Radiation-induced lumbar plexopathy
| The lumbosacral nerves | |
|---|---|
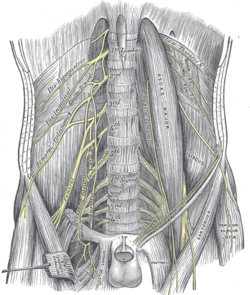 Lumbar plexus and its branches | |
 Dissection of pelvis showing sacral and pudendal plexuses | |
| Anatomical terms of neuroanatomy |
Radiation-induced lumbar plexopathy (RILP) or radiation-induced lumbosacral plexopathy (RILSP) is nerve damage in the pelvis and lower spine area caused by therapeutic radiation treatments. RILP is a rare side effect of external beam radiation therapy[1][2][3] and both interstitial and intracavity brachytherapy radiation implants.[4][5] RILP is a Pelvic Radiation Disease symptom.[6]
In general terms, such nerve damage may present in stages, earlier as demyelination and later as complications of chronic radiation fibrosis. RILP occurs as a result of radiation therapy administered to treat lymphoma or cancers within the abdomen or pelvic area such as cervical, ovarian, bladder, kidney, pancreatic, prostate, testicular, colorectal, colon, rectal or anal cancer.[7][8] The lumbosacral plexus area is radiosensitive and radiation plexopathy can occur after exposure to mean or maximum radiation levels of 50-60 Gray[7] with a significant rate difference noted within that range.[9]
Signs and symptoms
[edit]Lumbosacral plexopathy is characterized by any of the following symptoms; usually bi-lateral and symmetrical, though unilateral is known.[8]
- Lower limb dysaesthesia, abnormal sensations of touch or feeling
- Lower limb weakness
- Lower limb numbness
- Lower limb paresthesia, e.g., foot drop, muscle atrophy
- Lower limb pain
Symptoms are typically a step-wise progression with periods of stability in between,[1][3] weakness often appearing years later.[8] Weakness frequently presents in the lower leg muscle groups.[8] Symptoms are usually irreversible.[10]
Initial onset of symptoms may occur as early as 2[8] to 3[11][1] months after radiotherapy. The median onset is approximately 5 years,[8] but can be highly variable, 2-3 decades after radiation therapy.[8] One case study recorded the initial onset occurring 36 years post treatment.[12]
Cause
[edit]The treatment's ionizing radiation is an activation mechanism for apoptosis (cell death) within the targeted cancer,[13] but it can also impact nearby healthy radiosensitive tissues, like the lumbosacral plexus. The occurrence and severity of RILP is related to the magnitude of ionizing radiation[10] and the radiosensitivity of peripheral nerves may be further aggravated when combined with chemotherapy, like taxanes and platinum drugs, during treatment.[14]
Pathophysiology
[edit]The pathophysiological process behind radiation's RILP nerve damage has been discussed since the 1960s[10] and is still without a precise definition.[1][13] Consensus does exist on a progression of RILP symptoms, with a stepping (a time delay) between two periods of plexopathy onset, the first from radiation injury and the later from fibrosis. Proposed mechanisms of the early nerve damage include microvascular damage (ischemia) supplying the myelin,[1] radiation damage of the myelin,[15] and oxygen free radical cell damage.[1][15] The delayed nerve damage is attributed to compression neuropathy[1] and a late fibro-atrophic ischemia from retractile fibrosis.[1][15]
Diagnosis
[edit]The more common source of lumbar plexopathy is a direct or secondary[2] tumor involvement of the plexus with MRI being the typical confirmation tool.[15] Tumors typically present with enhancement of nerve roots and T2-weighted hyperintensity.[2] The differential consideration of RILP requires taking a medical history and neurologic examination.[15]
RILP's neurological symptoms can mimic other nerve disorders. People may present with pure lower motor neuron syndrome, a symptom of amyotrophic lateral sclerosis (ALS).[4][16] RILP may also be misdiagnosed as leptomeningeal metastasis often showing nodular MRI enhancement of the cauda equina nerve roots or having increased CSF protein content.[4]
Other differential diagnoses to consider are Chronic Inflammatory Demyelinating Polyradiculoneuropathy, neoplastic lumbosacral plexopathy, paraneoplastic neuronopathy, diabetic lumbosacral plexopathy, degenerative disk disease (osteoporosis of the spine), Osteoarthritis of the spine, Lumbar Spinal Stenosis, post-infectious plexopathy, carcinomatous meningitis (CM), mononeuritis multiplex, and chemotherapy-induced plexopathy.[1]
The testing to resolve a RILP diagnosis involves blood serum analysis, X-rays, EMG, MRI and cerebrospinal fluid analysis.[2][1][15]
Prevention
[edit]Since RILP's neurological changes are typically irreversible and a curative strategy has yet to be defined, prevention is the best approach.[1] Treating the primary cancer remains an obvious requirement, but lower levels of lumbar plexus radiation dosing will minimize or eliminate RILP.[1][15]
One method to reduce the lumbosacral plexus' dosing is to include it with other at-risk organs that get spared from radiation.[17][18]
Key to prevention is resolving the lack of clinical evidence between radiation treatments and the onset of neurological problems. That relationship is hidden by RILP's low toxicity rate, the lack of a large monitored population size and the lack of data pooling across multiple institutions.[1][19]
Management
[edit]Treatment of RILP is primarily supportive[15] with mental,[2][10] physiological[2][1][10][15] and social aspects[10] and consideration of any aggravating (synergistic) neurological factors.[1][10]
To prevent compounding existing RILP symptoms and to minimize further progression
- Remove co-morbidity factors[1][10]
- control acute inflammation. Pharmaceuticals that may be effective are corticosteroids (Dexamethasone)[2][1][15]
- avoid stretching a plexus immobilized by fibrosis, e.g., carrying heavy loads or extensive movements, which may cause sudden neurological decompensation.[1]
The effect on the person with the condition, depends upon the type of impairment. Handicaps may include physical challenges, bowel and/or bladder dysfunction and may occur in multiple settings of work and home.[10] Physical and occupational therapy are important elements in maintaining mobility and use of the lower extremities, along with assistive aides such as Ankle-Foot-Orthotics (AFOs), cane, walkers, etc.[2][10][15] Sensory reeducation techniques may be necessary for balance[2] and lymphedema management may be required.[10]
Pharmaceuticals that may be effective for RILP's neuropathic pain are
- tricyclic antidepressants (TCAs) (amitriptyline)[2][10][15]
- Antiepileptics or anticonvulsants (gabapentin, pregabalin, carbamazepine, valproic acid)[2][10][15]
- Selective serotonin re-uptake inhibitors(SSRIs) (duloxetine) to preserve normal norepinephrine and serotonin levels[2][10][15]
- Analgesic drugs (pregabalin, methadone)[2][10]
- Opiates may used singularly or to potentate the concomitant use of TCAs.[2]
- Antiarrhythmics (mexilitine) for muscle stiffness[10]
Non-pharmaceutical RILP considerations are
- acupuncture for pain[15]
- massage for pain[1][15]
- transcutaneous electrical nerve stimulation (TENS) for pain[2]
- Benzodiazepines may be used for paraesthesia[1]
- quinine may be used for cramps[1]
Functional impairment and residual pain can lead to social isolation.[10] Cancer support groups are valuable resources to learn about the syndrome and therapeutic options, and are a means to voice emotions related to having cancer and surviving it.[10][1][15]
Outcomes
[edit]With increasing cancer treatment survival rates, the quality of life for its survivors has become a public health priority.[1] The effects of RILP can be debilitating. With no effective treatment to control radiation damage's progressive nature, limb dysfunction is the likely result.[10]
Radiation damage's outcome is related to its initial onset time.
- Acute symptoms, occurring in the first few days, have the most favorable outcomes, likely diminishing within a few weeks.[20]
- Early-delayed symptoms, occurring within the first months, typically include myelopathy. These issues frequently resolve without treatment.[20]
- Late-delayed symptoms, occurring several months or years after treatment, may also include myelopathy, but its severity level is more likely to worsen, resulting in permanent paralysis.[20] Significant neurologic morbidity is typical, with a very slow neurologic recovery.[15]
Epidemiology
[edit]An exact occurrence rate has not been established. Literature on the topic is sparse.[21] Clinical occurrences of RILP are rare, affecting between 0.3 and 1.3% of those treated with abdominal or pelvic radiation.[2] The incidence rate is variable, dependent upon the irradiated zone, dosage level and method of delivery. For example, when alternate dosing levels were compared, higher rates were observed, from 12 to 23%, the higher RILP rates occurring with higher dosages.[21]
History
[edit]As of 1977 lumbosacral neuropathy arising from radiation therapy had been rarely reported. One of the earliest cases was in 1948.[7][11][22]
The incidence rate of peripheral neuropathy has been demonstrated to decrease when lower therapeutic radiation dosing levels are used.[21][1] A similar nerve injury, Radiation-induced Brachial Plexopathy (RIBP), may occur secondary to breast radiation therapy.[23] Studies on RIBP have observed the brachial plexus' radiosensitivity. Injury was observed after dosages of 40 Gy in 20 fractions and RIBP significantly increased with doses greater than 2 Gy per fraction.[21] RIBP is more common than lumbosacral radiculoplexopathy[4] and has a clinical history with reduced dosing levels. RIBP occurrence rates were in the 60% range in the 1960s when 60 Gray treatments were applied in 5 Gray fractions; RIBP occurrences in the 2010s approach 1% with 50 Gray treatments applied in 3 Gy fractions.[1]
RILP occurrence rates are estimated at 0.3% to 1.3%, though the actual rate is likely higher. The soft tissue damage leading to RILP is more commonly seen with exposure levels over 50 Gy, though has occurred with as little as 30 Gy.[24] A major step toward reducing RILP occurrences is by limiting the lumbosacral plexus' dosing level when treating pelvic malignancies, limiting the mean dose to < 45 Gy. One approach to reduced levels, the plexus' mapping with other organs at risk, was clinically evaluated during the 2010s.[17][18]
Clinical evidence of the cause-and-effect for prevention and the management of radiation induced polyneuropathy is limited.[10]
In 2011 the Radiation Oncology Institute (ROI) announced the National Radiation Oncology Registry (NROR). ROI and Massachusetts General Hospital would initially focus the NROR on prostate cancer, collecting efficacy and side effect information (like radiation induced neuropathy, RILP) from people treated with radiotherapy.[25] In 2013 the American Society for Radiation Oncology (ASTRO) joined the effort[26] and the number of data collection sites increased to 30 for a 1-year pilot project. Pitfalls of medical data collection arose with only 14 sites being able to provide data and all those requiring significant manual entry efforts.[27] The first NROR project conclusion was that future registries would need to cope with Big data analytics. In 2015 ASTRO, the National Cancer Institute and the American Association of Physicists in Medicine sponsored a Big Data Workshop at the National Institutes of Health.
Research
[edit]Experimental approaches for RILP treatment and management include:
- Hyperbaric oxygen (HBO) has had mixed results restoring nerve function, some studies showing benefit,[15][24] others without.[2][1][10]
- Anticoagulant therapy (warfin, heparin) has been tried for ischemia and capillary restoration, some without clear benefit,[1][15] others with improved motor function.[2]
- PENTOCLO therapy- a combination of Pentoxifylline (PTX), vitamin E and clodronate, a bisphosphanate; the PTX for inflammation, vitamin E as a scavenger for oxygen free radicals that can lead to fibrosis and clodronate which may inhibit myelin nerve destruction.[1][15]
- Myofascial release may reduce compressive effects of fibrouses, freeing trapped nerves.[10]
- Mobilization of injured limbs via exoskeletal systems or hybrid assistive devices can provide the mobility lost to nerve damage, offering a workaround until new medical therapies e.g. tissue engineering can repair peripheral nerve injury.[28]
See also
[edit]- Radiation poisoning
- Radiation therapy
- ICD-10-CM World Health Organization's Code G62.82: Radiation-induced polyneuropathy
- ICD-11-MMS (2018 version) World Health Organization's Code 8B92.0: Post radiation lumbosacral plexopathy
References
[edit]![]() This article incorporates text in the public domain from the 20th edition of Gray's Anatomy (1918)
This article incorporates text in the public domain from the 20th edition of Gray's Anatomy (1918)
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae Delanian, S; Lefaix, JL; Pradat, PF (December 2012). "Radiation-induced neuropathy in cancer survivors". Radiotherapy and Oncology. 105 (3): 273–82. doi:10.1016/j.radonc.2012.10.012. PMID 23245644.

- ^ a b c d e f g h i j k l m n o p q r Yadav, Rajesh R (July 14, 2017). "Radiation-Induced Lumbosacral Plexopathy: Background, Pathophysiology, Epidemiology". Medscape. Retrieved 21 May 2018.
- ^ a b Dyck P, Thaisetthawatkul P (2014). "Lumbosacral Plexopathy". CONTINUUM: Lifelong Learning in Neurology. 20 (5): 1343–58. doi:10.1212/01.CON.0000455877.60932.d3. PMID 25299286. S2CID 5538712.
- ^ a b c d Pradat, PF; Delanian, S (2013). "Late radiation injury to peripheral nerves". Peripheral Nerve Disorders. Handbook of Clinical Neurology. Vol. 115. pp. 743–58. doi:10.1016/B978-0-444-52902-2.00043-6. ISBN 9780444529022. PMID 23931813.

- ^ Lauritsen, Liv; Petersen, Peter; Daugaard, Gedske (15 August 2012). "Neurological Adverse Effects after Radiation Therapy for Stage II Seminoma". Case Reports in Oncology. 5 (2): 444–8. doi:10.1159/000341874. PMC 3433016. PMID 22949908.

- ^ Faithfull, Sara; Maher, Jane; Andreyev, Jervoise (2022). "Best Practice Pathway for Pelvic Radiation Disease" (PDF). www.prda.org.uk. Pelvic Radiation Disease Association. pp. 111–118. Retrieved 26 October 2024.
- ^ a b c Americo, J.; Filho, M. Fernandes; Ubogu, Eroboghene (2013). "Atypical Motor Neuron Disorders". In Katirji, Bashar; Kaminski, Henry J.; Ruff, Robert L. (eds.). Neuromuscular Disorders in Clinical Practice (Second ed.). New York, NY: Springer. p. 456. doi:10.1007/978-1-4614-6567-6_22. ISBN 978-1-4614-6566-9.
- ^ a b c d e f g Rutkove, Seward B.; Sak, Tracy W. (2013). "Lumbosacral Plexopathies". In Katirji, Bashar; Kaminski, Henry J.; Ruff, Robert L. (eds.). Neuromuscular Disorders in Clinical Practice (Second ed.). New York, NY: Springer. p. 1030. doi:10.1007/978-1-4614-6567-6_47. ISBN 978-1-4614-6566-9.
- ^ de Figueiredo, B. Henriques; Huchet, A.; Dejean, C.; Mamou, N.; Sargos, P.; Loiseau, H.; Kantor, G. (July 2010). "Normal tissue tolerance to external beam radiation therapy: Peripheral nerves". Cancer/Radiothérapie. 14 (4–5): 405–410 (Abstract). doi:10.1016/j.canrad.2010.03.012. PMID 20580590.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y Christian Custodio; Cody Christian Andrews (August 1, 2017). "Radiation Plexopathy". American Academy of Physical Medicine and Rehabilitation. Retrieved 14 April 2018.
- ^ a b Ashenhurst EM, Quartey G, Starreveld A (1977). "Lumbo-Sacral Radiculopathy Induced by Radiation". The Canadian Journal of Neurological Sciences. 4 (4): 259–63. doi:10.1017/S0317167100025087. PMID 597799.

- ^ Krkoska, Peter; Kazda, Tomas; Vlazna, Daniela; Adamova, Blanka (2022). "Case report: radiation-induced lumbosacral plexopathy – a very late complication of radiotherapy for cervical cancer". BMC Neurology. 22 (1). United Kingdom: BioMed Central Ltd: 1–6. doi:10.1186/s12883-022-03013-5. ISSN 1471-2377. PMC 9743098. Retrieved 25 March 2024.
- ^ a b Rosen EM, Fan S, Goldberg ID, Rockwell S (2000). "Biological Basis of Radiation Sensitivity". Oncology (Williston Park, N.Y.). 14 (5): 543–50. PMID 10826314.
- ^ Frykholm GJ, Sintorn K, Montelius A, Jung B, Påhlman L, Glimelius B (1996). "Acute lumbosacral plexopathy during and after preoperative radiotherapy of rectal adenocarcinoma". Radiotherapy and Oncology. 38 (2): 121–30. doi:10.1016/0167-8140(95)01665-1. PMID 8966224.
- ^ a b c d e f g h i j k l m n o p q r s t u Merrell, R. (2015). "Radiation-Induced Lumbosacral Plexopathy". In Ehrenpreis, E; Marsh, R; Small Jr, W (eds.). Radiation Therapy for Pelvic Malignancy and its Consequences. New York, NY: Springer. pp. 181–90. doi:10.1007/978-1-4939-2217-8_13. ISBN 978-1-4939-2217-8.
- ^ Abraham, A.; Drory, V. E. (July 2013). "Postradiation lower motor neuron syndrome: case series and literature review". Journal of Neurology. 260 (7): 1802–1806. doi:10.1007/s00415-013-6881-7. PMID 23463367. S2CID 20152621.
- ^ a b Yadav, Rajesh R; Talavera, Francisco; Andary, Michael T; Meier III, Robert H (July 14, 2017). "Radiation-Induced Lumbosacral Plexopathy Treatment & Management- Other Treatment". emedicine.medscape.com.
- ^ a b Min M, Roos D, Keating E, Kerr L, Mukherjee R, Potter A, Shakeshaft J, Baxi S (February 2014). "External validation of the lumbosacral plexus-contouring protocol developed by Yi et al. (IJROBP 2012; 84: 376–82) for pelvic malignancies". Journal of Medical Imaging and Radiation Oncology. 1 (5): 117–24. doi:10.1111/1754-9485.12106. hdl:10072/390047. PMID 24529065. S2CID 23218404.
- ^ Kirkpatric, John P.; Marks, Lawrence B.; Mayo, Charles S.; Lawrence, Yaacov R.; Bhandare, Niranjan; Ryu, Samuel (2011). "Estimating normal tissue toxicity in radiosurgery of the CNS: application and limitations of QUANTEC". Journal of Radiosurgery and SBRT. 1 (2). p. 95-107, ISSUES & RECOMMENDATIONS: #3. PMC 5675466. PMID 29296303.
- ^ a b c Patchell, Roy A. "Damage to the Nervous System Due to Radiation Therapy". www.merckmanuals.com. Retrieved 29 May 2018.
- ^ a b c d Vasić, Ljiljana (2007). "Radiation-induced peripheral neuropathies: etiopathogenesis, risk factors, differential diagnostics, symptoms and treatment" (PDF). Archive of Oncology. 15 (3–4): 81–84. doi:10.2298/AOO0704081V.

- ^ Greenfield MM, Stark FM (1948). "Post-irradiation neuropathy". The American Journal of Roentgenology and Radium Therapy. 60 (5): 617–22. PMID 18895006.
- ^ Khadilkar, S V; Khade, S S (January 2013). "Brachial plexopathy". Annals of Indian Academy of Neurology. 16 (1): 12–18. doi:10.4103/0972-2327.107675. PMC 3644772. PMID 23661957.
- ^ a b Buboltz, Jerome B; Cooper, Jeffrey S (January 25, 2018). Hyperbaric, Soft Tissue Radionecrosis. Treasure Island, FL: StatPearls Publishing. p. Introduction. PMID 29489193.
- ^ Palta, JR; Efstathiou, JA; Bekelman, JE; Mutic, S; Bogardus, CR; McNutt, TR; Gabriel, PE; Lawton, CA; Zietman, AL; Rose, CM (2012). "Developing a national radiation oncology registry: From acorns to oaks". Practical Radiation Oncology. 2 (1): 10–7. doi:10.1016/j.prro.2011.06.002. PMID 24674031.
- ^ Efstathiou JA, Nassif DS, et al. (2013). "Practice-based evidence to evidence-based practice: building the National Radiation Oncology Registry". Journal of Oncology Practice. 9 (3): e90–5. doi:10.1200/JOP.2013.001003. PMC 3651578. PMID 23942508.
- ^ Boyd Goldberg, Kirsten (25 September 2015). "Radiation Oncology Looks to Collaboration for Big Data Systems". ascopost.com. The ASCO Post, American Society of Clinical Oncology. Retrieved 9 May 2022.
- ^ Jones, Noble; Stubblefield, Michael Dean (2 March 2017). "Radiation Plexopathy". now.aapmr.org. PM&R KnowledgeNOW. Retrieved 22 April 2023.
