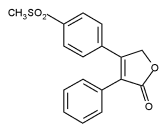
Rofecoxib
|
4-[4-(methylsulfonyl)phenyl]- | |
| Empirical formula | C17H14O4S |
| Molecular weight | 314.4 |
| Bioavailability (Oral) | 93% |
| Metabolism | hepatic |
| Elimination half-life | 17 hours |
| Excretion | biliary/renal |
| Pregnancy category | C (Australia) |
| Legal status | withdrawn |
| Routes of administration | oral |
Rofecoxib is a nonsteroidal anti-inflammatory drug (NSAID) that was used in the treatment of osteoarthritis, acute pain conditions, and dysmenorrhoea. Formerly marketed by Merck & Co. under the trade names Vioxx, Ceoxx and Ceeoxx, it was voluntarily withdrawn from the market in 2004 because of concerns about increased risk of heart attack and stroke.
Rofecoxib was one of the most widely used drugs ever to be withdrawn from the market. Worldwide, over two million people were prescribed Vioxx at the time. In the year before withdrawal, Merck had a sales revenue of US$2.5 billion from Vioxx.
Rofecoxib was available on prescription as tablets and as an oral suspension.
COX-2 selective inhibitor
Rofecoxib belongs to the group of NSAIDs known as COX-2 selective inhibitors or coxibs (CycloOXygenase-2 InhiBitors). Being COX-2 selective means that these drugs act specifically on one form of the cyclooxygenase (COX) enzyme, namely the COX-2, whereas previous NSAIDs inhibited both COX-1 and COX-2. This specificity allows rofecoxib and other COX-2 inhibitors to reduce inflammation and pain while minimizing undesired gastrointestinal adverse effects - peptic ulcers - that are common with non-selective NSAIDs such as aspirin, naproxen, and ibuprofen.
Interestingly, at the time of its withdrawal, rofecoxib was the only coxib with clinical evidence of its superior gastrointestinal adverse effect profile over conventional NSAIDs. This was largely based on the VIGOR (Vioxx GI Outcomes Research) study, which compared the efficacy and adverse effect profiles of rofecoxib and naproxen. (Bombardier et al., 2000).
Adverse drug reactions
Aside from the reduced incidence of gastric ulceration, rofecoxib exhibits a similar adverse effect profile to other NSAIDs.
Main article: Non-steroidal anti-inflammatory drug
Withdrawal from the market
The VIGOR study, published in 2000, had indicated a significant increased risk of acute myocardial infarction (heart attack) in rofecoxib patients when compared with naproxen patients. (Bombardier et al., 2000). The results of the VIGOR study were submitted to the United States Food and Drug Administration (FDA) in February 2001, which led to the introduction, in April 2002, of warnings on Vioxx labelling concerning the increased risk of cardiovascular events (heart attack and stroke).
In 2001, Merck commenced the APPROVe (Adenomatous Polyp Prevention on Vioxx) study, a three-year trial with the primary aim of evaluating the efficacy of rofecoxib for the prophylaxis of colorectal polyps. Celecoxib had already been approved for this indication, and it was hoped to add this to the indications for rofecoxib as well. An additional aim of the study was to further evaluate the cardiovascular safety of rofecoxib.
The APPROVe study was terminated early when the preliminary data from the study showed an increased relative risk of adverse cardiovascular events (including heart attack and stroke), beginning after 18 months of rofecoxib therapy. In patients taking rofecoxib, versus placebo, the relative risk of these events was 1.97 (rofecoxib 3.50% vs placebo 1.92%). The results from the first 18 months of the APPROVe study did not show an increased relative risk of adverse cardiovascular events. (Bresalier et al., 2005) Previous Phase III clinical trials had also not shown this trend. (Swan, 2004)
In sum, the APPROVe study suggested that long-term use of rofecoxib resulted in nearly twice the risk of suffering a heart attack or stroke versus patients receiving a placebo.
In addition to its own studies, on September 23, 2004 Merck apparently received information about new research by the FDA that supported previous findings of increased risk of heart attack among rofecoxib users (Grassley, 2004). Merck publicly announced the withdrawal of the drug from the market worldwide on September 30.
On November 5 the medical journal The Lancet published a meta-analysis of the available studies on the safety of rofecoxib (Jüni et al., 2004). The authors concluded that, owing to the known cardiovascular risk, rofecoxib should have been withdrawn several years earlier. The Lancet editorially condemned both Merck and the FDA for the continued availability of rofecoxib from 2000 until the recall. [1] Merck responded by issuing a rebuttal of the Jüni et al. meta-analysis (Merck & Co., 2004).
Whether all COX-2 inhibitors cause increased risk of adverse cardiovascular events is still unknown. A recent article indicated that valdecoxib (Bextra/Valdyne) more than doubled the risk of myocardial infarction - though the evidence cited may have been relatively weak.
Newer and more specific COX-2 inhibitors, including etoricoxib (Arcoxia) and lumiracoxib (Prexige), are currently undergoing Phase III/IV clinical trials. It is likely that these trials will be extended in order to supply additional evidence of cardiovascular safety.
References
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, et al. (2000). Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med 343 (21), 1520-8. PMID 11087881
- Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. (2005). Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 352 (11), 1092-102. PMID 15713943
- Grassley CE (15 Oct 2004). Grassley questions Merck about communication with the FDA on Vioxx. Press Release.
- Jüni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M (2004). Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. The Lancet (published online)
- Merck & Co., (5 Nov 2004). Response to Article by Juni et al. Published in The Lancet on Nov. 5. Press Release.
- Swan L, Merck Sharp & Dohme (Australia) Pty Ltd. (1 October 2004). Urgent Medicine Recall VIOXX® (rofecoxib) - Merck Announces Voluntary Worldwide Withdrawal of VIOXX.