunoglobulin light chains secreted by neoplastic plasma cells, such as in multiple myeloma, can be called Bence Jones protein when detected in the urine, although there is a trend to refer to these as urinary free light chains.
Increased levels of free Ig light chains have also been detected in various inflammatory diseases. It is important to note that, in contrast to increased levels in lymphoma patients, these Ig light chains are polyclonal. Recent studies have shown that these Ig light chains can bind to mast cells and, using their ability to bind antigen, facilitate activation of these mast cells.[10] Activation of mast cells results in the release of various pro-inflammatory mediators which are believed to contribute to the development of the inflammatory disease. Recent studies have shown that Ig light chains not only activate mast cells but also dorsal root ganglia[11] and neutrophils,[12] expanding their possible role as mediators in inflammatory disease.
See also
[edit]References
[edit]- ^ Katzmann JA, Clark RJ, Abraham RS, Bryant S, Lymp JF, Bradwell AR, Kyle RA (2001). "Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains". Clin Chem. 48 (9): 1437–44. doi:10.1093/clinchem/48.9.1437. PMID 12194920.
- ^ Das S, Nikolaidis N, Klein J, Nei M (2008). "Evolutionary redefinition of immunoglobulin light chain isotypes in tetrapods using molecular markers". Proc Natl Acad Sci U S A. 105 (43): 16647–52. Bibcode:2008PNAS..10516647D. doi:10.1073/pnas.0808800105. PMC 2575474. PMID 18940927.
- ^ a b Janeway CA, Jr.; et al. (2001). Immunobiology (5th ed.). Garland Publishing. ISBN 0-8153-3642-X. (electronic full text via NCBI Bookshelf).
- ^ IMGT Index Archived 2007-04-27 at the Wayback Machine Antibodies (or Immunoglobulins).
- ^ Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa E, Bendahman N, Hamers R (1993). "Naturally occurring antibodies devoid of light chains". Nature. 363 (6428): 446–8. Bibcode:1993Natur.363..446H. doi:10.1038/363446a0. PMID 8502296. S2CID 4265902.
- ^ Flajnik, MF; Deschacht, N; Muyldermans, S (2011). "A Case Of Convergence: Why Did a Simple Alternative to Canonical Antibodies Arise in Sharks and Camels?". PLOS Biology. 9 (8): e1001120. doi:10.1371/journal.pbio.1001120. PMC 3149040. PMID 21829328.
- ^ Greenberg, A. S.; Avila, D.; Hughes, M.; Hughes, A.; McKinney, E. C.; Flajnik, M. F. (1995-03-09). "A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks". Nature. 374 (6518): 168–173. Bibcode:1995Natur.374..168G. doi:10.1038/374168a0. ISSN 0028-0836. PMID 7877689. S2CID 4304231.
- ^ a b Owen, Judith A.; Punt, Jenni; Stranford, Sharon (2013). Kuby Immunology. New York, NY: W. H. Freeman and Company. p. 85. ISBN 9781429219198.
- ^ Leong, Anthony S-Y; Cooper, Kumarason; Leong, F Joel W-M (2003). Manual of Diagnostic Cytology (2 ed.). Greenwich Medical Media, Ltd. pp. 283–285. ISBN 1-84110-100-1.
- ^ Redegeld, Frank A.; van der Heijden, Maurice W.; Kool, Mirjam; Heijdra, Bianca M.; Garssen, Johan; Kraneveld, Aletta D.; Loveren, Henk Van; Roholl, Paul; Saito, Takashi; Verbeek, J. Sjef; Claassens, Jill; Koster, Andries S.; Nijkamp, Frans P. (July 2002). "Immunoglobulin-free light chains elicit immediate hypersensitivity-like responses". Nature Medicine. 8 (7): 694–701. doi:10.1038/nm722. PMID 12068287. S2CID 1322642.
- ^ Rijnierse, Anneke; Kroese, Alfons B.A.; Redegeld, Frank A.; Blokhuis, Bart R.J.; van der Heijden, Maurice W.; Koster, Andries S.; Timmermans, Jean-Pierre; Nijkamp, Frans P.; Kraneveld, Aletta D. (March 2009). "Immunoglobulin-free light chains mediate antigen-specific responses of murine dorsal root ganglion neurons". Journal of Neuroimmunology. 208 (1–2): 80–86. doi:10.1016/j.jneuroim.2009.01.008. PMID 19232443. S2CID 46629005.
- ^ Braber, Saskia; Thio, Marco; Blokhuis, Bart R.; Henricks, Paul A. J.; Koelink, Pim J.; Kormelink, Tom Groot; Bezemer, Gillina F. G.; Kerstjens, Huib A. M.; Postma, Dirkje S.; Garssen, Johan; Kraneveld, Aletta D.; Redegeld, Frank A.; Folkerts, Gert (15 April 2012). "An Association between Neutrophils and Immunoglobulin Free Light Chains in the Pathogenesis of Chronic Obstructive Pulmonary Disease". American Journal of Respiratory and Critical Care Medicine. 185 (8): 817–824. doi:10.1164/rccm.201104-0761OC. PMID 22227380.
External links
[edit]- Immunoglobulin+Light+Chains at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Educational Resource for Immunoglobulin Light Chains

