Wikipedia:Reference desk/Science
|
Welcome to the science reference desk.
|
Choose a topic:
See also:
|
November 6
Hearing own voice
Why is it strange to hear our own voice? 217.129.241.186 01:10, 6 November 2007 (UTC)
- Because you normally hear your own voice as it is carried through the tissues and bones in your skull, as well as through the air, which is the only part you hear when you listen to a recording of yourself. Someguy1221 01:30, 6 November 2007 (UTC)
- Which helps to explain why people with hearing loss often find it hard to make themselves heard to other people. They can hear themselves ok through their head bones, and they think the listeners can hear them just as well, but what listeners hear when a person is talking is not what the person themself hears. Being often asked to "speak up" is sometimes a strong clue that it's not the listeners who are going deaf, but the speaker. -- JackofOz 20:27, 6 November 2007 (UTC)
Olestra formula
While reading my chemistry textbook, I passed a short reference to Olestra, which made me think about going to that article. When there, I wondered about the chemical formula; since it's not in the article, I searched Google, but found virtually nothing. Anybody know the chemical formula (or formulæ, since it appears to have variable numbers of fatty acid chains) for Olestra? Nyttend 01:52, 6 November 2007 (UTC)
- And just to make it completely obvious: I'm not asking for homework help here :-) It's not even something I'll be tested on. Nyttend 01:57, 6 November 2007 (UTC)
DNA polymerase requires a "primer"?
I was looking into any information I could find on DNA polymerase since I'm currently taking a Cell Biology course, and I was wondering why exactly does DNA polymerase require a primer? I already understand the basics, but I am looking for a little more in depth explanations. Wilt0057 01:52, 6 November 2007 (UTC)wilt0057
- Did you look at the DNA polymerase article? It currently reads: "DNA polymerase can only add free nucleotides to the 3’ end of the newly forming strand. This results in elongation of the new strand in a 5'-3' direction. No known DNA polymerase is able to begin a new chain (de novo). They can only add a nucleotide onto a preexisting 3'-OH group. For this reason, DNA polymerase needs a primer at which it can add the first nucleotide". I'm not sure you need more than that. Or do you need to know why it requires a 3'-OH substrate? If so, you need to research about substrate specificity for active sites in enzymes. David D. (Talk) 02:40, 6 November 2007 (UTC)
Magnetopneumodynamic Drive - I'm gonna build me one! (Maybe)
Thanks again to Graeme and Carnildo for the answer above.
Now I know it's possible (in principle) I'm going to try to build a zeppelin model with a battery-powered MPD.
What's next is whether it's possible to use a circular form for the thruster rather than triangles/rectangles. I'm thinking of a concentric circle or cylinder system that would be mounted in pairs parallel to the long axis of the zep. Remote-controlled rudders at the rear of the drives would give directional control. Any comments/suggestions appreciated.203.21.40.253 02:51, 6 November 2007 (UTC)
- You won't succeed. There is absolutely zero chance of you getting something that works from a design like that. These 'lifter' things provide absolutely microscopic amounts of thrust - they have to be made of the lightest possible materials and be driven by huge voltages and currents. This means you couldn't even power one from a car battery - let alone a couple of AAA's. Look at some of the experimental setups people use. Not one of them has the power supply actually on the lifter - they always trail a couple of wires from some god-awful-large transformer with mains supply going into them! If you truly mean to use the lifter as the 'thrust' motor (as opposed to a lift motor) for a lighter-than-air balloon, then at least the lifter doesn't have to carry the weight - but consider the size of balloon you'll need to lift something much heavier than a car battery - then consider how much drag a balloon that big is going to have - then look back at the microscopic amount of thrust you get from a 'lifter' and consider how even the lightest of breezes will overcome it's pathetic thrust. Honestly, you truly don't stand any chance whatever of getting this thing to work. This technology is nothing more than an amusing party piece. Beware of the large armies of nut-jobs out there claiming that there is something magical going on here - anything from "quantum levitation" to anti-gravity to using the earth's magnetic field to power the thing...the distinguishing feature of all of them is that the best they can do is a very light lifter with power coming from the ground that can just barely lift its own weight. SteveBaker 17:59, 6 November 2007 (UTC)
- No worries about the "nut-jobs" Steve; I knew what the Mythbusters would find before they started. However, the model set-up for the "hybrid" at the Blaze labs site (external link at Ionocraft article page) seems to show that the thing is do-able. Your point about the thrust to drag ratio is the obstacle I was concerned with. Exploitation of the on-board power supply is the crucial issue. I think varying the planar forms of the ionising system may be the way to go in enhancing air flow. That's where I'm looking for any guidance people can offer. Retarius | Talk 01:17, 7 November 2007 (UTC)
- Well, if you aren't yet convinced, let's crunch some numbers. According to ionocraft, the force (in Newtons) exerted by the motor is the current in amps multiplied by the air gap in meters divided by 2x10-4. It also says that the air gap needs to be about 1mm per thousand volts applied to it. So roughly - the force from the lifter in Newtons is one fifth the current multiplied by the number of kilovolts.
- Let's suppose you want you craft to run on ten AA batteries and let's assume you get 100% efficiency. You've got only 15 volts and you can't have 0.0015mm air gap! Let's assume you step up your voltage to 15kV using some kind of circuit so you can have a more reasonable 1.5 centimeter air gap. You get about 3 Amp/hours from a AA battery at 1.5 volts and you can pull about 15 Amps @1.5v without it melting or whatever. But with 1000 times that voltage, you get 1000 times less current - but you have 10 batteries - so you'll have about 0.15 amps at 15kV - sadly, the batteries will die after 20 minutes of flight time - but maybe that's OK.
- So the force from the lifter can be no higher than 0.15*15/5 = 0.45 Newtons - that means it could lift a weight of about 45 grams against gravity. Sadly, a AA battery weighs 23 grams and we we have 230g of batteries alone...so this simply isn't going to fly even with the most optimistic possible numbers. (In truth, to push the voltage up from 15v from 10 AA's in series up to 15,000 volts will require some fairly serious electronics - that'll add a ton of weight and it'll be horribly inefficient.
- But never mind - you don't want to use the lifter to lift the dead weight of the batteries - right? You're going to have a helium balloon carrying the weight and the lifter turned sideways pushing it forwards. A helium balloon can lift about 1kg per cubic meter of gas. By the time you have a quarter kilo of batteries, plus electronics, lifter and gas-tight envelope, plus radio control gear - you'll be well over 1kg. So we're looking at a zepplin-shaped balloon well over a meter in diameter.
- So - what drag will this thing have? The relevent formula (from Drag coefficient) is that Force = Cd x 1/2 x rho x A x V2. Force is the 0.45 Newtons our lifter will generate. Cd will probably be about 0.3 if you are really careful (better than most cars - worse than a perfectly smooth sphere). Rho is air density - about 1.22, A is the cross section - let's say about one square meter. So the speed your zepplin will go (with the most optimistic possible numbers) will be: sqrt(0.45/(0.3x0.5x1.22x1)) ...hmmm - maybe 2 to 3 miles per hour. Wow! That's actually a lot faster than I guessed. However, in even a 1mph cross-wind - the thing will be utterly unmanageable.
- But that's an insanely optimistic estimate. The trouble is that we have no estimate for the energy losses in boosting a 15 volt battery pack up to 15,000 volts. Sadly, from our article: "Ionocrafts capable of payloads in the order of a few grams usually need to be powered by power sources and high voltage converters weighing a few kilograms". Well a 'payload' of a few grams in a vertical lifter means a thrust of a few hundredths of a Newton in the horizontal direction. So if we toss out my battery calculations and say that we need a FOUR cubic meter balloon to lift a 3 kilo power supply plus one kilo of other stuff (bigger envelope, bigger control vanes, etc). And if we downgrade the thrust estimate to 'a few grams' - ie maybe 0.03N, then re-run my drag calculations with a much bigger cross-sectional area - and now your zepplin is moving at a half mile per hour. Even a light draft inside a building will stop the thing dead in it's tracks.
- I'm betting the thing would bob around in air currents and you'd hardly be able to tell whether the engine was turned on or off.
- Someone needs to double-check my numbers...but since it's going to cost you a lot of time and effort to find that out experimentally - it's really worth checking. IMHO, it's a waste of time to even try.
- I appreciate your time taken, Steve, and I don't dispute the above. That's why I'm looking to explore the basic design of the thruster. The extant models are too weak to bother with and I only intend to test-bed the thing unless and until it shows a useful result in improved thrust.
203.21.40.253 03:43, 8 November 2007 (UTC)
Artificial oil production
Assuming that all one needs is a source of carbon and hydrogen and energy, how much oil, natural gas, etc. could be produced per day using nuclear reactors for no other purpose? Dichotomous 04:27, 6 November 2007 (UTC)
- That's equivalent to asking "how much more energy do nuclear reactors produce than fossil fuels?" because the energy to form a fossil fuel would be around that released by breaking its bonds. So from [1] we see that most fossil fuels release about 50kJ/g, and from [2] we see nuclear reactors release about 2x10^9 kWh/ton = 7x10^6 kJ/g. Great, but we don't use much material to power our reactors. The power output of a nuclear reactor is about 3x10^5 kW by quick google search, so we find that a nuclear plant can ideally produce about 10^4 g/s of fossil fuels, or 10kg per second. However, I don't think we actually have a process that can make fossil fuels in any efficient way, so our actual yield will be much less, but this is the theoretical maximum yield. SamuelRiv 04:53, 6 November 2007 (UTC)
- I believe a couple of scientists in the UK about ten or fifteen years ago demonstrated a method of crude oil production using high pressure and temperature. From your calculations it appears retaining an oil based energy distribution system is not threatened by exhaustion of naturally occurring petroleum, oil, natural gas, etc. Dichotomous 05:07, 6 November 2007 (UTC)
- I have always wondered why it is not possible to synthesise hydrocarbons from their constituent parts of carbon and hydrogen. For example Propane (C3H8) is just 3 carbon atoms and 8 hydrogen. How would one make them join up and form a propane molecule? I am not an expert at chemistry by any means but know the very basic basics. Also, why can CO2 not be broken up into oxygen plus carbon? I'm guessing the energy input required to do these things would outwiegh the benefits? GaryReggae 10:24, 6 November 2007 (UTC)
- In terms of lubricants versus fuel the cost might be justified in the absence of substitutes or synthetics based on something else when naturally produced petroleum runs out. Even if there were more to it than just a high pressure, high temperature cooker and many steps involved, the need for carbon based lubricants and fuels may never go away. Dichotomous 12:20, 6 November 2007 (UTC)
- Plus hydrocarbons have other uses, such as in plastics, paints and other such thingsGaryReggae 13:00, 6 November 2007 (UTC)
- Imagine your great, great, great, great, great granddaughter complaining about the need to use so much fusion power to replace natural oil that the cost of her cosmetics are beyond reason, in addition to the cost of recharging her car... and blaming it all on the excesses of good old great, great, great, great, great granddad. Dichotomous 15:31, 6 November 2007 (UTC)
- Plus hydrocarbons have other uses, such as in plastics, paints and other such thingsGaryReggae 13:00, 6 November 2007 (UTC)
- My car already uses synthetic oil - it's great stuff - it lasts three times as long as regular motor oil and it can be recycled more easily. The more interesting question is how chemical plants that use hydrocarbons as feedstocks for making plastics and such would fare. But as has been discussed elsewhere on the reference desks recently, we simply cannot afford to burn all of the oil we have left because of the greenhouse problem - so if we DO manage to save the planet by ceasing to burn oil, we'll have PLENTY left for making plastics and such like. SteveBaker 03:22, 7 November 2007 (UTC)
Global warming
Until recently I never had problems with kudzu, weeds or grass if I was away for a month or six weeks and returned to find that period of growth in the back yard. Where can I find a chart or graph which shows the rate of increase in plant growth (such as grass and kudzu, land based, and algae and coral, water based) per rate of increase in atmospheric CO2, to view the point plant growth would simply be outpaced by the production of excess CO2? Dichotomous 04:44, 6 November 2007 (UTC)
Choice
I apologize for posting this here, believe me. It will give some of you a headache, and not the good kind. Beyond the two factors of genetics and environment (experiences etc.), is there anything else, some third factor, that can account for our next choice in life? Sappysap 04:50, 6 November 2007 (UTC)
Free will, according to proponents of religion, and quantum mechanics, according to proponents of the quantum mind. Neither is widely accepted by scientists as a phenomenological explanation. SamuelRiv 04:55, 6 November 2007 (UTC)
"Choice" can be a problematical way to describe behavior since it leads some people assume that humans have a supernatural ability to escape determinism. Philosophers such as Daniel Dennett have explored the idea that it is more neutral to say that our brains give us the ability to control our behavior based on past experience. --JWSchmidt 05:42, 6 November 2007 (UTC)
- See Randomness#In_biology. Besides the freckle example I gave there, another example is sexual orientation. Identical twins raised in the same home should have identical genetics and environment, yet don't always have the same sexual orientation. Thus, we know there is a random influence at work. (Note that, in this case, it may be truly random or simply following a pattern too complex for us to recognize.) StuRat 11:21, 6 November 2007 (UTC)
- I'd rather attribute it to chaos (in the mathematical sense) than randomness - but I'm almost sure that our free will is an illusion. The mind clearly works on multiple levels and I strongly suspect that the conscious level that we're aware of is merely rationalising choices which a more mechanistic mind made at some lower level. There are so many ways in which lower levels of thought processes have been shown to actively disguise things from the higher levels. Things like when you move your eyes very rapidly across a scene, the signal from them is actually cut off - and your brain 'fills in' the experience of the "missing video signal" from memory and imagination. Similarly, the effects of our relatively slow reaction times are distorted by our minds to make it seem like things happen instantly without a delay. There are all sorts of ways in which our conscious mind is lied to by the lower levels - and choice or 'free will' seems to me to be just another one of those things. SteveBaker 22:52, 6 November 2007 (UTC)
- More reason to avoid thinking about free will only in terms of behavioral choice. We generally associate "choice" with conscious brain activity, but the fact is, we cannot be consciously aware of most of our own brain activity. If we think about free will in terms of behavioral control then we can be comfortable with the idea that our brains can produce useful behavior based on past experience ("we" are in control) even when much of that control over our behavior is rooted in unconscious brain activity taking place outside of our rather limited domain of introspective awareness. I think the powerful illusion is that we are conscious of what we take to be "free will". If we expand our concept of "free will" to include its foundation in unconscious brain activity, then we do not have to call the brain's ability to adaptively control behavior an illusion. --JWSchmidt 22:25, 7 November 2007 (UTC)
electric vehicles for the post office
If electric vehicles offer such and advantage why hasn't the post office started using them or the candidates started touting electric vehicles for the post office? With all the stop and go driving and the large number of vehicles, if converted to electric, do a lot for global warming, or is this just a farce as well? Clem 09:01, 6 November 2007 (UTC)
- In England, postal delivery rounds in suburban areas are often carried out on foot or by bicycle. However, electric milk floats have been used for many years for door-to-door deliveries of milk because they are quiet and their limited speed and range is not a problem. Gandalf61 10:52, 6 November 2007 (UTC)
- Electric vehicles don't necessarily help with global warming. If fossil fuels are burnt to make the electricity, and a good deal of that electricity is lost during delivery and storage, then they can even be worse than an efficient internal combustion engine. If the electricity is made by a nuclear reactor or some other method which doesn't release hydrocarbons, then electric cars may help. StuRat 11:11, 6 November 2007 (UTC)
- The huge advantage of electric vehicles in stop/start applications like door to door delivery is that you get regenerative braking more or less for free. This means that for postal runs, an electric vehicle would be massively more efficient than a gasoline car - even if its batteries are recharged by an oil-fired power plant. I don't know why they don't use them. Their routes are short enough that the limited range of an electric vehicle would not be an issue and they can have one central recharge point. As Gandalf61 says, electric milk delivery vehicles have been around in the UK for many decades (certainly they were around when I was a kid in the late 1950's). The only sound those things made was a 'kerchink, kerchink' of the milk bottles rattling...and when they switched to delivering milk in cartons...nothing...dead quiet. SteveBaker 13:00, 6 November 2007 (UTC)
- Assuming in the States (and probably applicable for most places), there's a both a cost and availability problem. For one, it's expensive to replace that large a fleet of vehicles, and the vehicles are individually more expensive than those they're replacing. For two, electric cars aren't readily available on the market. Making matters worse, postal vehicles generally prefer right-hand-drive (opposite the norm), making the availability problem worse. Unfortunately, the quick solutions for 1 and 2 are in opposition to each other: the solution to cost is to upgrade very gradually; the solution to availability is to commission a big run of specialized vehicles.
- The problem is worse for rural route carriers, who tend to use self-owned non-standard vehicles. In addition to the exacerbated cost/availability issues, they're also more likely to run into the problem of electric car range -- I would think a 200+ mile daily route is quite possible in some areas. — Lomn 14:14, 6 November 2007 (UTC)
- Besides the regenerative braking pointed out by Steve, the other huge advantage of an electric vehicle for postal delivery would be avoiding wasting all that energy idling an internal combustion engine while the vehicle is stopped. And both of those advantages are also available in hybrids, so issues about availability, range, charging time, and questionable cleanliness of the electrical supply don’t exist as excuses for not at least switching to use hybrids for all postal delivery. Unfortunately, in the U.S., a bill requiring a switch to hybrid postal vehicles would almost certainly just get vetoed by Bush, who only recently started admitting that global warming is even real. Hopefully we’ll get a president who takes global warming more seriously soon. (As a disclosure of my biases, I’m a very proud Prius owner.) MrRedact 17:39, 6 November 2007 (UTC)
- But the US post office already uses very non-standard vehicles. Getting manufacturers to build specialist vehicles is not that hard when you can put an order in for a few tens of thousands of them with a good likelyhood of them being in service for 30 years. Those funny little RHD jeep-like things are unique to the postal people - the only ones you see that aren't on postal routes are old beat-up ones people bought from the post office for very low dollar when they were completely worn out. Replacing one unique kind of vehicle with another on an 'as they wear out' basis shouldn't pose that many problems. I agree that on rural routes, an electric car would make less sense - but as you say, in those areas the mail carriers generally have some arrangement by which they use their own cars. Our post-lady drives a normal LHD SUV which she owns - but the post office pays for fuel, repairs and maintenance on it. One assumes they would leave those as-is and just replace the urban vehicles. A 200 mile range must be plenty since for most of the time they are driving at maybe 10mph and spending about half their time stopped - at those kinds of speeds you'd have to be driving for DAYS to hit the 200 mile range limit. SteveBaker 17:46, 6 November 2007 (UTC)
- A bit off the topic, but here in the UK some mail is delivered by the milkman on his electric float. I subscribe to several magazines and they are often through my letter box by 7:00am and the postie doesn't come until about 11:00am these days. I don't know if the delivery is definitely by the milko, but I can't see the postie making two rounds. To me it makes perfect sense to combine the roles of postie & milko, and receive all of our post nice and early like we used to.--193.195.0.102 20:46, 6 November 2007 (UTC)
- There are a whole pile of things that could be delivered in this way - anything that needs to be fresh or recent could be delivered in one handy trip. Sadly, here in the USA, the idea of milk delivery doesn't seem to have taken off (or maybe it did once but hasn't survived). Added to the virtual inability to buy long-life milk, this becomes a major pain for us Weetabix lovers. On the other hand, it's very convenient that the US post delivery service will pick up mail as well as deliver it. That's something the UK system could certainly benefit from. SteveBaker 22:45, 6 November 2007 (UTC)
- Unfortunately, milk deliveries are a dying breed in the UK as supermarkets have undercut the traditional milkman. I don't know why the post office have never tried using battery vehicles as they'd be ideal for mail deliveries and collections. Couriers could also use them for local journeys. UPS seem to have a fleet of highly customised vehicles that are unique to them so surely they could try it? GaryReggae 23:50, 6 November 2007 (UTC)
seeing blood vessels
The doctor had a waiting room full of patients so I did not ask him this question. After he gave me medication to make the blood vessels in my eye swell as a test for diabetes I could see after he stopped shinning a light in my eye in a flash blood vessels. How is this possible? Are the receptors in my eye covered by blood vessels that I can not see unless they are swollen and if so how is this possible. Wouldn't the blood vessels still block or interfere with the light hitting the receptors? Dichotomous 10:30, 6 November 2007 (UTC)
- This is an evolutionary mistake, that the blood vessels are in front of the light receptors. That is why we have a blind spot, that's where there is a cluster of them. Elsewhere they are normally thin enough that enough receptors are hit to give us an image. We aren't normally aware of this flaw in our vision because our brains "fill in the gaps" with a best guess as to what's there. However, when the blood vessels are larger than normal, the brain can't overcome this. StuRat 11:06, 6 November 2007 (UTC)
- Don't call it an evolutionary mistake. There are no mistakes in evolution. Evolution is merely horribly inefficient, and not all the traits lifeforms possess are necessarily good or useful. They simply exist because the species can live long enough to reproduce despite the bad traits. 64.236.121.129 14:40, 6 November 2007 (UTC)
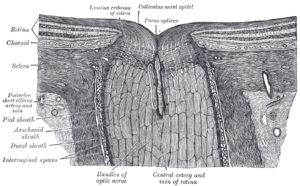
- Please see Blind spot (vision). The blind spot is
not due to blood vesselsmostly due to the axons of the retinal ganglion cells (and there are also some small blood vessels passing through the optic disc). The retina has a good design because it places the photochemically active rods and cones at the back where they can shed their older photo-sensory components into the pigment epithelium. --JWSchmidt 15:07, 6 November 2007 (UTC)
- 64, calling evolution inefficient has the same basic problem as calling something an evolutionary mistake: they both imply that it's actually trying to do something, rather than something that just happens. — Daniel 01:12, 7 November 2007 (UTC)
- Any phrasing seems to imply intention. Fault, error, mistake. The way eyes work is one of many unfortunate ways the human body works. Good design? Not a design, also ours is not the best way eyes work. Birds have much better eyes than us. Also, there's one part of our eyes which isn't obscured by blood vessels, and that's the only bit that does any worthwhile seeing (yeah, okay, the low quality stuff that our eyes also do is worthwhile too) - that three-dee panoramic full colour view most of us think we see is more of an illusion than anything else, at any moment, you can only see in detail an area about the size of the full moon as seen from Earth. See saccade for more info on how that works. Also have a look at change blindness. --Psud 09:23, 7 November 2007 (UTC) (my comments edited at 09:37, 7 November 2007 (UTC))
Astronomy, living far north
Hi, I tried to search Wikipedia for some material on celestial navigation. I currently live at a latitude of about 71deg north, and thus am experiencing complete darkness for about three months now. While it would be nice to acquire a stargazing map that details what I see (from northern Norway) at roughly midnight (first request), it would also be nice to have one that detailed what I see at noon (second!). Not that it is dark enough at noon that I can see most stars, but I imagine I can make the two maps 'overlap' in such a manner that I can follow celestial objects beyond simply those that can be seen at night.
If you can help me out with this, thanks in advance. :) 213.161.190.228 11:09, 6 November 2007 (UTC)
- This thing is great. It's made for 40 north, but you could cut it for your horizon. That combined with a real star atlas like Sky Atlas 2000.0 or Becvar's Atlas of the Heavens should get you there. --Milkbreath 12:32, 6 November 2007 (UTC)
- Of course one of the problems with living that far north is that your view of the sky doesn't change all that much depending on time of day. If you live on the equator, you get a total view of the entire sky over 24 hours (except, of course the sun rises and blots out half of it) - if you live at the poles, then the planet is essentially just spinning you on the spot so the sky seems to spin - but no new stars appear through your long night. At your latitude, the sky is going to "wobble" but there will be less difference between noon and midnight than at more equatorial latitudes. My imagination tells me that some of the stars nearest to the horizon at noon probably going to be obscured by sky glow because the sun is only just below the horizon - but I don't know for sure - a lot depends on how clear the atmosphere is where you live. SteveBaker 12:46, 6 November 2007 (UTC)
- There are a lot of options for generating a view of your night sky. Sky and Telescope magazine has an online interactive sky chart applet: [3]. Starry Night (planetarium software) has (or had, the last time I checked) a downloadable free version that had stars down to fifth or sixth magnitude. Some fully-featured free options include Stellarium and Celestia. I can't comment on the suitability for printing of the output from any of the applications I've listed, but all will allow you to view the sky at an arbitrary time of day from an arbitrary location on Earth; they will also allow you to run the clock back and forth so you can see the 'wobble' that SteveBaker describes. TenOfAllTrades(talk) 13:20, 6 November 2007 (UTC)
- Actually, I know a better link. It's the longitude for Oslo plus the Latitude for your location. Now, scroll down, and select "universal time", and replace the xx:xx:xx time with 23:00:00 for midnight and 11:00:00 for noon. It does not require any download, and probably doesn't need java either. Remember results might not be accurate completely. It's also better because you can scelect the coordinates exactly. Hope this helps. Thanks. ~AH1(TCU) 01:26, 8 November 2007 (UTC)
space diamonds
What exactly are space diamonds? Are they physically identical to diamonds found on earth? How are they collected? Where are they distributed? How do you verify their origin? How do they compare in price? What do existing diamond suppliers think about space diamonds? Etc..., I'm curious. —Preceding unsigned comment added by Mangosunshine (talk • contribs) 14:58, 6 November 2007 (UTC)
- Presumably this means stellar and/or planetary cores that have been compressed into diamond. They're an interesting find astronomically and have no impact whatsoever on the diamond market. — Lomn 15:18, 6 November 2007 (UTC)
- Carbonado or "black" diamonds may have an extraterrestrial origin, according to some researchers. And the core of white dwarf BPM 37093 is thought to be crystallised carbon, which you might loosely call diamond - so it has been nicknamed Lucy (after Lucy in the Sky with Diamonds) - but BPM 37093 is 50 light years away. You can even buy Space Diamond Gift Certificates, but I suspect they are nothing more than rather expensive pieces of paper. Gandalf61 16:09, 6 November 2007 (UTC)
- Wow. "Here you go darling, it's all that is left from an entire star compressed into a diamond." Keria 16:58, 6 November 2007 (UTC)
- There have been many speculations about various planetary cores being mainly carbon (that's part of the plot of the one of the sequels to "2001: A Space Odyssey (novel)" - I think it was 2061: Odyssey Three....mmmm...yes, that was it) - and at those temperatures and pressures, that would make a diamond of impressive size. Really, diamond isn't all that rare or special. We can now make synthetic diamonds that are better quality than "the real thing" - and which have to have artificial impurities introduced to them to make them look more real. The only reason diamonds are as expensive as they are is because the De-Beer folks make them so by hoarding them and only letting them out in quantities they control. It's a nasty monopoly. There is no doubt that diamonds should be commonplace and as cheap or cheaper than cubic zirconium. Another way that diamonds may become common is via nanotechnology - it's often said that if we had nano-bots going around making stuff for us, probably the easiest material for them to make would be diamond. Since the stuff is amazingly hard and could be made utterly smooth, we might one day find that many large-scale structures are most efficiently made from diamond. At this point, the value of diamond will be no more than a piece of coal. The same thing would happen if a large chunk of diamond were to be hauled in from space or impact on the earth as a meteorite. SteveBaker 17:31, 6 November 2007 (UTC)
Can chewing your lips cause cancer?
My dentist once told me that if you chew your lips (like chew the chapped, dried parts off), it can cause cancer. Is this true? 64.236.121.129 15:06, 6 November 2007 (UTC)
- !!!THIS IS NOT A MEDICAL DIAGNOSIS!!! - Some users feel it is necessary to delete any and all mentions of medical information because they perceive it as being a medical diagnosis. This is in no way a diagnosis. It is a note on medical studies that have been done on the topic asked about.
- See leukoplakia. Some studies have linked chewing on the lips or inside of the cheeks with leukoplakia and other studies have identified leukoplakia as a precancerous condition. Put together, that means that a very small number of studies have linked chewing on your lips to cancer in a rather roundabout way. This comes down to the definition of "can". Does it mean "it is likely to cause cancer" or "it is possible in a very rare circumstance that it will cause cancer"? -- kainaw™ 15:46, 6 November 2007 (UTC)
- Thanks for the notification, I appreciate that. I've found that most people tend to chew on their lips because they become chapped. What causes chapped lips? 64.236.121.129 15:54, 6 November 2007 (UTC)
- See chapped lips. -- kainaw™ 15:57, 6 November 2007 (UTC)
- Err, that article is pathetically bad dude... 64.236.121.129 16:05, 6 November 2007 (UTC)
- See chapped lips. -- kainaw™ 15:57, 6 November 2007 (UTC)
When the mucous membranes of the lips become dry they both become brittle and shrink resulting in superficial cracks. This is chapped lips. Dehydration and wind are two common mechanisms for the drying out. 83.147.141.69 19:49, 6 November 2007 (UTC)
Selection of yeast by growth media
I grew yeast which contained a vector coding for Leu and Trp allowing them to grow on media lacking leucine and tryptophan. However, when grown on media -Ura-Leu-Trp how come the yeast were able to grow, Ura was not coded for? Any ideas would really help...
--67.71.12.246 18:54, 6 November 2007 (UTC)Cat
- I'm not sure, but first you know that Ura is not an amino acid, as are Leu and Trp, but rather a pyrimidine. Uracil can be synthesized by the hydrolysis of Cytosine, (see Uracil#Synthesis). Hope that helps. (EhJJ) 21:05, 6 November 2007 (UTC)
- Hum okay...still not sure though...i had completely forgotten though that uracil was not an amino acid (rather a nucleic acid) but thanks for reminding me of that...but like then why do we have to exclude it from the medium...I'm not getting the reasoning behind it...
--67.71.12.246 21:38, 6 November 2007 (UTC)Cat
- Maybe the reason was to confirm that they can make their own uracil? A lot of classroom science experiments don't make any sense at all. Someguy1221 22:49, 6 November 2007 (UTC)
- Yeah I thought about that too...hehe but I figured there must be some point to it (guess I won't mention it in my lab report like that there's no chance of getting it wrong) i just would like to understand the point...because it's been playing with my mind for like 3 days!
--67.71.12.246 23:04, 6 November 2007 (UTC)CAT
- You have to remember that wildtype yeast can grow perfectly well in very minimal media. That is, if you plate most wildtype yeast strains onto -Ura -Leu -Trp media, you'll get colonies. What yeast researchers have done is isolated yeast auxotrophs which require Ura, Leu & Trp supplementation. These strains are each deficient in a single enzyme in the biosynthetic pathway for their respective compounds (separate enzymes for each pathway). Since they can't make it themselves, they have to get it from the media. This allows you to introduce plasmids encoding the deficient gene as a selectable marker. If the cells don't have the plasmid, they don't have the gene, can't make the compound, and can't get it from the media, so they die. If they have the plasmid, they can replace their deficient enzyme with the one on the vector, make the compound, and grow colonies. All of this is predicated on transforming the vector into a yeast strain which is a Ura/Leu/Trp auxotroph. My guess is that the yeast strain you used was *not* a Ura auxotroph ... that wouldn't explain why you didn't just use -Leu -Trp media instead, though. -- 22:18, 7 November 2007 (UTC) —Preceding unsigned comment added by 128.104.112.105 (talk)
Axial muscles
Probably a very simple question for many of you: what are axial muscles?
(I've checked muscle but axial muscles isn't mentioned there, and I checked Wiktionary, which says: axial = "Belonging to the axis of the body; as, the axial skeleton; or to the axis of any appendage or organ; as, the axial bones" - but I still don't understand which muscles are meant by axial muscles.) Lova Falk 19:30, 6 November 2007 (UTC)
- It means the skeletal muscles on the trunk of the body or the head - basically excluding the limbs and non-skeletal muscles. -- kainaw™ 19:37, 6 November 2007 (UTC)
- Thank you! Lova Falk 20:17, 6 November 2007 (UTC)
Why do we call alcohol, ethanol when talking about fuel?
Umm, it's the same thing. So why the different name? 64.236.121.129 19:47, 6 November 2007 (UTC)
- See alcohol and ethanol. They are not the "same thing". -- kainaw™ 19:50, 6 November 2007 (UTC)
- Yea but, ethanol is the alcohol found in alcoholic drinks. When people say alcohol, they refer to that. 64.236.121.129 20:08, 6 November 2007 (UTC)
- When people "who are ordering a beverage" say alcohol, they are referring to ethanol. The most common use of "alcohol" where I work is a reference to methanol. I wouldn't call alcohol and methanol the same thing simply because of my subjective experience at work. -- kainaw™ 20:12, 6 November 2007 (UTC)
- And if you drink fuel ethanol, you may get very sick (or even die) because it has impurities (such as methanol) that are not safe for human consumption. Fuel ethanol and drinking alcohol are not interchangable. Dragons flight 20:14, 6 November 2007 (UTC)
- Wait a minute, if the fuel ethanol also has other alcohols like methanol, shouldn't we call it alcohol anyway because it contains more than one alcohol, and not ethanol exclusively. Ethanol powered cars should be called alcohol powered cars because there's more than one alcohol in the mixture. 64.236.121.129 20:20, 6 November 2007 (UTC)
- That's like saying we shouldn't call the stuff I put in my tractor "diesel fuel", because it also contains a red dye. The other alcohols are impurities that are present in small quantities, not essential components of the fuel. You still call the stuff that comes out of your faucet "water" even though there's minerals and maybe fluoride in it, right? -- Coneslayer 20:30, 6 November 2007 (UTC)
- Those are bad analogies dude. Diesel fuel and red dye don't have a general term to refer to both of them. Where as, both ethanol and methanol are alcohols. Hmm if methanol is not an essential component, I guess that makes sense though, but why would impurities like methanol exist in ethanol. You don't accidentally get methanol in your beer. If you did, you'd be dead. 64.236.121.129 20:38, 6 November 2007 (UTC)
- My guess would be that it's a difference between fermentation by yeast, and industrial processes for producing ethanol. But if you're making an argument that we should use "alcohol" instead of "ethanol", you should give us a good reason for doing so. There is already an excellent reason for using "ethanol": there are other alcohols that are used as vehicle fuels, and you can't use ethanol in an engine that's designed to run on methanol, or vice-versa. Thus, using the precise name is advantageous. What would be the advantage of saying "alcohol" instead, as you suggest? -- Coneslayer 20:44, 6 November 2007 (UTC)
- So then why not ferment the ethanol by yeast instead of the industrial processes? No argument dude. Those are fine reasons to call it ethanol. 64.236.121.129 20:50, 6 November 2007 (UTC)
- Actually, I stand corrected--according to Ethanol, a lot of the fuel ethanol is produced by fermentation. The toxic components may be introduced deliberately for denaturing. There may also be toxic components besides the alcohols, such as detergents. -- Coneslayer 21:00, 6 November 2007 (UTC)
- Yeasts etc make a variety of alcohols, the main one's ethanol, but methanol's in there in noticeable amounts too. Actually, there's a bit of effort put into removing methanol from the drinking stuff when it's distilled, they wouldn't bother with that for fuel or industrial use. Actually, I note that nothing's done to remove other alcohols from beer either. --Psud 09:03, 7 November 2007 (UTC)
- Wow, I can't believe they will make that stuff toxic on purpose just to avoid taxes. 64.236.121.129 21:05, 6 November 2007 (UTC)
- It doesn't matter how toxic it is, because it's not intended for human consumption. Seems like a pretty obvious business decision: don't pay more than you have to. DMacks 21:55, 6 November 2007 (UTC)
- Wow, I can't believe they will make that stuff toxic on purpose just to avoid taxes. 64.236.121.129 21:05, 6 November 2007 (UTC)
- NO! REELY?! We know ethanol fuel is not for human consumption dude. Don't waste time pointing out obvious facts. 64.236.121.129 14:24, 7 November 2007 (UTC)
- In that case, why are you feigning astonishment about how toxic a non-foodstuff is ("Wow, I can't believe they will make that stuff toxic on purpose just to avoid taxes.")? DMacks 17:06, 7 November 2007 (UTC)
- NO! REELY?! We know ethanol fuel is not for human consumption dude. Don't waste time pointing out obvious facts. 64.236.121.129 14:24, 7 November 2007 (UTC)
- LOL! That's this kid's M.O: Ask a perfectly valid "why" question, then slam everyone who answers because he either doesnt understand, or doesnt want to hear that answer. Ok, here's the real reason we don't mix the terminology between Ethanol and Alcohol: Because the Battle Mechs with the spherical wheels, that fly using ducted fans over to drain Loch Ness in order to see if a parisitic twin got cancer from biting its lip would rather use Ethanol as an alternative to gasoline. You rock kid... don't ever lose your imagination! :) (But don't bite the folks who try to get to the bottom of your "whys" either). :) —Preceding unsigned comment added by 198.172.206.151 (talk) 19:12, 7 November 2007 (UTC)
- Whoever's alt account is 198.172, you are cracking me up with trying to hide behind an ip so you can flame and not be given a scarlet letter on your real account. Haha. I can take 3 guesses as to who you really are. 64.236.121.129 21:22, 7 November 2007 (UTC)
Alcohol is a chemical family. Ethanol is a type of alcohol, referred to as "alcohol" because it is the most common type encountered. —Preceding unsigned comment added by 83.147.141.69 (talk) 19:53, 6 November 2007 (UTC)
- In other words, ethanol is a type of alcohol, but not all alcohol is ethanol. So maybe your question might have been - Why do we not talk about drinking ethanol rather than the more general term alcohol? Why don't we have Ethanolics Anonymous? -- JackofOz 20:18, 6 November 2007 (UTC)
- Exactly. And it's sometimes clear from context- as pointed out above, if people are talking beverages, it's clear that alcohol means ethanol. When you're talking about industrial usage, it's less clear so people tend to use more specific terms. Friday (talk) 20:22, 6 November 2007 (UTC)
- Yeah - there are lots of kinds of alcohol. Ethanol is one of them, Methanol is another. When you are talking about fuels, the word "alcohol" has considerable ambiguity - did you mean methanol or ethanol or butanol or pentanol or 2-methylbutanol or...? When you are talking about things people drink however, there is no ambiguity because the only alcohol that's even close to being safe to drink is ethanol - so when we talk about alcoholic drinks - we're always talking about ethanolic drinks and there is no ambiguity worth mentioning. Language is sloppy - and when a laymans term intersects with a scientific term, it's never a good thing. Do you use the brakes and steering wheel on your car to accellerate it? In scientific terms 'accellerate' means 'change velocity' - so the answer is yes - both steering and brakes accellerate your car. Messy! SteveBaker 20:23, 6 November 2007 (UTC)
So while it's certainly true that the word ethanol is more specific than the word alcohol, I wouldn't take it for granted that that's the reason the E-word is used at the pumps. These naming decisions are made by marketing people, legal departments, and bureaucrats, more than by scientists and engineers. They may have been worried that, if they called it "alcohol", someone would try to drink it. --Trovatore 21:04, 6 November 2007 (UTC)
- I agree - that would certainly have been a risk. Even E85 ethanol (which is 85% ethanol) would be suicidally dangerous to drink - the E10 stuff we have now would be much, much worse! If we ever start to use E100 (as they do in Brazil) I dare not imagine what might happen! 70.116.10.189 22:11, 6 November 2007 (UTC)
- Is there a typo there? How could 10% be much, much worse than 85%? -- JackofOz 22:17, 6 November 2007 (UTC)
- Because the other 90% is gasoline! 70.116.10.189 22:22, 6 November 2007 (UTC)
- Is there a typo there? How could 10% be much, much worse than 85%? -- JackofOz 22:17, 6 November 2007 (UTC)
- I must be a little slow this morning; would you indulge me as I try to get this straight? E10 is dangerous, mainly due to the 90% gasoline content. E85 is dangerous, mainly due to the 85% ethanol content. E100 is dangerous, because it is pure ethanol. Is that it? -- JackofOz 23:01, 7 November 2007 (UTC)
- E10 is dangerous, mainly due to the 90% gasoline content. E85 is dangerous, mainly due to the 15% gasoline content. E100 would be dangerous, because it's intentionally denatured (made toxic), so that it can't be used as a beverage—but idiots might think it's safe because it's called "100% ethanol". -- Coneslayer 23:11, 7 November 2007 (UTC)
- Well, 100% ethanol is not exactly "safe". It will seriously irritate your mucous membranes, certainly enough to be painful and possibly enough to be dangerous. --Trovatore 03:01, 8 November 2007 (UTC)
- E10 is dangerous, mainly due to the 90% gasoline content. E85 is dangerous, mainly due to the 15% gasoline content. E100 would be dangerous, because it's intentionally denatured (made toxic), so that it can't be used as a beverage—but idiots might think it's safe because it's called "100% ethanol". -- Coneslayer 23:11, 7 November 2007 (UTC)
You might try Alcohol#Etymology; the word "alcohol" certainly predates the word "ethanol". shoy (words words) 20:42, 7 November 2007 (UTC)
- Did you know? In Brazil, both the beverage and the fuel ethanol are just called "alcohol". We can even say something like "to increase the percentage of ethanol in the alcohol fuel" 200.255.9.38 13:23, 12 November 2007 (UTC)
If we can drain lakes, why don't we drain Loch Ness to see if the Loch Ness Monster is there
Why not? 64.236.121.129 20:28, 6 November 2007 (UTC)
- Why bother? Who cares? If they that do can raise the money, overcome environmental and other concerns, figure out what to do with the water, and get the necessary permits, I say go for it. DMacks 20:38, 6 November 2007 (UTC)
- Lots of people care dude. Lots of people care. 64.236.121.129 20:40, 6 November 2007 (UTC)
- Loch Ness is very deep (>230m) and it would be very difficult to drain. Besides, as the water level dropped, Nessie would simply entomb herself in the solid rock in the same way a toad can and avoid detection.--193.195.0.102 20:51, 6 November 2007 (UTC)
- If the monster exists, it probably needs the lake. Drain the lake and kill the monster, meaning the monster would not exist. Might as well just declare the monster does not exist. Then we can skip draining the lake. Johntex\talk 20:53, 6 November 2007 (UTC)
- What's to say that they (c'mon, if there is something down there, there'll have to be a breeding population of them - it's an unknown animal, if anything, not an immortal 'magical beast') even spend all their time in the loch? --Kurt Shaped Box 21:46, 6 November 2007 (UTC)
- These questions have been asked many times in the past. Any serious debate is over. We have all the proof we need to know there is nothing there. The entry and exit to the Loch are fairly shallow streams and go through well populated areas. Nothing of any size could get up and down there without being very visible indeed. For a breeding population of any species to survive, you need at least 500 individuals in order to have enough genetic diversity. 500 creatures the size of the hypothetical Nessie could just maybe hide in the Loch (although how they'd have avoided being found in sonar studies and other careful searching is hard to imagine) - but the effect they'd have on the local fish populations would definitely have been noticed. So, no Nessie. Sorry - it's just not possible. 70.116.10.189 22:21, 6 November 2007 (UTC)
- What's to say that they (c'mon, if there is something down there, there'll have to be a breeding population of them - it's an unknown animal, if anything, not an immortal 'magical beast') even spend all their time in the loch? --Kurt Shaped Box 21:46, 6 November 2007 (UTC)
- Lots of people care dude. Lots of people care. 64.236.121.129 20:40, 6 November 2007 (UTC)
- Why don't we cut down the rainforest to see if there are any endangered species there...? TenOfAllTrades(talk) 22:08, 6 November 2007 (UTC)
- Because that would destroy the rainforest! DUH! Man... Think a little man, it's not that hard to figure out :). 64.236.121.129 14:32, 7 November 2007 (UTC)
- I think you missed the sarcasm. I guess the point is, if you remove the creature's living environment (loch ness or rainforest) then whatever you do find is kind of screwed. Besides, the mystery is 99% of the fun with Nessie! :) ArakunemTalk 18:19, 7 November 2007 (UTC)
- He was being sarcastic?! NO! REELY?! I just like to crush pathetic attempts at smart assery :). 64.236.121.129 21:25, 7 November 2007 (UTC)
- An excellent idea, think of all the Bigfoots we could find! -- MacAddct 1984 (talk • contribs) 23:15, 6 November 2007 (UTC)
- Ohhhh - I was assuming we'd be training them to wield the chainsaws. OK, nevermind. SteveBaker 03:25, 7 November 2007 (UTC)
- An excellent idea, think of all the Bigfoots we could find! -- MacAddct 1984 (talk • contribs) 23:15, 6 November 2007 (UTC)
Diaphragm
Is Diaphragm a smooth muscle??thanks--82.105.205.27 21:17, 6 November 2007 (UTC)14mala
No. It is striated, and is under voluntary control. Make yourself cough! Voila! :-) Fribbler 21:24, 6 November 2007 (UTC)
Nuclear Ingredients
i am unable to find out (as a list) all the ingredients to a nuclear bomb this is for my physics homework. i would really appreciate if you could help in any way. i have been through all of the wikipedia nuclear articles but i am not successful. please can you help me. all the information that i have gathered about nuclear ingredients were plutonium and uranium. thank you very much —Preceding unsigned comment added by 87.74.98.190 (talk) 21:21, 6 November 2007 (UTC)
- Ahmadinejad? Is this you? —Preceding unsigned comment added by Fribbler (talk • contribs) 21:25, 6 November 2007 (UTC)
- You might be having problems because you expect the recipe to be more complex than it is. Really, you just take some plutonium and use conventional explosives to squish it. The ingredient list gets bigger if you want to increase yield, but in general nuclear explosions are pretty simple compared to chemical explosions. It has more to do with the technique of the chef than the recipe. --Mdwyer 21:42, 6 November 2007 (UTC)
- This article will give you step-by-step instructions for a fission device. Delmlsfan 21:54, 6 November 2007 (UTC)
- The abstract principle of a fission weapon is very simple. You need to take several chunks of radioactive material (plutonium or uranium) that are each, individually, much less than the critical mass for that substance - and you need to slam them together into one large chunk that's bigger than the critical mass so it goes bang. Everything else is annoying engineering details.
- The most significant problem is that if you just take two half-critical mass chunks and push them together with a lever or something - they'll get so amazingly hot that they'll melt and distort so much that you won't be able to get them close enough together and the thing will 'fizzle' - make a horrible radioactive mess - but not much of an explosion. So the next trick is to slam them together using high explosives so they move so fast that they don't have time to heat up an fizzle. But now you have to set off those explosives at precisely the same moment and have them slam the pieces together just right - or no bang. Then the components of the bomb have to be assembled in such a way that the person putting them together doesn't die of radiation poisoning and they have to be in a chunky enough bomb casing that the finished device can be handled safely. These weapons aren't cheap and you certainly can't afford to have one not go off when you want it to - or go off when you don't - so the fuses and timers and other precautions are much greater than with a regular bomb. You can't use normal computers and such inside the bomb because the radiation can corrupt their memories.
- The whole very simple idea just turns into a gigantic pain in the ass when you try to actually build one. SteveBaker 22:34, 6 November 2007 (UTC)
- Eh, you've made things more complicated than you need to. You don't need computers inside a gun-type bomb. You don't even need to have well-timed explosives—just send one of the sub-critical bits into another, don't have them slam together simultaneous, that just complicates things. You're confusing the gun-type weapon—which is really quite simple—with the plutonium bomb—which is not. The finished device—which in a very primitive bomb is going to be quite large—is not going to be terribly radioactive as long as you keep the two sub-critical bits properly apart from each other at all times. The hardest part here is going to be getting enough high-enriched uranium; the design could be done by someone with the engineering knowledge of repairing motorbikes. You only need more sophisticated knowledge if you want to say, drop it out of a plane, which requires being able to fit a lot of things into a relatively small package and make sure the wires won't come undone, etc. (A plutonium bomb is quite different and requires very sophisticated engineering on top of everything else.) --24.147.86.187 04:22, 7 November 2007 (UTC)
- It should be noted for completeness, though, that there are a couple of significant tradeoffs with the Little Boy design. One, you have to use uranium. Plutonium won't work. Two, you need (relatively speaking) a lot of uranium -- over 60kg vs less than 5kg plutonium in an implosion-type bomb. — Lomn 05:49, 7 November 2007 (UTC)
- True enough. It's actually a great blessing that from an engineering and physics perspective these things are balanced as they are—uranium is easy to make a bomb with, but hard to produce; plutonium is easy to produce, but hard to make a bomb with. It might not be too hyperbolic to say that these simple facts have done more for non-proliferation than anything else out there. --24.147.86.187 15:11, 7 November 2007 (UTC)
- It should be noted for completeness, though, that there are a couple of significant tradeoffs with the Little Boy design. One, you have to use uranium. Plutonium won't work. Two, you need (relatively speaking) a lot of uranium -- over 60kg vs less than 5kg plutonium in an implosion-type bomb. — Lomn 05:49, 7 November 2007 (UTC)
- Eh, you've made things more complicated than you need to. You don't need computers inside a gun-type bomb. You don't even need to have well-timed explosives—just send one of the sub-critical bits into another, don't have them slam together simultaneous, that just complicates things. You're confusing the gun-type weapon—which is really quite simple—with the plutonium bomb—which is not. The finished device—which in a very primitive bomb is going to be quite large—is not going to be terribly radioactive as long as you keep the two sub-critical bits properly apart from each other at all times. The hardest part here is going to be getting enough high-enriched uranium; the design could be done by someone with the engineering knowledge of repairing motorbikes. You only need more sophisticated knowledge if you want to say, drop it out of a plane, which requires being able to fit a lot of things into a relatively small package and make sure the wires won't come undone, etc. (A plutonium bomb is quite different and requires very sophisticated engineering on top of everything else.) --24.147.86.187 04:22, 7 November 2007 (UTC)
- The whole very simple idea just turns into a gigantic pain in the ass when you try to actually build one. SteveBaker 22:34, 6 November 2007 (UTC)
- The article Nuclear weapon design is pretty thorough. There are more ingredients mentioned there than you have. What about the trigger? Give it a read. --Milkbreath 22:53, 6 November 2007 (UTC)
- For an even more simple assessment, see the pages on the first two atomic bombs: Little Boy and Fat Man. They have pretty straightforward "ingredient" lists, blueprints, etc. The hard part is not the design, for the most part. The hard part is in getting the materials. --24.147.86.187 04:18, 7 November 2007 (UTC)
What's the name of that multicolored thing on TV?
It's a screen where you have several different bars, and they are all different colors, and the TV emits a high pitch noise. What is that? 64.236.121.129 21:22, 6 November 2007 (UTC)
- I call it a test pattern, or "Bars and Tone". Here's a link: SMPTE color bars --Mdwyer 21:34, 6 November 2007 (UTC)
- Historically, it was called a test card and was used in the early days of television to make sure the colours all looked correct. When the TV station closed for the night, the test card was broadcast although nowadays it is a rare sight. GaryReggae 23:43, 6 November 2007 (UTC)
- They were broadcast during the daytime in the UK before all-day broadcasting was instituted. The idea was that TV repair shops could make use of them to figure out a range of different problems inside the TV set. These days you can cheaply buy a little box to generate a range of different TV test patterns for this purpose - so there is no need for them to be broadcast - although I believe some cable and satellite services still dedicate a channel to transmitting them. SteveBaker 03:53, 7 November 2007 (UTC)
- Very rare on broadcast TV though. We've got a couple of sub-24 hour/day TV stations where I live, neither of which show a test pattern overnight. I did see a test pattern for about three seconds during a system fault a few months ago on one of our major commercial TV stations, but their standard "Sorry for the inconvenience" screen replaced it quickly. --Psud 08:44, 7 November 2007 (UTC)
- In countries using the NTSC television system, such as the United States, a color bar test signal is still used as a reference to adjust analog receivers and frame synchronizers to fine tune four basic video levels to match the transmitted NTSC signal: Video level (contrast on home receivers), black level (brightness), chroma saturation (color) and chroma phase (hue or tint). Many satellite uplinks transmit this signal when the main program is not in progress, so that those users downlinking their signal can adjust their receivers.
- The "high[-]pitched noise" referred to by the OP is a generally a standard audio reference tone, 1 kHz in frequency and transmitted at a standard amplitude of 0 VU. Again, this is used to adjust the level of the receiver's audio gain to match the standard level sent from the source. Thomprod 19:34, 7 November 2007 (UTC)
Could a parasitic twin lead to an evolutionary change?
I was just watching this bizzare video on CNN, http://www.cnn.com/2007/HEALTH/11/06/india.girl/index.html#cnnSTCVideo ,and I was wondering if a parasitic twin could potentially lead to a evolutionary change where an species of animal has more limbs because of failed twinning. 64.236.121.129 21:43, 6 November 2007 (UTC)
- See conjoined twin for some background info. I don't think this situation is guided by genetics- it's more of a physical accident. So I don't see room for genes that cause this to be passed on. Also, this condition doesn't improve one's chances of survival and reproduction- quite the opposite. Friday (talk) 21:48, 6 November 2007 (UTC)
- I agree with your first point, but your second point assumes too much. Malamockq 00:52, 7 November 2007 (UTC)
- Empirically, Friday's second point is quite correct. The parasitic twin extra limb thing (as opposed to conjoined twins) is invariably detrimental to the person. In this case, various articles have noted that the child is entirely unable to walk and likely to die before adolescence without radical surgery. While we can theorize a beneficial Doc Ock-style extra limb thing, evidence suggests that such benefits are confined to the realm of fiction. — Lomn 02:29, 7 November 2007 (UTC)
- Surely the key point here is Friday's first point, that the condition of having a conjoined twin/parasitic twin is not genetic. Therefore, regardless of whether it might convey any benefits to the individual concerned, it is not inherited, and so cannot play any part in the evolution of a species. Gandalf61 16:15, 7 November 2007 (UTC)
- Empirically, Friday's second point is quite correct. The parasitic twin extra limb thing (as opposed to conjoined twins) is invariably detrimental to the person. In this case, various articles have noted that the child is entirely unable to walk and likely to die before adolescence without radical surgery. While we can theorize a beneficial Doc Ock-style extra limb thing, evidence suggests that such benefits are confined to the realm of fiction. — Lomn 02:29, 7 November 2007 (UTC)
- I agree with your first point, but your second point assumes too much. Malamockq 00:52, 7 November 2007 (UTC)
Yes that's what I thought. Just wanted to make sure. Btw lomn, I think your assumptions are based on conditions right now rather than changing circumstances in the enviroment. If a nuclear war, ice age, etc happens, there might be circumstances where many limbs would be preferable. Or not. I just think it's bad to assume things just based on current circumstances. Poor science pally boy. 64.236.121.129 21:15, 7 November 2007 (UTC)
- While the possibility of changing environments is good to keep in mind (very wierd things can happen, like the loss of an appendage or sense becomes advantageous), the inability to survive adolescence is simply not beneficial. And even if she could survive without surgery, I don't see how not being able to walk is advantageous. Maybe if a strange animal appears that eats anyone with less than 8 limbs... Someguy1221 21:24, 7 November 2007 (UTC)
- Dude, I wasn't talking about that girl. I was talking about a mutation where you have lots of limbs. 64.236.121.129 21:38, 7 November 2007 (UTC)
Altruistic act? I dont think so
I was having a discussion the other day about whether all acts are truly motivated by self interest, I am for the motion, and was looking to see if anyone could help me by provide some research into the area, I am particularly interested in finding a piece of research I read a while ago into how your brains distorts positions to your point of view in order to justify decisions you have previously made, which could lead to you believing acts were altruistic when they are not. Thanks. 172.200.188.149 22:02, 6 November 2007 (UTC)
- I think a key problem with saying that is you need to very firmly define "motivated by self-interest." Certainly all actions beyond accidents are motivated by something and we can always contort our reasoning to call that self interest, for even if it does not benefit a person in any obvious way, there is some reason they "want" to do it. Someguy1221 22:07, 6 November 2007 (UTC)
- The Principles of NLP say that Behind every behavior is a positive intention. Whether that intention is positive towards your own welfare without regard to others', or towards others without regard to your own, or some of both, would depend on the circumstances. -- JackofOz 22:15, 6 November 2007 (UTC)
- While this won't help you with the second part of your question, our article here on Objectivism might be a good place to start your research on this topic, and will at least give you some food for thought about the philosophy supporting your thesis. Jeffpw 22:20, 6 November 2007 (UTC)
- The Principles of NLP say that Behind every behavior is a positive intention. Whether that intention is positive towards your own welfare without regard to others', or towards others without regard to your own, or some of both, would depend on the circumstances. -- JackofOz 22:15, 6 November 2007 (UTC)
- Maybe you were thinking of Cognitive_dissonance#Origins_and_experiment? Altruism#Altruism_in_ethology_and_evolutionary_biology has some good places to start too. -- Diletante 22:41, 6 November 2007 (UTC)
The distinction you're concerned with here is one of behavior versus motivation for that behavior. We can define altruistic behavior and then give examples of humans behaving altruistically; someone risking their own life to save a stranger's should satisfy most definitions and is certainly not unheard of. However, trying to answer the question of whether or not such behavior was selfless and disinterested true altruism and not motivated by some conscious or unconscious selfishness is problematic. To prove or disprove such a thing would require knowing the internal thoughts and motivations of the person in question, something not currently available to science. See the Criticism section of Psychological egoism for more. So, it's an unwinnable arguement for both sides from a scientific perspective. My take is that, yes, from a biological/evolutionary perspective selfishness is a hard-wired human trait, but genetic traits are not fixed or unmodifiable; humans are a very unique species and I wouldn't discount out of hand the possibility of true altruism in humans. Either way, there's worse things to aspire to than acting altruistically, regardless of true motivation. Azi Like a Fox 17:45, 7 November 2007 (UTC)
- Saving a stranger raises social your standing, earns you 'brownie points' as such, also you expect people to do the same thing for you, and prolongs the survival your species. And acting altruistically is a bit of an oxymoron as something must be motivating you and if it is something else, then its is altruism and not acting, and if its selfish then its not altruistic at all. ΦΙΛ Κ 21:45, 7 November 2007 (UTC)
- Right, and by the Original Poster's definition these ulterior motivations all preclude the original act of saving someone as "true" altruism. The expectation of someone doing the same for you would be reciprocal altruism (talk about an oxymoron). I was trying to explain that it's unknowable/unmeasurable whether someone's altruistic action is ever truly devoid of self-interest; given human nature it's not unreasonable to think that such actions aren't or can't be, a bit cynical but not an unrealistic position. Regardless, my point was that even if you say that no human action can be devoid of self-interest, it's still better that people behave generously and altruistically even if such behavior is motivated at some level by self-interest. My giving to local charities is none the less beneficial to the community and the recipients for being predicated on what I consider rational self-interest and an investment in the mid to long-term future rather than a disinterested and altruistic desire to be charitable. If instead I gave because it simply made me feel good to help others, then again it could be argued my motivations are self-interested (my own pleasure in helping others) rather than purely altruistic. So be it, teaching and cultivating altruistic behavior is all the more important and remarkable when it happens in light of our "selfish" nature. Hope that clears up what I mean by describing an action as altruistic even when the motivations behind it aren't always. Azi Like a Fox 23:58, 7 November 2007 (UTC)
In vitro mitochondrial respiration states
What are the different respiration states of mitochondria (1,2,3,4)?
One paper says: "In the present study, state 2 respiration is defined as the rate of respiration in the presence of substrate but before the addition of ADP." but a website says regarding state 4: "State IV respiration is defined as oxygen consumption by isolated mitochondria on a particular substrate, in the absence of ADP..." So what's the difference? --Seans Potato Business 22:44, 6 November 2007 (UTC)
- I believe that the difference is that state 4 (mito + reduced substrate without ADP) is reached as a phase during the experiment after all the ADP has been converted to ATP. Whereas state 2 is a starting condition prior to ADP being added. Subtle, I know but you have to consider them with respect to phases during an experiment.
- It is common to start with mito alone, state1. Then spike with substrate to get to state 2 (limited respiration will occur). Then add ATP to move into state 3 (maximal respiration) and when the ADP runs out you enter state 4. If you respike with ADP will reenter state 3 and then back to state 4 when the ADP is all converted to ATP (all assuming you have inorganic phosphate as a substrate in excess). David D. (Talk) 03:25, 7 November 2007 (UTC)
- Original 1955 article & see this. In terms of the original Chance & Williams protocols, I think a typical "state 2" experiment involved plenty of ADP but no added substrate such as succinate. A "state 4" experiment involved having high levels of a substrate such as succinate while ADP was rate limiting and driven to zero concentration during the experiment by conversion to ATP. Other people define "state 2" differently. You have to look at the details of how each "state" is defined by the people doing the experiments. --JWSchmidt 03:42, 7 November 2007 (UTC)
- This is an excellent point: "You have to look at the details of how each "state" is defined by the people doing the experiments". However, i might add I was going from memory and since JWSchmidt has a nice link quoting the Chance/Williams experiment that is clearly the definitive answer. David D. (Talk) 04:55, 7 November 2007 (UTC)
November 7
my girlfriends disorder?
Diversity Antenna?
I remember way back in college that there was some discussion about diversity antennas (Using two antennae to increase signal strength or reduce noise, I think). It's was used in disccussion about wireless mics in TV (why many ENG news cameras have two floppy antennae sticking out of their back) I can't seem to find any entry on diversity in regards to radio waves on Wikipedia. What's the science behind it and what's it called? --24.249.108.133 00:08, 7 November 2007 (UTC)
- See Antenna diversity. --Milkbreath 00:14, 7 November 2007 (UTC)
- The quality or strength of the field can fluctuate wildly from point to point, so if you get the signal from a bad position you can compensate by using the good signal. Also two different polarizations can be used. The signal varies due to diffraction and destructive interference Graeme Bartlett 00:44, 7 November 2007 (UTC)
Sun and moon at midday
I live in the state of Virginia (US). A few days ago I went out to lunch from work on a pristine, blue-skied, bright autumn day...and there it was. The moon, 3/4's full and as clear as if I were looking at its craters on the clearest of nights; only the background wasn't black, it was a blinding bright blue. I looked back and forth at the sun and the moon and my watch (which read 12:45 pm) for quite a while and couldn't believe it. It was beautiful. Sadly, in just an hour the phenomena ceased.
I have lived here all my life (a respectable 27 years) and have never seen the sun and the moon together so prominently sharing the sky of midday. Is there any way to predict when this rare optic event will happen? —Preceding unsigned comment added by Sappysap (talk • contribs) 01:14, 7 November 2007 (UTC)
- It sounds as though you saw a sun dog. Possibly due to ice crystals Graeme Bartlett 01:56, 7 November 2007 (UTC)
- Actually, the sun and moon share the sky approximately half the time. When the moon is full, it's on the opposite side of the Earth from the sun. When it's at either quarter, it's at a 90° angle relative to the sun and thus above the horizon for about 6 hours of night and 6 hours of daylight. As the moon approaches its new phase, it's in the sky almost entirely during the day.
- So as you can see, this isn't rare at all -- it's just a matter of stopping to look up. Additionally, the moon isn't the only celestial object visible in full daylight. Early this year, Comet McNaught was visible in full daylight, and Venus is often visible with binoculars, and with the naked eye at sufficient altitude. — Lomn 02:13, 7 November 2007 (UTC)
- (after edit conflict) It's not a rare optical event at all, even though it may seem to be because the Moon is much less noticeable against a bright blue sky than against a black background. The Moon is up exactly as often during the day as it is at night.
- Anyways, during your lunch break, the Moon will be up every day from full moon to new moon. The atmospheric seeing wil change from day to day and when you'll see the Moon cripsly will depend on chance. --Bowlhover 02:25, 7 November 2007 (UTC)
- The only thing you won't see is a full moon in the daytime sky along with the sun. Just about anything else is possible. (But I agree, a daytime moon can be a lovely sight.) —Steve Summit (talk) 03:16, 7 November 2007 (UTC)
- That's not true - at dawn or at sunset, the moon can be on the opposite horizon from the sun and in that case it will be a full moon. Someone reading this is just thinking to argue that it can't be quite 100% full if the sun and moon are both actually visible - and when they do that I'm going to find a large baseball bat and beat them into a quivering heap whilst yelling "AT-MOS-PHER-IC DIFF-RAC-TION YOU ID-I-OT!!". I'm hard - but fair. SteveBaker 03:37, 7 November 2007 (UTC)
- Yep. What struck me about the original poster's report is that when the moon is close to full, it spends most of its time in the night sky and not so much in the day sky. (For a crescent moon, of course, it's the opposite.) So to see the moon 3/4-full close to noon actually is fairly unusual. Arbitrarily guessing Richmond for the poster's location, I plugged some different dates into this page and figured out that the date in question must have been Tuesday, October 30. The moon was 73% full and in Richmond it set at 12:50 pm. And of course the moon being close to the horizon triggers the moon illusion and makes the sighting even more impressive. --Anonymous, 07:33 UTC, November 7, 2007.
- I saw he same thing on the same day at same time from near Dulles airport. I have lived here since 19770. What was unusual about that day was that the air was extraordinarily free of any pollution. What I noticed first was that the sky far from the sun as the darkest I have ever seen at midday, and there was a very obvious gradient (darker to lighter blue) toward the sun. I attribute the lack of pollution to the fact that we had just had a rain after the longest drought in about 20 years. We usually have a lot of urban pollution and a lot of terpene "pollution" from the mountains to the west. The moon was about half-way from the sun to the darkest part of the sky. -Arch dude 17:42, 7 November 2007 (UTC)
Bindeez to GHB??
Apparently these toys can be converted to 4-Hydroxybutanoic acid when swallowed... The toys apparently can be arranged into some pattern and will set when you put water on them..
http://www.theage.com.au/news/national/victoria-bans-toxic-toy/2007/11/07/1194329268448.html
Anyone know what the beads are made of? 1,4-Butanediol according to http://www.news.com.au/perthnow/story/0,21598,22712449-2761,00.html
So how about a conversion mechanism? Assumingly it has acid is involved in the conversion. Been a while since I've done organic chem but this looks like a very simple conversion... How did this ever get on the market??— Shniken1 03:07, 7 November 2007 (UTC)
Obviously first one of the hydroxy groups gets protonated... draws electon density from the carbon... promotes nucleophillic attack..?Shniken1 03:10, 7 November 2007 (UTC)
- Oh well, not to worry - you can still buy them online here: http://www.redsave.com/products/Bindeez-Super-Studio-Design-Centre,,22 (eeeekkkkk!!!!). —Preceding unsigned comment added by SteveBaker (talk • contribs) 03:47, 7 November 2007 (UTC)
- [4]. It's a fairly common chemical reaction, although I'm not sure how this would happen in the body, or if it would necessarily happen in this manner. (and damn you, Steve Baker, your edit conflict somehow crashed my web browser.) Someguy1221 03:52, 7 November 2007 (UTC)
- In the body it looks like it's converted using alcohol dehydrogenase and aldehyde dehydrogenase (from the 1,4-butanediol article. GHB = 4-hydroxybutanoic acid). Maybe someone with more biochem experience can give more info --Bennybp 03:59, 7 November 2007 (UTC)
- The account I read suggested that the toxic versions were made of different stuff to the normal versions, which were a non-toxic glue. So the ones you buy online might be safe. Or not. Skittle 04:01, 7 November 2007 (UTC)
The Counterpartness (Not a Real Word, But You Know What I Mean) of Human Races and Animal Breeds
I know that the topic of whether or not the idea of "human races" is biological or scientific is controversial in general, but what about when comparing them to breeds of animals? The idea of being a "breed of animal" is considered biological and scientific, is it not? And aren't human races and breeds of animals counterparts? And, if so, then why do the words "breed" and "race" exist separately and are not one word, as humans are scientifically animals (I know this has to do with history, but, if my assumptions are right, then why hasn't a word been been developed)? Also, if human races and animal breeds are counterparts, then why is there controversy over whether or not human races are biological and scientific? I know that included a lot of questions, but any help on any part would be great! Thanks! —Preceding unsigned comment added by Pitman6787 (talk • contribs) 05:43, 7 November 2007 (UTC)
- A brief answer, but this undoubtedly go further. As you suggest the origin of the words is undoubtedly historical rather biological as the concepts are similar - it's just another case of a different word being used largely through the antiquated ideas of humans not being animals, and the consequences of that mode of thought. Now if you read the breed article you'll see the second sentence says "For a type to be recognised as a breed, there should be a viable true-breeding population." This has always been a big issues with human races - there is not a viable true-breeding population in any so-called race - basically given any contact there is immediate crossbreeding, and there is huge variation within populations. One of the other significant problems is that definitions of human races are distortions - claimed characteristics of given races rarely, if ever, hold true across that 'race'. Another problem is simply the misuse of race given almost any chance - why look for tenuous and largely non-existent differences and claim them as scientific when they will be mainly used for political and discriminatory purposes, and when the truth is that our similarities are far greater than our differences? --jjron 08:14, 7 November 2007 (UTC)
- "Now if you read the breed article you'll see the second sentence says....", why bother go that far. The first damn line says a breed is a DOMESTICATED subspecies. So by definition, a human population can't be a breed simply because humans are never domesticated. So we are talking about subspecies. And it is thought, biologically, that the differences between the so called, races, are not significant enough to be classified as different subspecies. Different races have different colored skin, some differences in noses, hair texture, and eyes, but that's it. That's not significant enough to classify as a different subspecies. Perhaps if these groups were isolated and remained isolated for hundreds of thousands of years, then we would observe more pronounced differences between the different human populations. 64.236.121.129 21:10, 7 November 2007 (UTC)
- There are several differences between breeds and races, some of which are more a matter of degree rather than clear-cut distinctions:
- For one thing, with dog breeds for instance, in general the genetic variation between individuals within a breed is much smaller than the genetic variation between breeds. This is reversed with race: the genetic variation between races is much smaller than the genetic variation of individuals within one race.
- Looked at another way, dog breeds in general consist of very clear-cut, genetically isolated populations. In contrast, with humans the prevalence of various genotypes and phenotypes often changes gradually over a geographic area, with less in the way of clear-cut boundaries. Because of the above and other complications, it’s very difficult to try to define and test for race genetically. See Race and genetics.
- Within one geographical area, it may feel like there are reasonably clear-cut distinctions between races, if that geographical area contains large groups of people that had been relatively genetically isolated within recent history. For example, it works at least to some degree to group at least a lot of people in the U.S. based on whether most of their ancestors came from Europe, from western Africa, or from southeast Asia. But that moderately workable grouping to a large extent disappears if you consider all the people in the world as a whole, in which there are more gradual genetic variations over geographical areas.
- Another difference between breeds and races is that there is a cultural aspect to race. Whether two populations of humans are grouped as one race or two depends a lot on how culturally similar or dissimilar the two populations are perceived as being. This is yet another reason why it is very difficult to try to define race in any rigorous way. See Race (classification of human beings). MrRedact 08:39, 7 November 2007 (UTC)
Material Properties in outer space
Hi, In hearting about the ISS unfurling their solar array and having it tear and all that - it got me wondering... Aren't these materials near Absolute_zero? Doesn't metal and plastic freeze solid at absolute zero? I guess not. —Preceding unsigned comment added by InverseSubstance (talk • contribs) 06:46, 7 November 2007 (UTC)
- Why do you think that the temperature at the ISS is near absolute zero? The atmosphere at this height is quite hot. Not that this would matter - the temperature of the ISS is far more dependent on radiative heat transfer. Icek 07:06, 7 November 2007 (UTC)
- The problem with this stuff is that somewhere, somehow, some scientist let slip the idea that space is cold. The truth is that space isn't any temperature. Temperature is (essentially) a measure of the average speed that atoms are moving around within some substance. No atoms - no temperature. So let's get rid of that idea. The temperature of spacecraft comes about in the same way things do down here at sea level - you add up the energy coming in (mostly sunlight - but also reflected earth-light and heat generated on-board the craft) - and you subtract the energy going out (by reflection of sunlight, and emission of infra-red light). If there is more energy coming in than going out, the temperature goes up and if more is going out than coming in, the temperature goes down. If the temperature increases, then more and more infra-red light is emitted and eventually you reach a temperature at which sufficient IR light is leaving that the temperature strikes a perfect balance. Similarly if the temperature falls, so does the IR emission and again, the temperature eventually stabilises. This is the same mechanism we have down here on earth - except that we also gain and lose energy by conduction into and out of the surrounding atmosphere and the ground. Also we get more incoming heat from scattered light from the sky.
- OK - so those are the mechanisms. What happens with the solar panels? Well, as sunlight comes in, they heat up, the temperature slowly rises and they heat up - and when the spacecraft orbits around to the dark side of the planet, there is no more sun - so the temperature slowly decreases. The space station orbits (from memory) about once every 75 minutes. So the temperature of the craft goes up for about 37 minutes then cools off for 37 minutes over and over again. The large metal components take a long time to change temperature by much so they are going to tend to settle down to some more reasonable average temperature. So imagine a large chunk of metal down here on earth - if you take a large chunk of steel (a car perhaps) at some nice toasty temperature (because you've been driving it) and park it out in the snow in mid winter, would it cool down to freezing in 30 minutes? I don't think so. It certainly won't cool down to absolute zero over just 30 minutes! In earth, the car is cooled both because it's radiating IR light - but ALSO because it's heating up the air around it - that hot air rises and is replaced by cold air - so that's quite an efficient way for the car to lose heat. But up in space, there is no air - so it can only lose heat by radiation. That means that our solar panel will take MUCH longer than the car to drop to freezing point - and VASTLY longer to cool of to anywhere close to absolute zero - and it's only in the dark for 37 minutes in each orbit.
- Another way to think about this is to watch the movie Apollo 13. Those guys had to live for nearly a week in an unheated spacecraft. The lunar lander got cold - but no so cold that they couldn't survive in their flight suits. Those spacecraft had to be spun so that one side of the craft wasn't perpetually in sunlight and the other perpetually dark. The ISS orbits - so that's not necessary for them.
- Regarding the original question, the relevant metals and plastics are of course already frozen (solid) at room temperature.
- Regarding 70.116.10.189's answer, a little nitpicking: There are atoms - and even molecules - up there, but the density is too low to have a significant impact on the ISS's temperature. Even at more than 780 km height, where ERS-2 orbits, the atmospheric density is at least several hundred particles per cubic millimeter.[5]
- And the space station's orbital period is 91 minutes.
- Icek 14:25, 7 November 2007 (UTC)
Two Questions about Gravity.
Okay. Why isn't there a "nuetral" gravitational force? Okay. Let me explain. I think I was told by a teacher that, if there were no gravity on a planet, then it would be virtually impossible to reach it, as no force would be pulling you in. But, at the same time, that would seem as if there were a "negative" force keeping you away from the planet. But, why would there HAVE to be a force pulling you INTO a planet to be able to reach it, when there is NO "negative" gravitational force keeping you AWAY from it? That doesn't make any sense to me. Why isn't there "neutrality" in gravity?
Also, wouldn't we not have been able prove that gravity existed until we proved there were "gravities" that have different levels of force than Earth's? My thinking is that, until we proved there were "gravities" with different levels of force than Earth's, the only "gravity" that we knew existed was on Earth, with equal gravitational force on everything. So, basically, as far as we knew, gravity had only only level of force, but, knowing that, how could we prove it existed? I wish you could see into my mind, as my thinking is much better than that --- I just can't put it into words. It's like, since we knew there were different levels of force, we knew there had to be a force at all. Okay. Lets say humans were the only were the species that existed (work with me here), and we still classified the human being as a "species" --- like saying gravity existed when there was only, as far as we knew, one level of gravitational force. So, if there were only one type of species, then how could we prove that the concept of a "species" existed? Or, let's say EVERYTHING in the universe moved at the exact same speed (work with me here). We couldn't prove that a concept of "speed" existed, as, only one speed existed, right? We use the word "speed" because there are different speeds. If everything moved at the same speed, then we couldn't say "He's running fast" or "He's running slowly" or "He's moving at [number]/[unit] [length of time]." We could only say "He's moving", right? Or, if there were only color in the universe, we couldn't call it a color, right? We couldn't prove that the concept of color existed, unless we found a different color, right? It's like, if EVERYTHING in the universe had a quality about it that was the EXACT SAME, then how could you call it or prove that it was a characteristic at all? --- there would only be one type. I hope you know you what I mean. And I'm not saying that, even if there were only one level of gravitional force in the universe, that gravity wouldn't exist. I'm just saying that, before we found out that there are different levels of gravity, we really didn't have any proof that gravity existed --- knowing that there are different levels of gravity REALLY proves that there some kind of force exists, right?
Any help would be great! Thanks! —Preceding unsigned comment added by Pitman6787 (talk • contribs) 06:58, 7 November 2007 (UTC)
- Re the first question, that's nonsense. Firstly you wouldn't have a planet without gravity, but let's just say you did. Saying that you couldn't get there because the gravity wouldn't pull you in is like saying you can't navigate to an arbitrary place in empty space because there's nothing pulling you in - but you can navigate through empty space to any given point. Your instincts are right, that there's nothing preventing you getting there. I'll leave the second question to others. --jjron 07:54, 7 November 2007 (UTC)
- I would say for the second one, the problem is the inability to test variables that are beyond our capability to create or measure. The ancients knew well that gravity pulled anything that wasn't too light towards the center of the earth, but until Newton, no one figured a way to test gravity outside of the earth. And then finally, Henry Cavendish found a way to both eliminate the earth's gravity from affecting measurement, and measure the gravity between metal spheres. Similarly, until the 20th century, testing the extremes of small distances and great velocities was beyond anyone's capabilities. As such, while we were certainly capable of imagining classical mechanics as applying just the same to such conditions, this was entirely not the case. When people found ways to test them, they confirmed laws that changed the way physicists thought about the world. Someguy1221 08:39, 7 November 2007 (UTC)
- Excellent questions ! Why does the gravitational attraction between two bodies depend on their masses but not what they are made from ? Why isn't there such a thing as anti-gravity paint ? Why doesn't anything have negative mass (as far as we know) ? Why is gravitational mass equivalent to inertial mass ? It was thinking deeply about these sorts of questions that led Einstein to the general theory of relativity in which gravitational forces are due to the curvature of spacetime. Gandalf61 10:57, 7 November 2007 (UTC)
- Yes, you do some real thinking. What you say about our not seeing a thing that has no variation is quite true. That's part of the reason it took so bloody long to figure it out. Hats off to old Isaac, eh? You might be the one to see the remaining things of that kind. Keep us posted. --Milkbreath 12:17, 7 November 2007 (UTC)
- We (well, people like Kepler and Newton) observed the orbits of other moons and planets and deduced from that that there must be a force attracting planets and comets towards the sun and a force attracting moons towards the planets. It's not much of a stretch to deduce that the smaller, lighter things are producing less force than the bigger, heavier things. You can go on to calculate how much force the earth is exerting on the moon - and with a sufficient leap of imagination - that the apple that just fell onto your head was being pulled by the same exact force (well, the apple story is probably apocryphal - but you get the idea). From the outset, we knew of many different sources of this mysterious attractive force. It was hard to quantify it because you can't easily figure out the mass of something that you can only see through a telescope as a blurry dot - but it must have been very clear that the gravity due to (say) Jupiter was vastly greater than that of Mars because you can SEE that Jupiter is huge, even in a primitive telescope. So they knew there was some kind of size/strength relationship. They could also figure out the decreasing pull of gravity as a function of distance. It took more careful experiments with big iron balls and very accurate measurements to get the details and the math exactly right - but the fact that there were differences and that the earth wasn't the only thing that had gravity must have been known for a very long time. SteveBaker 18:56, 7 November 2007 (UTC)
- I wouldn't say so. While the idea may have occurred to people, the Aristotelian/Platonic system of celestial spheres was widely accepted (in the West) until Galileo began his astronomical observations in the early 17th century. Someguy1221 19:02, 7 November 2007 (UTC)
Omega-3 fatty acids in water-inhabiting animals
Why are omega-3 fatty acids so prevalent in water-inhabiting animals? They are prevalent in unrelated species, like whales, fishes and crustaceans. I guess they are produced somewhere near the bottom of the food chain. Is there some evolutionary advantage for omega-3 producing water-inhabiting animals? Icek 07:13, 7 November 2007 (UTC)
- Quite a few prevelant microalgae produce omega-3 fatty acids. And yes these are on the bottom of the oceanic foodchain, so anything consuming them will also be rich in omega-3 fatty acids. As a matter of fact many fish can barely synthesise their own omega-3 fatty acids, and thus are almost fully dependent on microalgae. As to the evelutionary advantage of producing these specific fatty acids as opposed to producing others I do not know. PvT 12:54, 7 November 2007 (UTC)
Correct design?
Little Boy design is shown as having a round bullet with a cylindrical hole being fired at a cylinder shaped plug that fits in the hole. This arrangement seems counter intuitive to firing the cylinder plug into the cylindrical hole so as to avoid the problem of a rounded projectile twisting out of alignment versus the plug only being able to rotate in the gun barrel. Why was the arrangement shown used instead of the other way around? Clem 07:51, 7 November 2007 (UTC)
- Little Boy#Counter-intuitive design. I think your link answered your own question. Someguy1221 08:43, 7 November 2007 (UTC)
- The first picture does not seem to show the projectile as a hollow cylinder but rather as a hollow ball.
Even with the alignment question answered it is still not totally clear to me why the hollow cylinder has to move instead of the plug....until you include the Tungsten-Carbide tamper as part of the movable projectile or stationary target. Clem 09:12, 7 November 2007 (UTC)
- The first picture does not seem to show the projectile as a hollow cylinder but rather as a hollow ball.
- I think the first picture is just meant to be schematic in a cartoony way. --24.147.86.187 15:30, 7 November 2007 (UTC)
- The page basically says that the larger area is actually more than one critical mass (which is certainly true) but kept from being critical by being in a tube shape (it has a hole in the middle so the uranium isn't too close to itself). If you put that into the large neutron-reflecting tamper you'd run the risk of too many neutrons spontaneously floating around and creating a criticality accident. So you keep that part far away from the neutron reflectors, and only when you bring it together is it subjected to their reflection. I don't know if that is true or not but it sounds plausible to me. You'd have additional problems created by such an arrangement but it might let you use more uranium to compensate. --24.147.86.187 15:33, 7 November 2007 (UTC)
- One of the scarier experiments they did when designing that bomb was described in one of Richard Feynman's books - they actually dropped the cylindrical tube part over the core part experimentally to try to measure the amount of neutrons that would be produced - but they arranged that the tube would not stop when it was in the right position for an explosion - but merely keep on going. The theory being that if you could do this fast enough, you could avoid the chain reaction getting big enough to make a nuclear explosion. However, since they weren't 100% sure what the outcome of the experiment would be until they did it, it was always possible for something to go horribly, horribly wrong! Needless to say, this was a pretty dangerous experiment because if anything got stuck or moved just a bit too slowly...KABLOOIE! The building where they did those tests was way, WAY out in the desert! SteveBaker 18:28, 7 November 2007 (UTC)
- Actually, they managed to kill at least a couple of people working on the Manhattan Project through careless manipulation of near-critical masses of radioactive material. See Criticality accident#Incidents, Harry K. Daghlian, Jr., Louis Slotin. TenOfAllTrades(talk) 19:01, 7 November 2007 (UTC)
derivation of phrase sex maniac
would like to know what the specific derivation is as not common in english language to use maniac with other terms eg dont describe someone as a chocolate maniac or a drug maniac.86.4.107.45 10:05, 7 November 2007 (UTC)
- See Hypersexuality. The concept of hypersexuality replaces the older concepts of nymphomania (or furor uterinus) and satyriasis. Lanfear's Bane | t 10:37, 7 November 2007 (UTC)
Chicken-eating spiders
To my astonishment, I found this statement in the Raising Chickens Wikibooks:
"In south America there is a type of spider that will prey on chickens"
What kind of spider is that? It must be huge... --Taraborn 11:10, 7 November 2007 (UTC)
- The Goliath birdeater is native to South America and seems to be a contender for the largest spider in the world [6]. However, despite its name it seems it does not normally eat birds - our article says it got its name because one specimen was once seen to eat a hummingbird. So very doubtful that it, or any spider, could prey on chickens. Gandalf61 12:16, 7 November 2007 (UTC)
- ...said he a moment before the Gargantua wizardeater, a species as yet unacknowledged by Western science, sank its glistening poisoned fangs into the nape of his neck. --Milkbreath 12:47, 7 November 2007 (UTC)
- Don't forget good old Shelob. Lanfear's Bane | t 13:15, 7 November 2007 (UTC)
- It can prey on a chicken and still be a pretty modest spider. If it has a venomous bite - then it could certainly kill a chicken and yet be pretty small - that a small spider might eat a small amount of a dead animal might not be surprising. I don't think we can dismiss this just because we can't imagine a two foot spider pouncing on the chicken and ripping it limb from limb with inch long fangs! SteveBaker 18:20, 7 November 2007 (UTC)
Civil engineering: Factor of safety?
in Civil engineering- what is a Factor of Safety ? and why do we use it ? —Preceding unsigned comment added by 82.69.206.166 (talk) 12:26, 7 November 2007 (UTC)
- Welcome to Wikipedia. You can easily look up this topic yourself. Please see factor of safety. For future questions, try using the search box at the top left of the screen. It's much quicker, and you will probably find a clearer answer. If you still don't understand, add a further question below by clicking the "edit" button to the right of your question title. .--Shantavira|feed me 13:44, 7 November 2007 (UTC)
- Put simply: If you are building (say) a bridge and the heaviest truck you expect to cross it weighs 20 tons, you shouldn't build a bridge that can only support 20 tons because you know damned well that some idiot is going to drive a 21 ton truck across it - and you probably suspect that the steel you built it with is only 95% of the strength they said it would have at the steel mill, so it'll only really support 19 tons anyway. Then you know that the bridge will corrode some over it's lifetime and maybe the crew who build it make a little mistake and don't put the rivets quite close enough together - and maybe you didn't realise that in 100 years time, the bridge will have been painted 10 times and so it's now got half a ton of paint to support as well as that truck. So right at the outset, you look at the 20 ton limit and you say to yourself "I'd better build a bridge that can support 30 tons - and that way I'll be OK no matter what". The extra 10 tons you added is the "Safety factor" for the bridge. Of course you shouldn't over-do this because it's likely that a bridge that can cope with (say) 40 tons will cost twice as much as one that'll only support 20 (or was that 19?). That's all there is to it - nothing complicated. SteveBaker 18:01, 7 November 2007 (UTC)
Too obvious to be realizable?
What is the process by which plant cells transform carbon to oxygen, and can it be synthesized? If so, is that technique applicable to reducing carbon in our atmosphere? If not, how unrealistic an idea is this? Beekone 14:04, 7 November 2007 (UTC)
- Photosynthesis is the process by which plants use light energy to convert carbon dioxide and into oxygen and glucose. Your idea of carbon sequestering is a good one...see also our Carbon dioxide sink article. DMacks 14:23, 7 November 2007 (UTC)
- See photosynthesis for details on the process. Note that this reaction requires energy (sunlight) to occur, so we would need enormous amounts of energy for significant results (think of how many trees there are in the world, and multiply that by at least 100 to get the total weight of algae, which produce about 80% of our oxygen). From the article, "Through photosynthesis, sunlight energy is transferred to molecular reaction centers for conversion into chemical energy with nearly 100-percent efficiency. The transfer of the solar energy takes place almost instantaneously, so little energy is wasted as heat." This means that not only would we need huge solar panels or lots of nuclear plants to do this cleanly, but we still would be nowhere near as efficient as plants. That's not to say it isn't possible: certainly the reaction produces food on the order of Soylent Green in artificial sugar-rich goodness, which might be very economical in the future. But as far as significantly impacting the CO2, I sincerely doubt it would have much effect. We're better off by first and foremost switching all our power plants to renewable and nuclear sources, which would be necessary for the reaction to have net effect anyway. SamuelRiv 14:30, 7 November 2007 (UTC)
- Since when was photosynthesis efficient? Biological processes are notorious for being inefficient. I heard a retired biology professor say that photosythesis was inefficient. 64.236.121.129 16:22, 7 November 2007 (UTC)
- Depends what you mean by "efficiency". To quote the article: "This chemical energy production is more than 90% efficient with only 5-8% of the energy transferred thermally." but "Not all wavelengths of light can support photosynthesis.". DMacks 19:21, 7 November 2007 (UTC)
It just struck me as such an obvious solution that surely someone had thought of the implications. I thought I'd leave it up to you guys to drop some knowledge on me. Thanks for pointing me in the right direction, very interesting stuff! Beekone 14:52, 7 November 2007 (UTC)
- I do vaguely recall some experimental setup with large polythene bags full of some liquid that you'd lay out in the sun and bubble air through to capture the CO2 and produce some kind of useful product - it's not in widespread use though which suggests that there was some problem with it. But the answer is simple enough - cover a greater percentage of the earth's surface with plants (restoring the sahara to greenery would help for example) and stop cutting down the rainforests and starting forest fires! You can even harvest the plants (so long as you plant new ones immediately afterwards) and either use them to make ethanol/biodiesel to run your car sustainably or (if you actually want to sequester CO2), toss the dead plants into an anaerobic landfill someplace (so they don't produce methane as they rot).
- Sadly, the very effect we're trying to prevent (global warming) is the exact same effect that's causing deserts to increase in size and making it harder to grow crops in those kinds of places. SteveBaker 17:52, 7 November 2007 (UTC)
Psychology: person A evaluates B by the evaluation of C, D, E.... for person B.
This happens quite often. Is there a name for this please? And are there any known personality traits of person A that make them more likely to indulge in this? Thanks 80.2.214.75 15:19, 7 November 2007 (UTC)
- Could you give an example, or a more detailed question? I'm having a lot of difficulty understanding what concept you might be looking for. On the off chance that I've got it right, is projection the term you seek? TenOfAllTrades(talk) 16:37, 7 November 2007 (UTC)
- This sounds like gossip to me! That is, making judgements about people solely based on what you've heard others say about them. 83.249.121.85 16:41, 7 November 2007 (UTC)
- "Checking a job applicant's references"? -- Coneslayer 16:42, 7 November 2007 (UTC)
An example: when I was at college girl X was attracted to me in proportion to the amount of esteem that she observed other people had for me; while girl Y was attracted to me irrespective of what other people thought. I am curious about how girl X and Y may differ in personality. I have seen this in men also: I was interviewed for a job once by the boss of my future boss, and when my future boss saw that his boss was taking an interest in me, his behaviour towards me improved greatly. I imagine that this behaviour is associated with authoritarianism, in-groups and out-groups, perhaps Machiavelimism and so on.
Another example could be germans who evaluated people of the jewish faith according to nazi propoganmder, and others who were more resistant to the propoganda. Another example is here http://news.bbc.co.uk/1/hi/scotland/north_east/6273231.stm where it says women are attracted to men according to the attention they receive from other women.80.2.211.126 23:12, 7 November 2007 (UTC)
Energy
We are told that energy can neither be created or destroyed but say a person throws a ball which eventually comes a complete stop, where has that energy gone? —Preceding unsigned comment added by 195.188.208.251 (talk) 17:53, 7 November 2007 (UTC)
- It all winds up as heat, thermal energy. Whenever energy vanishes from a mechanical system (throwing a ball is a simple such system), it mostly becomes heat. Someguy1221 18:00, 7 November 2007 (UTC)
- Look at potential energy, and also energy in particular 'forms of energy'. it has a bit on a bouncing basketball. and further down the conservation of energyny156uk 18:02, 7 November 2007 (UTC)
- Yes - and in the case of the ball, what happened was that the successive final bounces of the ball deformed the rubber that it's made of and that caused the rubber to heat up and the ball to bounce a little less high the next time - the ground too would flex a tiny bit and gain some heat at the expense of the ball. Also every time it bounced, the ball made a noise - which required some of the kinetic energy to be consumed in making the air vibrate - but the moving air has some internal friction which heats the air up a tiny bit as the sound dies away. In the end, it's turning all of that interesting kinetic energy into generalised, diffused heat. But it might take a while - we can come up with some scenarios in which it takes an INSANELY long time to finally turn all of the energy of the ball into heat. Suppose the ball landed on (of all things) a WintOGreen flavored Life Saver and crushed it. A weird property of that particular candy (called triboluminescence) would actually result in a small pulse of blue and UV light to be radiated outwards as the candy was crushed. Of course as that light is absorbed, it too will turn into heat - but a small fraction of the light would head upwards, escape from the earth's atmosphere and head out into space. It could be trillions of years before the light hits a stray bit of cosmic dust - but when it does, you just get some heat. This tendancy for everything interesting to eventually turn into heat is called Entropy and it's really rather depressing. Can we talk about something else now? :-( SteveBaker 18:15, 7 November 2007 (UTC)
Related question
If one were to invent a 100% perfectly efficient machine, would it not produce any sound, as sound would be a result of friction and wasted energy? -- MacAddct 1984 (talk • contribs) 19:36, 7 November 2007 (UTC)
- Yes, exactly. (Except of course if the machine were designed to produce sound, like a loudspeaker or musical instrument.) —Keenan Pepper 19:49, 7 November 2007 (UTC)
The way intelligence grows on the "nurture" side
Hi! After studying the "nature vs. nurture" aspect of intelligence in a high school psychology class, I came up with my own "theory [in respect that I have never read or heard anything related to the ideas in this "theory" before]" on how intelligence grows on the "nurture" side of things. I know this isn't the place to post personal theories and want them to be proven or disproven, but, after thinking about this one, I thought it was a basic enough concept that it would have to have had already been proven or disproven by now --- so I am now simply looking for someone to confirm or deny this.
Okay. My "theory" is that, indirectly, the more you know, the more intelligent you are --- not simply because you know more, but because, knowing that you DO know more, we know that you have used your brain more, which is the reason you're more intelligent. I am relating this to physical fitness. Physical muscles [as opposed to the mental muscle (i.e., the brain)] grow --- along with phsyical fitness level --- as you exercise more. Why wouldn't the same be true with the brain, except with gaining more knowledge other than exercising? The brain is a muscle, too, right? And, so, using that muscle more (i.e., gaining more knowledge) would increase THAT muscle's competency too, right?
Going along with this, I know a lot of people criticize I.Q. tests, saying "Well, the I.Q. test included questions with information I just never learned before! It wasn't fair! Just because I never learned information doesn't mean I'm less intelligent!" But I criticize that criticism, with the information I posted above. Also, or people will say "Just because I didn't receive that high a level of education doesn't mean I'm not intelligent". But, again, I am trying to refute that.
And I think that the statements in the paragraph above are made and thought of because of people's emotions. I know that it isn't always people's faults that they didn't receive that high of a level of education --- and because it wasn't their fault, it would be "mean" to think a person is less intelligent because of a reason they couldn't help. But, whether or not you can help your level of education, (according to what I am thinking) the higher the level of education you have, or the more knowledge you know, the more intelligent you --- indirectly. Again, not because you learned and know more but because you used your brain --- a muscle --- more, which increased its competency.
And I do realize that, with the "nature" side of intelligence, people can still be intelligent, despite low level of education, or lack of knowledge, as their base --- or genitic factor of --- intelligence might have been high at birth. I am just saying that knowing more information is a GENERAL indication of a higher intelligence.
Again, I thought this was a basic enough concept that it had to have been proven or disproven by now. So, which is it? —Preceding unsigned comment added by Pitman6787 (talk • contribs) 19:37, 7 November 2007 (UTC)
- Just giving you a few of my thoughts here. Anybody who exercises more, will grow more muscles, but only a few can become top athletes - the ones that have excellent genes. Yes, if you know more, you will get a higher result on some intelligence tests, but people differ in how much information they can gain and reproduce (and by testing this you would rather test memory instead of intelligence), and, more importantly, having a huge fund of information doesn't mean for instance improved reasoning skills or perceptual abilities, which also are considered to be important parts of intelligence. —Preceding unsigned comment added by Lova Falk (talk • contribs) 19:48, 7 November 2007 (UTC)
- I'm not sure if you saw this page section: Intelligence quotient#Environment. --JWSchmidt 20:59, 7 November 2007 (UTC)
- Re: education. I don't think anyone would argue that a mind well-tested as a mind sharpened. If you are in the habit of dissecting logical arguments, for example, you are going to be better at it than if you were not in that habit. The question is whether that measures what we think of as "intelligence" or not. Expanding that definition to include education makes sense in some cases, but not in others. Look at the test questions from the Army Alpha Intelligence Test (1914-1918). Try and tell me with a straight face that knowing who Rosa Bonheur is, what product Velvet Joe was the mascot for, or what a Wyandotte is measures anything other than exposure and retention of simple factual information? Those are the extreme examples, but everything else in the "informational" section falls under that category; it's entirely possible to be quite bright and just never learn where exactly the pancreas is in one's body; and on the converse it's possible to be quite dumb and know simple things like that. It's well known that total dolts can excel at bar-room trivia; that's not a measure of intelligence of any sort. So clearly some line must be drawn between being asked questions that are purely "educational" and those that are not; knowledge of miscelleanous facts is not what anyone generally considers "intelligence" to be. --24.147.86.187 01:08, 8 November 2007 (UTC)
- Re:Sorting it out. We have somewhat sorted out, the answer is "it's complicated, and it's something of an artificial question." Genes provide the template for an organism which then must develop in an environment; they are not mutually exclusive categories in many respects. They are in a complicated relationship, some times more complicated than others. With very simple traits (single-point mutations) it is not too hard to untangle in most cases; when talking about more complicated genetic traits then development starts to play a big role. When talking about something like "intelligence", the exact definition of which is quite murky, then it becomes exceptionally hard to untangle, and many environmental and genetic factors start being at play. But it isn't a simple "x% this, x% that" sort of equation; it's the complexity that comes from having an organism develop, where genetic code tells individual cells what to do, but somehow we are supposed to understand complicated aggregate behavior of an entire organism. It's not that we don't know how to sort some of these things out, but sorting them out in a straightforward way has proven pretty difficult, especially if we are talking about groups that exist in complicated social dynamics that cannot be really reasonably escaped for the purposes of testing. --24.147.86.187 01:08, 8 November 2007 (UTC)
Human Evolution
Well, Heroes is on again tonight. I was just wondering if there are any clues as to how H. sapiens will evolve in the medium term. Obviously i am not expecting spontaneous levitation, telekenesis or other such nonsense - more physiologically. Are humans now capable of purposefully selecting a route of evolution to their own ends? --russ 20:13, 7 November 2007 (UTC)
- The things we are evolving are likely to be small tweaks that make us better suited to our environments...not major stuff like being able to fly. One example of a "recent" evolved change has been adult lactose tolerance. Most other mammals (and humans as recently as the ancient Egyptians) are lactose intolerant as adults. When we started farming and milking goats and cows, there was a clear advantage for humans who were able to digest milk. So we started to evolve lactose tolerance and the lactose intolerant people started to decline in numbers. However, in our modern world, it seems very unlikely that you could die without leaving offspring just because you are still lactose intolerant - so it seems like that genetic change hasn't spread throughout the population yet. It wouldn't surprise me if we started to adapt in ways that helped us in the modern world...but genetic changes take thousands of years and our environment has changed so dramatically in just the last 100 years that we havn't had time to evolve to support that change. If I had to go out on a limb and predict something, I'd suggest that we might evolve a way to avoid obesity. That kills off a lot of younger people and certainly reduces their attraction to the opposite sex - so it ought to have a serious impact on reproductive capability. If a gene came along at random that helped avoid that problem then I imagine it would sweep the western world in a matter of just a few thousand years. But you can't KNOW that this will happen.
- As for whether we are purposefully driving evolution - in a sense, we always have. By being selective with whom we mate with - we make that choice quite a lot. In terms of using gene replacement therapy we MIGHT make a change. As a matter of fact though, it's possible that we're inadvertently screwing things up rather badly. Think about this. What happens if a married couple want a baby and can't have one? They go to the scientists and magic wands are waved, incantations spoken and (in quite a few cases), a baby is the result. In fact, multiple babies may result in an unnaturally large percentage of the cases. So if there were a genetic cause for infertility that would normally be strongly selected against, we could quite easily accidentally make it not only NOT be selected against - but because of the possibility of multiple births due to fertility drugs, we might even make genetic infertility become a trait that tends to increase over time. That's pretty worrying - but it's exactly the kind of thing that we'd be just stupid enough to do.
- SteveBaker 20:27, 7 November 2007 (UTC)
- The ancient Egyptians were lactose intolerant? Them and 70% of adults alive today. Algebraist 20:41, 7 November 2007 (UTC)
- That 70% seems very high. Even more so are some of the population-specific numbers -- none of the many various Asian Americans I know have ever identified themselves as lactose intolerant, and I've certainly seen them consuming cow by-products before. jeffjon 21:21, 7 November 2007 (UTC)
- The ancient Egyptians were lactose intolerant? Them and 70% of adults alive today. Algebraist 20:41, 7 November 2007 (UTC)
- If milk causes such problems in so many people, why is it so popular? Do only 30% of the population drink milk? Surely people wouldn't drink it if they were afflicted? --212.204.150.105 21:18, 7 November 2007 (UTC)
- The degree of lactose intolerance is highly variable in both its severity and its age of onset. Some people don't begin suffering until their 30s or 40s, and even then, many lactose intolerant individuals can drink a cup or two a day without suffering any symptoms. Someguy1221 21:31, 7 November 2007 (UTC)
- 70% has got to be *WAY* too high. The article says that there are multiple causes - we're talking here about people who are congenitally lactose-intolerant as adults - not people who are that way because of diseases. But even so, there simply is no way that number can be right in any of the parts of the world I've lived in. Out of all of the people I know well enough to have been able to tell - at least 20 or 30 people - I can only think of one who suffers from that condition. If it's 70% then the number should be 15 to 20 of them...there is no way I can not know those people that badly. We've had every one of them over to our home for dinner on multiple occasions - my wife's French - everything has cream sauces and such. No - it's not 70% in Europe or North America. It's gotta be way less than 10%. 70.116.10.189 23:38, 7 November 2007 (UTC)
- If 70% seems way too high to you, it’s because of which people you’re typically exposed to. The mutation that allowed lactose to be digested by adults happened quite recently (on an evolutionary scale) somewhere in northern Europe. So if you hang out around people of primarily northern European descent, then the 70% is going to seem high to you. But if you lived in China or Thailand, that figure would seem very low to you. MrRedact 00:32, 8 November 2007 (UTC)
- 70% was for the whole world. See Image:LacIntol-World2.png. Someguy1221 00:34, 8 November 2007 (UTC)
- 70% for the world seems right when you factor that there are two large groups that traditionally had lactose-intolerance issues - Africans and Asians. There are a hell of a lot of Africans and Asians in the world, compared to Europeans which have less of an issue traditionally. Americans have a far, far lower rate of lactose intolerance - probably due to various worldwide cultures intermixing genes to allow them to spread faster. Kuronue | Talk 04:54, 8 November 2007 (UTC)
- Historically, the idea of humans "purposefully selecting a route of evolution to their own ends" has often been a bad idea, especially with Nazi eugenics. MrRedact 00:49, 8 November 2007 (UTC)
- Of course, if we have eliminated natural selection by using fertility clinics, that makes genetic research that much more important. It seems to me that if we want to survive as a species, eventually we are going to have to manipulate our genes, either before or after we are born. 68.231.151.161 01:49, 8 November 2007 (UTC)
- I'm not sure that we need any help on that. See also Population, Carrying capacity, Sustainability, and Earth. I think the better question is why are we doing so well as a species, and at what point should we stop trying to let people live longer. All things considered, we're simply going to run out of physical space in which to fit everyone. --slakr\ talk / 04:40, 8 November 2007 (UTC)
- Some argue that humanity’s ecological footprint already exceeds one Earth.[7] But there’s no problem with still trying to let people live longer, as long as we simultaneously reverse the population explosion through voluntary population control measures such as improving access to family planning and reproductive health care and information, eliminating incentives to have larger families, and public education about the consequences of continued population growth. MrRedact 05:21, 8 November 2007 (UTC)
Special Wheel Supported on the Lower Rim
I remember seeing somewhere a wheel that was supported on the rim below the wheel's center point. The vehicle using the wheel had no axles. I assume the rim at the point of support had some kind of bearings. The wheel had nothing inside the rims. This had the benefit of lowering the vehicle's center of gravity. Can anyone point me to a reference for this arrangement? ThreeE 20:54, 7 November 2007 (UTC)
- Have you tried Hubless wheel? --TrogWoolley 22:31, 7 November 2007 (UTC)
- Perfect. Sbarro (http://www.burningart.com/meico/moto/sbarro/) was the designer I was looking for. Many thanks. ThreeE 23:38, 7 November 2007 (UTC)
Why is the waste disposal system the same as the reproductive system?
Not the same, biologically speaking, but using the same body parts. Why is it like that? Do you peeps think it has some kind of evolutionary function or it's merely a bad design that just evolved that way because it works without any major malfunctions (although there are sometimes malfunctions). 64.236.121.129 21:33, 7 November 2007 (UTC)
- Er, not sure what you mean. In mammals the reproductive system is separate in all respects except for the dual-purpose male phallus. In other vertebrates the two systems do vent at the same place, the cloaca.--Eriastrum 23:12, 7 November 2007 (UTC)
- And the reason for that arrangement is basically that the original chordate body plan consists of a muscular tube with a hole at each end. Any elaborations on that, such as limbs or extra orifices, are later additions, and are unlikely to occur unless there has been some selection pressure for them at some point. We can thus deduce that, for example, at some point in the evolution of placental mammals there was an evolutionary advantage to females having a separate vaginal opening. Figuring out what the cause of the pressure might've been is left as an exercise for the reader. —Ilmari Karonen (talk) 01:12, 8 November 2007 (UTC)
- You don't know what I mean? Drop your pants and see where your sexual organs are, and where your waste disposal organs are. DUH. Come on, this ain't rocket science peeps :). 64.236.121.129 15:31, 8 November 2007 (UTC)
- I think this argument is questionable. Sexual reproduction exists outside of phylum Chordata and presumably predates it, so one might as easily discover that the development of a spine was an elaboration on some proto-tubeworm's reproductive system. I don't know what the relationships actually were, but they are presumably very, very old. Dragons flight 03:10, 8 November 2007 (UTC)
- That could well be; the point I was making is that the default condition inherited from our proto-chordate ancestors is "one hole in front, one in the rear". That our present body plan, in many ways, still resembles this ancestral model should not be surprising; rather, it's the deviations from this basic body plan that require an evolutionary explanation. Presumably, then, the reason the human reproductive organs are close to the anus is that they used to share the same opening (the cloaca), and that there hasn't been sufficient evolutionary pressure to move them any further from each other than they are. —Ilmari Karonen (talk) 22:09, 8 November 2007 (UTC)
I Cannot think of a good reason why poop is RIGHT by the fun stuff! PS dude above, MAMMALS: NOT SEPERATE at all...Hands and feet,..now that's seperate —Preceding unsigned comment added by 76.168.69.208 (talk) 02:09, 8 November 2007 (UTC)
I'm actually curious as to this as well. Not just humans - most female mammals that I'm familiar with have both vaginal and anal openings within a few inches of each other. Considering how irritated the vaginal opening can get when feces is accidentally introduced (personal experience here - always wipe front to back!), this seems counter-intuitive. Kuronue | Talk 04:58, 8 November 2007 (UTC)
Perhaps, there were some advantages in the prenatal development of these organs that let it evolved in this way. See Development of the urinary and reproductive organs. What about digestive and respiratory systems? They seem to have some overlaps as well. --Vsion 05:43, 8 November 2007 (UTC)
- Because this enables humans to cover for most eventualities with just one item of underwear.--Shantavira|feed me 09:07, 8 November 2007 (UTC)
- I think it is merely just a bad evolutionary design that stuck merely because it worked satisfactorily, although it is anything but ideal. 64.236.121.129 15:36, 8 November 2007 (UTC)
- You shouldn't think of evolution as a design. No men in white suits mulling over the latest design models for the new species. Evolution happens by accident, and for things to change a mutant child must be born whose genetalia are farther removed from their bowels. Maybe move them up to the stomach? It'd be more convenient for child birth. (Every time I think of it, an image pops into my head from the original Alien movie) Man It's So Loud In Here 21:48, 8 November 2007 (UTC)
- Looks like a devine example for unintelligent design
--Cookatoo.ergo.ZooM 19:21, 13 November 2007 (UTC)
- Looks like a devine example for unintelligent design
series of vaccine for rabies
Following an encounter with a rabid fox, I received a series of vaccine for rabies as treatment. A month later I was planning to donate blood at the local Red Cross. Prior to my scheduled time at the Red Cross, I had contacted a physician who had said that it was okay for me to donate my blood. However, the people at Red Cross thought differently and told me that I have to wait up to one year to donate again. Do you know how long a person who has received the series of vaccine for rabies needs to wait and why?Pumpkin68 21:37, 7 November 2007 (UTC)
- Time limits are pretty much arbitrary. The (U.S.) Department of Defense concurs with your Red Cross information: they require a wait of 1 year before blood donation if the rabies vaccination was given for rabies exposure (an animal bite, for example); there is no wait if the rabies vaccine was given for other reasons, and the donor is symptom-free. Thus the wait seems to be dictated by the animal bite and not the vaccine. - Nunh-huh 22:27, 7 November 2007 (UTC)
Are other mammals attracted to female mammary glands?
Human males are attracted to girls with big boobs. Is there any scientific evidence that shows other mammals attracted to mammary glands? Like maybe a male Bull poking its nose against a cow's big puffy thing at the bottom (utter? whatever you call it). 64.236.121.129 21:45, 7 November 2007 (UTC)
- Not really what you're looking for at all, but slightly related: Koko, the female Gorilla that liked to see and touch human nipples (both male and female). jeffjon 21:57, 7 November 2007 (UTC)
- It is my understanding that the male attraction to breasts is part genetic, part culturally trained. The genetic part I always assumed worked on the basis that larger breasts made a female look like they would produce enough breast-milk to rear a large family - thus making them more attractive to the males who wish to spread their seed. Now i cannot say this is scientific, i probably heard it some place and took it as true, and i'm sure there'll be science that shows breast-size has little impact on the amount of breast-milk a woman can produce, but that has been my idea. If this angle is correct then other animals will have an interest in any female/male attributes that show-off to the group/pack that they are fertile/strong, whatever the other sex wishes them to be. So things like Peacocks showing off their fantastic tails to attract peahens (lots of birds have coloured plumage to attract other birds) or the male lions fighting for pack-dominance because that will often lead to them having the best female-mates. Look at sexual attraction ny156uk 00:41, 8 November 2007 (UTC)
- I think I can say with a great deal of certainty that other mammals are not attracted to female mammary glands. Check out our cleavage article. And be sure to read the linked citations too (actual pages from the book, I think). --Cody Pope 02:32, 8 November 2007 (UTC)
- I think I've heard it speculated that the phenomenon of enlarged female breasts in homo sapiens is an example of an evolutionary arms race, or more specifically, runaway evolution. (This is basically what ny156uk was saying.) —Steve Summit (talk) 03:16, 8 November 2007 (UTC)
- All other mammals are attracted to mammary glands. That is the single defining behaviour of mammals (the clue is in the name!) However, in most mammals the attraction wanes considerably on weaning. As far as we can tell, only adult humans consider breasts sexually attractive. Incidentally, investigating how and why mammals find breasts attractive (for suckling, not for staring at in Playboy) and the sensory mechanism through which that attraction is mediated, is what I do for a living. We have some interesting and surprising findings, but I'm afraid I can't tell you what, is it is not yet published. At work today I spent the afternoon timing babies crawl around on naked female breasts. Its a tough job! Rockpocket 09:07, 8 November 2007 (UTC)
- Cats which like milk would be attracted to the smell of milk from a lactating female human. Edison 13:11, 8 November 2007 (UTC)
- Unweaned mammals (meaning young which still want to nurse) don't seem to care where the meal comes from, as long as they get fed. Simple experiment: Have a post-puberty female human pick up a nursing puppy or kitten. Expected result (because I've seen it many times): If allowed, the puppy or kitten will attempt to nurse. A hungry baby mammal just doesn't care who or what owns the teat; he/she/it just wants a meal. The news is full of cross-species nursing. Even our own legends (founding of Rome) are full of it. -SandyJax 16:13, 8 November 2007 (UTC)
- That isn't entirely true, the literature shows that the smell of milk from different species does not always promote suckling in others, irrespective of Romulus' experience. For example, its the smell of 2-methylbut-2-enal in rabbit milk the promotes suckling in rabbits. Human, cat or mouse milk does not promote suckling in rabbits because, presumably, they do not express 2MB2 in their milk. The suckling promoting odors of other mammals have not yet been identified. Rockpocket 19:20, 8 November 2007 (UTC)
- I once read a story in the newspaper about a woman who breast-fed a litter of orphaned kittens. That is about all I have to contribute to this thread. --Kurt Shaped Box 20:04, 8 November 2007 (UTC)
- It seems that enlarged mammae - at least in other mammals - are the result of ovulation having taken place. As such they are a prime sign of fertility, which, in mammals including homo sapiens, is periodic and not constant. So, the attraction of bigg(er) boobs makes evolutionary sense as it maximises the offspring per copulation.
--Cookatoo.ergo.ZooM 19:43, 13 November 2007 (UTC)
- It seems that enlarged mammae - at least in other mammals - are the result of ovulation having taken place. As such they are a prime sign of fertility, which, in mammals including homo sapiens, is periodic and not constant. So, the attraction of bigg(er) boobs makes evolutionary sense as it maximises the offspring per copulation.
a reaction is either oxidative or non-oxidative, never inbetween
AGEs can be formed via oxidative pathways (e.g. carboxymethyllysine [CML] and pentosidine), via non-oxidative pathways (such as pyrraline), or from highly reactive dicarbonyl precursors, such as glyoxal, methylglyoxal and 3-deoxyglucosone (3-DG). - a reaction is either oxidative or non-oxidative; how can they give three categories then? The dicarbonyl precursors either react oxidatively or they don't... --137.120.53.67 21:47, 7 November 2007 (UTC)
- What's an AGE? Delmlsfan 23:42, 7 November 2007 (UTC)
- Could be Advanced glycation endproduct, what you get when you cook carbohydrate with protein. Graeme Bartlett 01:05, 8 November 2007 (UTC)
Amnesia
What is the name of the illness where the sufferer experiences amnesia every few minutes? Keria 22:23, 7 November 2007 (UTC)
- I forget. -- Coneslayer 22:38, 7 November 2007 (UTC)
effect heat has on rubber
</math>
- In oxygen atmospheres, combustion is the usual result. Delmlsfan 23:44, 7 November 2007 (UTC)
If you can exclude oxygen, it will give off hydrocarbon gasses and liquids, and leave behind some kind of charcoal. Graeme Bartlett 01:02, 8 November 2007 (UTC)
- Vulcanization? Heat equation and heat transfer will get you started on how the energy transfers along the bulk, but your relevant parameters ("k") are going to be temperature dependent and sample deformation will be an issue. At low temperatures, rubber becomes frangible (yet another way to have fun with liquid N2). At some temperature depending an what you mean by "rubber" (i.e. the stuff that comes out of the tree or any of several commercial products and colloquial usages meaning materials having the characteristic bouncyness of rubber), your sample will melt. Very shortly thereafter, it ceases being rubber, as above. Eldereft 05:44, 8 November 2007 (UTC)
November 8
cells
what would happen if you place a blood cell in a hypertonic solution —Preceding unsigned comment added by Dmx123 (talk • contribs) 00:37, 8 November 2007 (UTC)
- The reference desk it not for answering homework questions, but if you can't figure this one out on your own, you should read osmotic pressure. Someguy1221 00:49, 8 November 2007 (UTC)
- ... or Osmosis or Diffusion. --slakr\ talk / 04:35, 8 November 2007 (UTC)
- ... or better yet, hypertonic. (The article answers this very question.) -- 20:21, 8 November 2007 (UTC) —Preceding unsigned comment added by 128.104.112.105 (talk)
- ... or Osmosis or Diffusion. --slakr\ talk / 04:35, 8 November 2007 (UTC)
navel
hey how come for some people when they're fingered in their belly button, it hurts and for others it tickled them?Jwking 01:00, 8 November 2007 (UTC)
- Because some people don't know how hard to poke, and just stab you with their finger. HYENASTE 01:17, 8 November 2007 (UTC)
Reception of off air frequency standards
May be a stupid question, but why, when receiving, do you need a local oscillator to phase lock to the incoming signal? Only reason I can think is that the transmitted signal is not constant in amplitude. Why cant you use the incoming frequency dorectly? Also, why do you need a quartz Xtal osc to be locked to the incoming frequency, won't a normal vco do? —Preceding unsigned comment added by 79.76.246.62 (talk) 01:42, 8 November 2007 (UTC)
- It's for FM reception. The radio signal is varying slightly up or down in frequency depending on the amplitude of the sound wave it's trying to transmit to you (Frequency Modulation). You tune the radio's local oscillator to the nominal center frequency and it's easy to produce an audio signal that's proportional to the difference in frequency of the local oscillator and the radio signal. SteveBaker 01:59, 8 November 2007 (UTC)
- Er, no its AM. [8] —Preceding unsigned comment added by 79.76.246.62 (talk) 02:20, 8 November 2007 (UTC)
- You need a very low bandwidth. Typically the signal will be 5kHz wide, at a frequency of 10MHz. A VCO is no where near stable for this and will drift off in a few minutes. You can see this on old cheap shortwave radios, which will need retuning every so often. The crystal oscillator is much more stable. A VCO locked to a crystal is one way to get flexibility. Another important thing for a frequency standard is low phase noise. The best way would be to have a narrow crystal filter at 10 MHz, but even so the ionosphere causes fading and phase shifts. For 60kHz standard a LO would not be needed. Graeme Bartlett 05:57, 8 November 2007 (UTC)
- So you can use the ultra stable 60 kHz freq directly (or multiplied up to 1 MHz or 10 MHz or whatever)? Is that what your saying? If so, why do most designs use a local oscillator locked to the incoming frequency? —Preceding unsigned comment added by 79.76.246.62 (talk) 12:06, 8 November 2007 (UTC)
- See Superheterodyne receiver. Changing the frequency of the local oscillator is what tunes the radio to a station. Its frequency is beat against everything coming in from the antenna. The resulting harmonics are filtered for the intermediate frequency, 455kHz in the case of AM. A big advantage to this system is that from there on the amplifiers need only pass the one relatively low frequency. I don't know what you mean by a crystal oscillator locked to the incoming frequency, but it has been a long time, so please clarify. --Milkbreath 03:33, 8 November 2007 (UTC)
- Crystal control is absolutely not needed in an AM radio. I have owned several old AM radios which would stay tuned to a station for a year or more without re-tuning. Older car radios had pushbuttons which mechanically rotated a tuning cap to the desired stations, and did not need re-tuning for years at a time. Edison 13:10, 8 November 2007 (UTC)
- For a frequency standard, a Local Oscilator is not stable enough. You would need to down convert to the intermediate frequency, filter, and then up convert to the original stable frequency to get the reference. On HF frequencies around 10 MHz drift is ten times bigger than it is on the AM band around 1 MHz that you get on an old car radio. If you just want to listen to the time pips all this extra stability is not needed, you just need to keep the radio tuned to the station. Graeme Bartlett 20:38, 8 November 2007 (UTC)
- Crystal control is absolutely not needed in an AM radio. I have owned several old AM radios which would stay tuned to a station for a year or more without re-tuning. Older car radios had pushbuttons which mechanically rotated a tuning cap to the desired stations, and did not need re-tuning for years at a time. Edison 13:10, 8 November 2007 (UTC)
- I think the answer is that the off air frequency references have excellent long term stability, but are subject to the vagaries of radio reception such as : noise, timing uncertainty due to reflections from the ionosphere etc, and unwanted amplitude modulations, breaks in reception etc. OTOH, Local crystal oscillators can be made to have excellent short term stability and low phase noise etc but are subject to long term drift from component aging. Put the two together and get the best of both worlds!
Minerals
Is it possible to make a mineral (in this case a molecule containing iron) magnetic by running an electric current through it, or around it? I have reference here to the specific mineral asbestos, or one of its several 'subspecies'?76.182.3.188 01:56, 8 November 2007 (UTC)
- Very few minerals can be magnetized in a way such that they remain magnetic after the inducing field is removed. See Magnetization. A good many minerals, including some without iron, such as salt, can be made to give a diamagnetic response while the inducing field is present, but I do not think asbestos is one of them (but I'm not certain about that). Cheers Geologyguy 03:03, 8 November 2007 (UTC)
- Most minerals do not conduct, so you cannot easily run a current through them. In the case of asbestos it is a good insulator so it will not conduct at any reasonable voltage. If you ran a current around it you would have an electromagnet. The atoms in the mineral would respond in some way, but most have no strong response. A few Iron minerals may respond with ferromagnetism and even be magnetised as in Magnetite Graeme Bartlett 05:52, 8 November 2007 (UTC)
microwave hyperthermia
any reason not to heat someone up (their whole body to 104 F - 107 F) with a very high tech microwave (in order to generate a healing fever)? It penetrates and is the same frequency as cell phones... —Preceding unsigned comment added by 76.168.69.208 (talk) 02:02, 8 November 2007 (UTC)
- Microwaves do not heat everything uniformly, and in particular they will heat organs with a high water content more rapidly. Of special concern are the eyes where high levels of microwaves can promote cataracts and other damage.
- On the more general point, I'm not sure that heat is necessarily helpful in fighting disease. Fever is one of a myriad of reactions the body produces to combat disease, but an externally produced hyperthermia might be more detrimental than helpful since it wouldn't necessarily be accompanied by the same suite of immune responses as a natural fever. Dragons flight 02:25, 8 November 2007 (UTC)
- Also note: Wikipedia does not give medical advice. Anyway, the Hyperthermia article states that temperatures above 104 degrees Fahrenheit are "life threatening". I wouldn't reccomend it either, unless you know what you are doing, and I would get a second opinion (from a qualified professional) either way. I also have never heard of inducing a fever to heal by this method (or any), as fevers are caused naturally by the body as part of the immune response. SmileToday☺(talk to me , My edits) 02:28, 8 November 2007 (UTC)
- He might have gotten this from last week's episode of House, in which a portion of a treatment for an individual was artificially raising his body temperature. Someguy1221 02:39, 8 November 2007 (UTC)
- Microwave heating of smaller regions of the body to fever-range temperatures has been tested for various therapeutic purposes. If you're envisioning putting the entire body in a microwave oven (even a high-tech one) to heat the entire patient at once, you're out of luck. Per Dragons flight, you would get dangerous local heating effects that are very difficult to control. There are other, lower-tech methods that are just as effective. Where I have seen microwave heating employed is to do rapid, local thermal ablation of smaller volumes—a microwave antenna is inserted into a solid tumour, and the temperature elevated high enough to 'cook' the tissue.
- If you go to ClinicalTrials.gov and search on the term hyperthermia, you'll find a number of trials – mostly for cancer – that are testing the use of whole-body hyperthermia as a way to sensitize the body to radiation or chemotherapy or to potentiate the immune system's response to malignant tissue (e.g. [12], [13], [14]). Techniques that have been used to achieve hyperthermia include induction heating, warm wax immersion, hot water blankets, and radiant infrared heating. Patients under general anasthesia can also be treated using extracorporeal hyperthermia—blood can be drawn from the body, warmed externally, and returned to circulation. TenOfAllTrades(talk) 12:45, 8 November 2007 (UTC)
- I'll note that in non-medical contexts, there have been various suggestions to replace a home's heating system with a (low powered) microwave generator. Instead of heating the air, you heat the body directly. Supposively, this would save on energy costs. A quick Google search turns up [15]. -- 20:25, 8 November 2007 (UTC) —Preceding unsigned comment added by 128.104.112.105 (talk)
Why is water transparent?
Why is water transparent? I did some searching, and the reason for this is because water is transparent to the visible spectrum of light. But why does it exhibit this property? Does it have something to do with its hydrogen bonds that are responsible for so many of its other special properties? Acceptable 02:37, 8 November 2007 (UTC)
- There’s some explanation of this in absorption spectrum. Which frequencies a water molecule can absorb depends on what possible quantum states the molecule has. The frequencies of photons that the molecule can absorb correspond to the possible differences in energy between pairs of states. The ratio of a difference in energy level to the corresponding freqency of light is known as Planck's constant. MrRedact 03:20, 8 November 2007 (UTC)
- I should point out, water is slightly blue. Malamockq 03:28, 8 November 2007 (UTC)
- Consider checking out Color of water. --slakr\ talk / 04:32, 8 November 2007 (UTC)
- Plasmon frequency? —Preceding unsigned comment added by TreeSmiler (talk • contribs) 03:15, 9 November 2007 (UTC)
- Eyeballs are mostly made of water. This would affect what light could be seen.Polypipe Wrangler 21:24, 13 November 2007 (UTC)
Tectospinal tract
Is it safe to conclude from this picture that the superior colliculus is connected with the inferior colliculus through the tectospinal tract? Lova Falk 10:29, 8 November 2007 (UTC)
- There are some axons that go from the superior colliculus to various brain locations (tectobulbar axons) but I think the vast majority of the axons in the tectospinal tract first go anterior (ventral) and then cross the midline before descending past the level of the inferior colliculus. A good neuroanatomy textbook will have a figure for the tectospinal tract showing a series of brain cross-sections, one at the level of the superior colliculus, one at the level of the inferior colliculus and several more going down to the spine. This is the best I could find online (and it is not very good). --JWSchmidt 18:51, 8 November 2007 (UTC)
Point and shoot digital camera
Is it true that camera manufacturer deliberately introduce shutter lag into PS digital camera to "encourage" their clients to buy the much much more expensive DSLR camera instead. 220.237.184.66 12:06, 8 November 2007 (UTC)
- Unlikely, as not all point-and-shoot manufacturers have a DSLR in their lineup. One reason that comes to mind is that contrast-detection autofocus is slower than phase-detection autofocus. It's also possible that "something" has to be done to the CCD or CMOS sensor before taking the shot, if the sensor has been used for a live preview. For example, CCDs may accumulate charge that needs to be cleared. Since DSLRs generally do not have a live preview, they can keep the sensor ready to shoot. Maybe people know of other factors that I'm not thinking of. -- Coneslayer 13:31, 8 November 2007 (UTC)
- Point-and-shoot cameras are slower because they use contrast detection rather than phase detection autofocus, and because they usually have a less-powerful focus motor to increase battery life. You can test this by pre-focusing a point-and-shoot: once it's focused, it's actually faster than a DSLR at taking the picture, because the DSLR needs to get the viewfinder mirror out of the way. --Carnildo 22:46, 8 November 2007 (UTC)
Inelastic collision
About Inelastic collision As we know that in inelastic collision the initial and final momentum,total energy are conserved but kinetic energy is not conservsed.this is why ,why kinetic energy is not conserved .Plz explain the example of the collsion of cars.also mentioned it that after collsion the two cars come to rest then how is initial and final momentum the same , as they are moving with a speed before collision .thanx ........usman —Preceding unsigned comment added by Star33 2009 (talk • contribs) 13:15, 8 November 2007 (UTC)
- It may be useful to compare inelastic collisions with elastic collisions, whereby kinetic energy is conserved (see the first two sentences of that article, then contrast with the lede in inelastic collision). As for the car collision, bear in mind that physics is not nearly so concerned with "speed" as it is with velocity, and consider that the momentum of the two-car system is what's being conserved, not the momentum of the two individual cars. — Lomn 14:00, 8 November 2007 (UTC)
- I actually ran a little experiment on this. Kinetic energy is always conserved as a physical law- if it's not, you haven't included everything in the system. So we have to know where the energy of collision goes to. I modelled this with a spring system, as springs are a good approximation for any interaction and well-established in the interaction of particles. So then we have energy initial = energy final, or . Now note that the energy of the spring includes both the spring potential energy, 1/2kx^2, and the kinetic energy of particle 1 and 2 oscillating against the spring. It is what is called a simple harmonic oscillator. So now
- ,
- and so energy is conserved. The spring model has some consequences: it implies both that there are oscillations between the two particles after collision and that there is a non-zero collision time with finite acceleration. In the intro physics lab which I TA, the data showed the finite acceleration which agreed to good approximation with the theoretical acceleration of the spring (a linear differential equation - if you want me to show you how it is solved, please let me know), but no oscillations after collision. This is because the oscillations are quickly damped out as heat (another differential equation), which should also be measurable with a calorimeter, but I haven't tried this experiment. Energy is still conserved, as heat is another form of energy for which we can account.
- For your second question, note that momentum is a vector quantity, so it has both magnitude and direction - that should put you on the right track. SamuelRiv 14:11, 8 November 2007 (UTC)
- Momentum is a vector - but kinetic energy isn't.
- If you crash two very stiff objects together (two billard balls for example) then they bounce off and the sum of the kinetic energies of the two balls is almost exactly the same before as after. When you whack two cars together, the energy is absorbed in bending and tearing metal and plastic - so they don't bounce off much - there is no kinetic energy left, it all turned into heat. If you bounce two rubber balls together, the result is somewhere between the two extremes. KINETIC energy is not conserved in real-world collisions - but TOTAL energy is. SteveBaker 03:38, 9 November 2007 (UTC)
camera pixel resolution
What is the engineering standard for stating the number of pixels that make up a camera? The actual number of individual sensors, i.e., the number of rows of sensors times the number of columns of sensors on the chip, or the number of different areas of a picture that are focused onto a single sensor or single small group of individual sensors in sequence to build the whole picture? Clem 13:54, 8 November 2007 (UTC)
- Megapixel ratings are just a description of the number of sensors. The fact that more goes into a good picture than that rating alone is one of the reason the rating system is seen as being somewhat deceptive. As the page points out, in cameras this is even more deceptive, since each sensor generally registers only one color, and so the final image resolution can be easily a third less than the MP rating. --24.147.86.187 15:03, 8 November 2007 (UTC)
Here is an example of a sensor with three layers one for red, one for green and one for blue.[16] Along with the powerpoint that outlines the old technology and explains this new technology.[17] It is marketed as a 4.5 Megapixel CMOS direct image sensor with a maximum picture size of 1420 x 1064 x 3 matrix as seen in the HanVision HVDUO-5M digital camera. David D. (Talk) 23:05, 8 November 2007 (UTC)
- The megapixel count on a camera is the number of pixels in the image it produces. The actual number of light-sensing elements depends on the sensor technology used and the relative influences of the marketing and engineering departments. Sensors using the Bayer pattern and the related CMYK pattern will typically have as many single-color sensor elements as there are pixels in the output image. Cameras using the Foveon sensor pattern can have one-third as many full-color sensor elements as there are pixels; cameras using Fuji's Super CCD pattern have one-half as many songle-color sensor elements as there are pixels. Cheap high-megapixel cameras will use a small sensor and scale the image up: a 20-megapixel camera might use a 5-million-element sensor and use interpolation to produce an image with more pixels. --Carnildo 23:35, 8 November 2007 (UTC)
Old cars carbon emissions
How does the average carbon output of a 1980 1.4l petrol engine compare to a recent one? I heard statistics saying that 20% of the oldest cars represent 60% of total emissions. Is this correct? In view of these statistics some countries want to tax (or are already doing it) old cars. If we add the carbon output of producing a new car and disposing of the old one to the equation, is it still so favorable to buying a new car vs. keeping an old one running longer? Should we add to this the carbon output necesssary to produce enough wealth to buy the new car or is this irrelevant? Thank you. Keria 14:06, 8 November 2007 (UTC)
- Please be sure to distinguish pollution from carbon emissions. Older engines emitted far, far more pollution (unburned hydrocarbons, carbon monoxide, and the like) but the amount of carbon ultimately emitted is a strict function of the fuel economy of the car. Your 1980 1.4L car is probably emitting much less carbon per mile than my 2003 4.2L Audi (which averages about 25 miles/US gallon).
- You only have to compare fuel consumption numbers - that's a pretty fair comparison because the amount of CO2 you get out of a gallon of gas is about the same no matter how you burn it. My 2007 MINI Cooper'S with a 1.6l engine manages 7.5l/100km city and 5.3l/100km highway. A 1986 Honda Integra (pretty similar in size) also had a 1.6l engine and manages 7.8l/100km city, 6.8l/100km highway. So what gives? We don't do much better now than we did then...well, the MINI manages 170hp - the Integra managed about 108hp. What's happening is that we're getting more power out of engines than we used to. So whilst the consumption of a 1.6l engine isn't much better than it always was - we're able to stick a 1.6l engine into a car that would have required a 2.5l engine 20 years ago. Having said that, my old 848cc 1962 Mini manages 5.1l/100km on the highway - fractionally better than the 2007 version - but the 1962 car only has 37hp and could only manage a top speed of 72mph - the 2007 car goes MORE THAN TWICE AS FAST on the same amount of gas. SteveBaker 03:24, 9 November 2007 (UTC)
Ladder to Space
There's this infinitely tall ladder in my backyard. When I climb three or four feet and let go of the rungs I obviously fall back down to the ground. What is the minimum distance I would have to climb so that when I let go of the rungs I would never fall back down to the ground? (I think the answer is 22,240 miles where I would join all the geostationary artifical satellites of the Clarke Belt, but I'm not sure. Orbits, and rocket science in general, confuse the bejeezus out of me.) Sappysap 14:08, 8 November 2007 (UTC)
- You might want to check out Space elevator. -- Coneslayer 14:17, 8 November 2007 (UTC)
- It'd be geostationary orbit altitude, but only if you're also at the equator. Climbing a ladder ascending vertically from New York will not put you in a stable orbit at that altitude; you'd have to go higher. Climbing a ladder from the North Pole confers you no velocity and you'll drop from any altitude. I'm guessing the tangent of latitude is relevant to exactly how high you've got to go, but I'm not sure. — Lomn 14:24, 8 November 2007 (UTC)
- Irrelevant Post Ahead: I just have to say, that Space Elevator article is extremely fascinating. Beekone 14:49, 8 November 2007 (UTC)
- That is true. See Coriolis effect. We can calculate the height needed to get you in orbit - the thing about a ladder is that it's rigid, so your angular velocity at the top of the ladder is equal to the angular velocity of the earth at that latitude, so your orbit will only occur at a geosynchronous distance: see Geosynchronous orbit derivation. SamuelRiv 14:52, 8 November 2007 (UTC)
- Where's a mathematician when you need one? Say your ladder is at 40 degrees north latitude. You will have to climb up to where your speed is the geostationary orbital speed. This will be higher than if your ladder were at the equator. When you let go, you will move toward the earth until you reach the geostationary altitude where you'll stay until perturbations mess things up. I'm sorry, but I don't feel like deriving the formula for all that, the relationship between angular velocity, altitude and latitude. Where's that mathematician? There are guys who can do this sort of thing in their head. --Milkbreath 17:13, 8 November 2007 (UTC)
- Perhaps they're all here?
- Atlant 17:17, 8 November 2007 (UTC)
- Right. I had a chance to think about this just now while watching a Labrador retriever do his business (hardly a Newtonian anecdote, eh?). The only way you'll be able to let go of the ladder and just stay there is if the ladder is at the equator. So, the nerds are off the hook. (I suspect that the answer to my pointless question above is a straight line tangent to the earth at a geostationary point above the equator.) --Milkbreath 17:41, 8 November 2007 (UTC)
- Atlant 17:17, 8 November 2007 (UTC)
- Not quite - there are important consequences when you get far enough out where the rotation of the Earth is too fast for gravity and you get vertical components of the coriolis effect. Integrate across the length of the ladder accounting for atmospheric effects and you'll get a mess. The ladder itself needs a counterweight to make it stable, which is why the space elevator needs such a large counterweight mass. Oh, and the math for latitude at angle phi just needs a sine term, sin(phi), to multiply through. See the formula at Coriolis effect. SamuelRiv 20:43, 8 November 2007 (UTC)
- Blaise Gassend computed whether, where and how hard an object falling from any level on an equatorial elevator will hit the ground. —Tamfang 18:49, 9 November 2007 (UTC)
Answering the actual question
We were not asked how high you would have to climb on the ladder in order to enter geostationary orbit. We were asked how high you would have to climb in order to never fall back down to the ground. Which means that any stable orbit would do.
Since the atmosphere has no sharp outer boundary, there is no specific altitude above which you have to orbit in order for orbital decay due to atmospheric drag to become negligible. However, I will assume for simplicity that a distance of 8,000 km above the Earth's center (that's roundly 1,600 km or 1,000 miles above the surface) is what you need to achieve. So you want to be in an orbit with its perigee at that distance and its apogee at the point where you jump off the ladder.
The answer clearly depends on your latitude. If the ladder is at the North or South Pole, it doesn't matter how high you climb: the ladder is simply rotating around its own axis and that doesn't put you in orbit, so you'll always fall back to Earth. But if the ladder is at latitude L and you climb to a distance A above the Earth's center, then you are moving horizontally in a circle of circumference 2 pi A cos L, completing one circle per sidereal day. From here on I'll suppress units; numerical values are based on distances in km, times in seconds, speeds in km/s, etc. Then your speed is V = KA where K = (2 pi/86164) cos L.
Now, using the formulas on this page with some changes of variable names, the orbit's apogee and perigee distances A and P (from the center of the Earth), and the apogee speed V, are related by
- V² = G M (2P/(A(A+P))
or in other words
- V² A (A + P) = 2P G M
where G is the gravitational constant and M is the Earth's mass, and the value of GM is known to be 398,600. But in this case we also know that V = KA, so we have
- K²A³ (A + P) = 2P G M
and since all the other values are known (for a specific latitude), we merely have to solve this for A. As it reduces to a quartic equation, this is not easily done by algebra, but it can be solved numerically by a simple computer program.
For example, suppose your backyard is at latitude L = 45°. Then we have K = (2 pi/86164) cos L = 0.00010313 and K² = 1.0635e-8. We are assuming P = 8,000, and we know GM = 398,600. So 2GMP = 2 x 8,000 x 398,600 = 6.3776e9, and we have
- (1.0635e-8) A³ (A + 8,000) = 6.3776e9
so
- A³ (A + 8,000) = 5.9968e17
with the numerical solution that A = 26,020 km to 4 significant digits. Taking the Earth's radius at your latitude as 6,370 km, the answer is that you would have to climb 19,650 km or say 12,210 miles in order to jump off and reach a stable orbit with the perigee mentioned above.
If your backyard is on the equator, you're in a much better position. In that case K² is larger by a factor (cos 0 / cos 45°)², which conveniently is exactly 2, which makes A³ (A + 8,000) smaller by the same factor, i.e.
- A³ (A + 8,000) = 2.9985e17
This has the numerical solution A = 21,630 km. If your ladder is at the equator, you must climb a mere 15,250 km or 9,475 miles to jump into a stable orbit. And if you wanted to enter a geostationary orbit (not a bad idea if you ever intend to come back down using the ladder!), then on the equator it would be possible by climbing to the height mentioned above. Anywhere else, of course, it would not be.
--Anonymous, edited 04:49 UTC, November 9, 2007.
The effect of stars on Earth
What if a genie were to withdraw all of the stars in the universe except the sun, and the photons in transit to Earth were taken away as well. Would there be a gravitational effect? Would there be a climate change on Earth? Essentially, do the stars in the night sky play any describable role in Earth's affairs? —Preceding unsigned comment added by 150.167.179.111 (talk) 17:00, 8 November 2007 (UTC)
- Well, stars provide a useful amount of light on a clear night (see night vision), but I think there's no other routinely discernable effect. The opinions of astrologers will differ, of course.
- Note: It is thought that a sufficiently close supernova would emit enough gamma radiation to toast us all, but I think we all hope that won't occur any time soon, so I'm excluding that as a current effect.
- The stars are all far enough away that gravity is not an issue; starlight doesn't make up an appreciable amount of radiation reaching Earth so it shouldn't have any effect on the temperature, climate, etc. My bet would be "no". The stars don't play any real physical role in Earth's affairs. --24.147.86.187 18:57, 8 November 2007 (UTC)
- For that matter, said genie could remove everything but Earth/Moon/Sun and we'd see no appreciable difference apart from the view in the nighttime sky. — Lomn 19:10, 8 November 2007 (UTC)
- In terms of climate, the effect of the loss of every single star is essentially nil over any short or medium time scale. (Over an extremely long period of time – hundreds of millions of years – there's the risk Atlant notes of a nearby supernova explosion.) This web page talks about using single stars as light sources of known, carefully-measured intensity for evaluating the sensitivity of digital camera sensors. Interestingly, it also provides the relative illumination provided by starlight (0.001 lumens per square meter) compared to full daylight (10 .000 lumens per square meter). Making the reasonable assumption that most of the energy we receive from starlight will be at visible and near-visible wavelengths, distant stars contribute less than a millionth of the incoming radiation to Earth.
- As for gravity, the effect is again negligible unless a massive star passes extremely close to the Earth-Sun system. (This would be a very rare event.) Since gravitational force follows an inverse square relationship, a star the size of the Sun only one light year away will pull on the Earth lss than one-billionth as strongly as the Sun does. TenOfAllTrades(talk) 19:52, 8 November 2007 (UTC)
- On the other hand, the effect could be catastrophic. See Ice_age#Causes_of_ice_ages. While I don't believe this theory, just remember that we are in some kind of orbit around a galactic center, and therefore there is a very real gravitational effect on the Earth and Solar System. The planets and outer solar system all have enormous influence regarding the slinging of comets and asteroids into Earth's path, and there are clear measurable gravitational effects from Venus, Mars, and Jupiter. The moral of the story is that in a chaotic system like climate and ecology, you cannot just ignore the small variables. SamuelRiv 20:28, 8 November 2007 (UTC)
On the one hand, the physical effects on the planet are described above. On the other hand, there is the potential effects on society and humanity. Consider the espers' trick on Ben Reich in The Demolished Man, multiplied by six billion, or the end of "Nightfall" in reverse... the sudden disappearance of every single star in the universe would be quite traumatic. The most calm would be rightly troubled, and the least calm would revert to base fears, thoughts of religiously inspired (or even very real) armageddon. When people panic on large scales, Bad Things Happen. If everyone in the world is exposed to the same instantaneous trauma (and even something as simple as the stars disappearing can be quite effecting, I'd imagine), its a safe bet that human society as a whole would have a fairly hard time coming through and recovering from such a scenario. --Jeffrey O. Gustafson - Shazaam! - <*> 10:54, 12 November 2007 (UTC)
growing crystals of copper sulfate
how would you make a hot, concentrated solution of copper sulfate? —Preceding unsigned comment added by 86.42.210.0 (talk) 17:24, 8 November 2007 (UTC)
- You would boil some water (perhaps in a kettle), pour it in a heat resistant glass or ceramic container, then add copper sulphate crystals and stir. Do not use aluminium or steel containers as copper will plate on to their surfaces. Then you decant the solution, leaving any undissolved stuff behind. Commercial copper sulphate probably has ferrous sulphate as well, so it may not be pure. As the solution cools you will get a growth of crystals. If you can hang a little crystal from a thread, you can make it grow into a big crystal. Other experiments you can do with copper sulfate solution are: add ammonia to get a dark blue solution which can dissolve cotton, add biuret to get a different dark blue solution, add a base like sodium bicarbonate to make a precipitate. Graeme Bartlett 20:14, 8 November 2007 (UTC)
Viruses
Virus is a DNA With a Protein Coat Protecting It Can a virus be destroyed if the protective protein coat is damaged so that it cant protect DNA anymore if yes then can an enzyme be used as protease to digest the protein coat thus destroying the virus ????? —Preceding unsigned comment added by 212.71.37.97 (talk) 18:04, 8 November 2007 (UTC)
- Proteases generally act, well, in a general way. They either consume a protein at one of its ends, or they cleave proteins at specific amino acids. So as you can see, a protease based antiviral measure would cause quite a bit of collateral damage if used to "carpet bomb" infected tissue. It could destroy or inactivate the virus; however, some viruses are even evolved with this in mind, and cleavage of viral proteins upon entering the cell can actually activate the virus (I can't for the life of me remember what article this is in, but it came up in a previous ref desk question I can't find in the archives). Far easier to target them with antibodies. Someguy1221 19:39, 8 November 2007 (UTC)
- Something like http://dx.doi.org/630030? DMacks 21:19, 8 November 2007 (UTC)
- Also, your definition of "virus" is not quite right. There are many RNA viruses. And the RNA of many of those viruses can infect cells as "naked RNA"; no protein coat is required for the virus to infect cells and produce new virions. - Nunh-huh 21:18, 8 November 2007 (UTC)
ecosystem
can you show me a picture of an ecosystem (example) that a 4th grader could use to help them do a project? —Preceding unsigned comment added by 72.18.102.36 (talk) 20:16, 8 November 2007 (UTC)
- I like the images and text here. But there were other suitable examples when I did a google image search. Man It's So Loud In Here 21:08, 8 November 2007 (UTC)
nuclear energy: a given
Let's face it: we are running out of oil. The federal "government" doesn't have a plan for the event known to the public (or is the coal industry now going to move in for what it's worth?). We are in a bad spot so we quickly fall back to our former nuclear technology, which could be a rescue except for the problem of "spent" nuclear fuel. So has there been any design for a facility that can "speed up the procees of nuclear decay" of a spent fuel on-site? Can a half-life be made into a quarter-life? There's energy there. LShecut2nd 23:29, 8 November 2007 (UTC)
- Radioactive decay is a quantum process and as far as I know there's no way to affect it one way or another. That being said, there are plenty of other ways that one can imagine dealing with the waste problem. Unfortunately the stakes are quite high and the need for government intervention quite high as well so as a result it is a rather toxic bureacratic issue, so to speak, and progress has been pretty slow and problematic. --24.147.86.187 00:21, 9 November 2007 (UTC)
- Breeder reactors are one technology that affects the quality and quantity of waste by transmuting some of the waste into other substances. The drawback is that this technology produces plutonium which is much easier to use as the core of a nuclear bomb than ordinary reactor materials. Dragons flight 01:39, 9 November 2007 (UTC)
- It is a fundamental mistake to say that we are running out of oil. If (hypothetically) we were to continue consuming it at the rate we do now then we would indeed run out in 40 to 150 years (depending on the economics of pulling oil from sands and shales as the price inevitably rises). However, if we burn oil at this rate for even 20 more years, the planet will die. So given that we don't intend to kill the planet - we WILL cut our consumption. So - the problem remains - somehow we have to stop using oil. Certainly there are alternatives - nuclear isn't wonderful - but the difficulties of safely storing nuclear waste is a lot more tenable than the the problems of collecting and storing millions of tons of CO2 gas. Nuclear waste will trash the environment wherever we put it - but it's a lot better than trashing the entire planet. So let's pick a place (right in the middle of a desert someplace might be good) and dump all of the stuff there. Sure, the consequences will be nasty - but a heck of a lot less than melting ice caps, rising sea levels, increasing human misery - annihilation of some terrifying percentage of the species of life.
- But you can't speed up the rate that the low level waste decays. Sure, it contains energy - but at a level that can't be economically recovered. There really isn't much you can do but let it decay. The plan ought to be to use nuclear as an emergency stop-gap. We URGENTLY need to get away from fossil fuels - and the efforts with wind, solar, wave, etc really aren't cutting it - and the one renewable resource that did anything (hydroelectric) has proven to be a problem, the dams silt up and the downstream environment suffers...argh! So we need to build a bunch of nuclear power plants - shutting down the coal, oil and gas plants as we do so. Then research - LOTS of SERIOUS research. Whatever happened to huge orbiting solar power plants with microwave downlinks? Why aren't there VAST numbers of windmills everywhere? Fusion power - perpetually "25 years away" from getting something working...we need a 'Manhatten Project' for fusion. For vehicles, we have other problems - cheap electricity could solve it - but I think ethanol may be the more likely answer.
- It is quite easy to speed up nuclear decay. First, you need to chemically separate the waste into elemental components, since they must be treated differently. Then, use a neutron source to bombard the correct set of your elements. You can get the neutrons from a fusion reactor. The process can be engineered as a net power source. None of this can be done today: the science is there, but the engineering is not. This is however a way that our grandchildren can eliminate the low-level nuclear waste that we must generate to avoid killing ourselves with oil. -Arch dude 03:04, 9 November 2007 (UTC)
- Well, have a look on Fusion power and ITER. Thermonuclear reactors are the next-generation-nuclear-device which would produce far less waste while consuming heavy water (D2O, more precisely, heavy isotopes of hydrogen: deuterium and tritium), i.e. they will be able to provide Humankind with cheap clean energy. —Preceding unsigned comment added by 62.63.76.14 (talk) 09:51, 9 November 2007 (UTC)
- Is fusion energy really all that clean? The fusion reactor itself becomes highly radioactive. "...most of the radioactive material in a fusion reactor would be the reactor core itself, which would be dangerous for about 50 years, and low-level waste another 100. By 300 years the material would have the same radioactivity as coal ash..." If fusion energy became viable on a world scale to power everything, how bad of a nuclear waste problem would we have? Sappysap 14:24, 9 November 2007 (UTC)
- Probably very little; there isn't anything important under the Sahara and Gobi deserts, or the Great Basin of Nevada. These would hold massive quantities of waste. —Preceding unsigned comment added by 98.196.46.72 (talk) 16:12, 11 November 2007 (UTC)
- Also see Integral Fast Reactor, and this Q&A here. Too bad the project was killed, eh? grendel|khan 15:36, 9 November 2007 (UTC)
The Reichsbrücke article does not state why the bridge collapsed and was anybody found guilty. Does anybody know any details concerning that collapse? Mieciu K 23:58, 8 November 2007 (UTC)
- Following the third link in the References section, I find this paragraph:
- Ursachen. Nachdem zunächst Gratz seinen Rücktritt angeboten hatte, übernahm der Wiener SP-Planungsstadtrat Fritz Hofmann die politische Verantwortung für den Einsturz und schied wenige Tage nach der Katastrophe aus dem Amt. Eine Expertenkommission gab kurz darauf bekannt, dass der linke Pfeiler der nach Ende des Zweiten Weltkrieges sanierten Brücke zum Teil mit Sand und "unverdichtetem Beton" gefüllt gewesen war. Durch das schlechte Material sei Wasser eingedrungen, was schließlich zu dem Einsturz führte.
- Combining pieces from two machine translations of this (Google Language Tools and Babelfish) and putting the word order into something more like English myself, I figure that this says:
- Causes. After Gratz [the mayor] initially offered his resignation, the Viennese FR planning town councillor Fritz Hofmann took political responsibility for the collapse and resigned from office a few days after the disaster. An expert commission shortly afterwards announced that the left column of tbe bridge, rehabilitated after the end of the Second World War, had been partially filled with sand and "uncompressed concrete". Water penetrated by the bad material, which ultimately led to the collapse.
- --Anonymous, 03:29 UTC, November 9, 2007.
November 9
Robinson projection
I'm beating my head against the wall. I want to come up with a simple script that will convert latitude and longitude coordinates to x,y coordinates on a given map in a Robinson projection of a given width with a given central meridian. Ideally this would be done in Actionscript but if I had it in any sort of code or pseudocode that would be fine (which is why I am asking here and not the computing desk—it is not a computational difficulty, it is a conceptual one. Once I have an idea of what I should be doing conceptually it will be trivial to code it).
There are a few map projection projects out there but they are all extremely complicated since they are designed for exporting ALL projections; I _just_ want Robinson. I've read the article and I grok that it's about a lookup table but I still have no idea how I'm supposed to convert that table into x,y coordinates with a given map.
Can anybody help? I just want instructions on the level of "take your number from column one, multiply it by something in column two, then do something else, do something with the central meridian and the width, and presto-chango you have x and y coordinates." I'm having trouble figuring out how to use the table. --140.247.10.141 00:18, 9 November 2007 (UTC)
The general method will be to get a formula that maps the points from one projection to the other. THen you invert the formula so that it maps the points in the reverse direction. Then scan all the points on your new map, say from top left to right and then going down in a raster pattern, use the formula to get a new coordinate to look up the original map. If the coordinates are on the map copy the pixel. If its off the map stick in a "missing data " colour - perhaps white or blue. Graeme Bartlett 01:19, 9 November 2007 (UTC)
- Graeme, A Robinson projection is not a formula driven mapping.
robval = [ 00 1.0000 0.0000 05 0.9986 0.0620 10 0.9954 0.1240 15 0.9900 0.1860 20 0.9822 0.2480 25 0.9730 0.3100 30 0.9600 0.3720 35 0.9427 0.4340 40 0.9216 0.4958 45 0.8962 0.5571 50 0.8679 0.6176 55 0.8350 0.6769 60 0.7986 0.7346 65 0.7597 0.7903 70 0.7186 0.8435 75 0.6732 0.8936 80 0.6213 0.9394 85 0.5722 0.9761 90 0.5322 1.0000 ]; robval(:,3) = robval(:,3) * 0.5072; robval = [robval(end:-1:2,:);robval(1:end,:)]; robval(1:90/5,[1,3]) = -robval(1:90/5,[1,3]); rvals2 = interp1(robval(:,1),robval(:,2),latitude,'cubic'); rvals3 = interp1(robval(:,1),robval(:,3),latitude,'cubic'); y = -rvals3; x = rvals2/2.*longitude/180*2;
- The above is taken from a Matlab program I wrote to generate a Robinson projection. Dragons flight 01:35, 9 November 2007 (UTC)
- does it use cubic interpolation? the article does not say what kind of interpolation is used. Graeme Bartlett 01:44, 9 November 2007 (UTC)
- My quick search didn't turn up specifics on the kind of interpolation (other than the phrase "simple interpolation method"), but this page shows an example Robinson map with a caption saying "...calculated with 3rd degree polynomial interpolation", for what it is worth. Pfly 06:29, 9 November 2007 (UTC)
- That looks like it is in the right ballpark. Here I admit to not being able to follow MatLab's syntax with dealing with Arrays. Could someone convert it into something a little more standard, or just pseudocode? I feel like I'm on the cusp of having it but researching how MatLab deals with Arrays is something I'm not very excited about... --140.247.11.32 16:09, 9 November 2007 (UTC)
Muteness
If you're born mute due to brain damage rather than throat/vocal cord damage, as an infant, do you still cry? Assuming you can hear, what happens when you learn to understand language? Do you merely stop crying but not be able to communicate until someone teaches you sign language? In fact, is it even possible to be mute but not deaf as a result of brain damage? Or would it have to be throat/mouth damage? Kuronue | Talk 02:06, 9 November 2007 (UTC)
- I'm not sure about the physiology of crying (there is no article on crying here, unfortunately), but it seems to be associated with contraction of the diaphragm, and should occur with or without vocal chords or vocalization control. Children can hear their own voices, and it's an extremely important part of their personal language development, so a mute child will probably have to learn some sort of gesture or sign language to compensate, and if one isn't taught, it will probably imitate or invent such a language from what it perceives from others (gestures, etc). Unfortunately the pages here for muteness and aphonia are also lacking. Brain damage can cause muteness if the damage is significant enough to somewhere such as Broca's area, which would affect speech production, though it would damage a lot of other linguistic abilities as well such that someone with such damage may not be able to perform sign language. A much more mild damage would be from, say, damaging one of the facial nerves that control the mouth and tongue, which would easily destroy comprehensible speech. Finally, of course, a laryngectomy of the sort that Steven Hawking went through would destroy vocalization completely. SamuelRiv 06:10, 9 November 2007 (UTC)
asbestos -microencapsulization healing?
Once fibers get in the lung, they can't be removed., sometimes leading to cancer. When re-modeling a house, asbestos that can't be removed is encapsulated with an impenetrable material. Why can't micro-encapsulization, biological or chemical, be used likewise to neutralize fibers in situ? Wiki articles on microen. and on 'self-healing' were good. Comments? —Preceding unsigned comment added by 76.182.3.188 (talk) 02:11, 9 November 2007 (UTC)
- Well, microencapsulation wouldn't remove the actual foreign particle from the lung, which I believe (not my field at all) is the primary cause of the cancer in the first place. See carcinogen. Aside from that, encapsulating the fibres should be feasible with molecules attracted the long mineral, but I'm not sure of any such engineering out there for individual strands. SamuelRiv 06:15, 9 November 2007 (UTC)
- Exactly. One of the things that asbestos does (Asbestosis) happens because the particles are essentially impenetrable. The lung detects the presence of the foreign particle, triggering an immune response. Essentially it releases some localized acid to try to dissolve the particle, which does not work on the asbestos particle, but over time does start to dissolve lung tissue. Coating the asbestos in some other impenetrable shell would likely result in the same thing, unless you could use something invisible to the immune system and non-irritating to the tissues to avoid that response. Not medical advice! ArakunemTalk 15:53, 9 November 2007 (UTC)
Time
- Will time "be faster" if an event caused the Earth to rotate faster? (and vice versa)
- Does the moon going farther out from the Earth affect the rotation of the Earth?
- Also, could this be happening now?
Time recently has been going by so freaking fast. I worship seconds now. 67.35.94.120 02:27, 9 November 2007 (UTC)
- No, No, Yes. Time wouldn't change if the earth rotated faster - the length of a day would get shorter - but the definition of a second (and therefore a minute and an hour) is defined by the rate of some atomic event or other...something that will never change. The orbit of the moon doesn't significantly affect the rotation of the earth - although doubtless there is some small effect or other. The moon is most definitely gradually moving away from the earth - but not at a rate that you could possibly notice even over many human generations. Sadly, your subjective rate of time flow is something we can't do much about - but it is entirely subjective. SteveBaker 02:39, 9 November 2007 (UTC)
- Actually tidal drag caused by the moon is the primary effect slowing the rotation of the Earth and is directly linked to the growth in the moon's orbit. It is still a very small effect though. Dragons flight 03:01, 9 November 2007 (UTC)
- (ec) Angular momentum is transferring from the spinning earth to the moon's revolution about the earth by a mechanism involving the tides. As the moon moves away, the earth is slowing down. Yes this is happening now. This causes the day to get longer, but the effect is small. We now must add about one second per year to keep the clocks and synchronized with the earth's rotation: see leap second. -Arch dude 02:41, 9 November 2007 (UTC)
- Actually, when I read the question I assumed anon was referring to special relativity effects of reduction in relative velocity to the perception of time. The centripetal acceleration that we feel makes the calculation non-trivial, but in general our time would be "slower" than that of an observer stationary relative to our rotation. SamuelRiv 03:38, 9 November 2007 (UTC)
- (By a negligable amount...yes) The point is that while there are such effects, they are all quite utterly negligable compared to human timescales. Our OP is claiming that time is going by faster than it used to within his/her lifetime...that's simple not true to any measurable degree no matter what complicated science you try to throw at it. The effect of the moon, the effect of relativity - these are all UTTERLY negligable - and it is wrong to suggest otherwise just because we can. The clear answer to the OP's question is "No". SteveBaker 14:11, 9 November 2007 (UTC)
absorption refrigerator
Look at this http://www.nh3tech.org/abs.html
Since absorption refrigerator uses heat to chill things down and since CPU generates a hot of heat. Why isn't absorption refrigerator uses the CPU heat to cool down hot CPUs? 202.168.50.40 04:15, 9 November 2007 (UTC)
- Expense, complexity, and environmental hazards. Basically the first two are the same reason computers don't come standard with liquid cooling. Air cooling is by far the simplest, cheapest, and safest answer for most processors. SamuelRiv 04:25, 9 November 2007 (UTC)
- Another reason is that you can't use the heat from the thing you're trying to cool as the energy source for the system. If you cool the CPU, it won't be hot, so it won't be able to boil off the ammonia, so the CPU will heat up until you cool it again, at which point it won't be hot...you get the idea. And you don't want the CPU to be hot enough to power the system in the first place. I remember learning about the ammonia system when I was tutoring my landlord's kid. It's a fascinating machine. It's one of those inventions that is NOT obvious once someone's done it the way the cat door is. --Milkbreath 14:16, 9 November 2007 (UTC)
paleontology & neuroscience
If the brain of a dinosaur or other prehistoric organism were discovered, perfectly preserved so as to be in the same state as moments after its death, what facts about its behaviour, intelligence, memory capacity, etc. would neuroscientists & paleontologist be able to confidently infer from the brain & nothing else.
Hypothetically-speaking; please disregard whether such a brain could possibly be found in reality. 3170s228 04:42, 9 November 2007 (UTC)
- From the perspective of neuroanatomy, an awful lot. From a cognitive perspective, next to nothing. Animal brains (which I know nothing about) are a lot different from human brains, but in general we could find out most innervation and structure of the brain, which would tell us quite a bit about a variety of processing abilities. Intelligence is usually measured in terms of cerebrum size, so we'd have an estimate of that. We may be able to infer some things about memory if limbic structures are present, including the apparent structure of long-term memory and perhaps even its synaptic plasticity. With present understanding, however, we could infer next to nothing about behavior - I don't see any real viable correlation of brain structure to behavior in a class of animals like reptiles. SamuelRiv 05:33, 9 November 2007 (UTC)
- Animals tend to have similar brain parts. Knowing the existence and relative sizes of different parts of the brain can tell you a lot. They hardly need a perfectly preserved brain. The cavity in a fossilised skill would do. I remember seeing something where they showed that the brain of some kind of dinosaur was similar to that of an alligator and that it probably behaved like one. — Daniel 01:29, 10 November 2007 (UTC)
How would one find out about the kind of society of prehistoric humans?
How would someone find out about the kind of society that existed among prehistoric humans 50,000 years ago? I am not asking what the answer actually is but rather how you would find the information. I do not mean by like research but how would like scientists find out this information. Any help would be appreciated. —Preceding unsigned comment added by 69.181.131.67 (talk) 05:16, 9 November 2007 (UTC)
- Great question! As you might guess, information about the social structure 50,000 years ago will be fairly limited. What kind of inference you make will be based on whatever physical evidence is preserved. By examining what kind of tools they had, and what wear was associated with them, along with bones or other trace detritus, it may be possible to guess at what they ate (some other techniques, such as Isotope analysis also can be telling in this regard). Sometimes human skeletal remains can give clues about how the person lived, although this is tricky at long ages. Occasionally a glimpse of the "soft culture" is given; One (Neanderthal) skeleton recovered from the Shanidar site indicates injury and healing. A reasonable guess, since he would have been unable to fend for himself, is that he was cared for by his companions. Was there a specific question which prompted the interest? --TeaDrinker 05:37, 9 November 2007 (UTC)
- Note bones also tell about religious practices and superstitions. Garbage (discarded bones of killed animals tell what they hunted and how) and housing are other important finds. Then one can look at probable migration patterns or recorded history to see where that society went: if a descendant society still exists, one could learn a lot from examining them and their own perception of history. Linguistic history of any kind could almost certainly not be traced that far back, unfortunately. SamuelRiv 05:41, 9 November 2007 (UTC)
- An overly broad and probably not very helpful answer: Look at the archaeological record and, using everything we know in modern science (particularly medicine) and current cultures with deep roots, make some "educated" guesses. --Bennybp 07:38, 9 November 2007 (UTC)
- A while back they did a reconstruction in Germany. Build a prehistoric village and have people live there in the way the prehistoric people did. They're interactions changed significantly from what they were used to in modern life. You can Google on SWR and "Das Steinzeit Experiment". - 131.211.175.100 12:26, 9 November 2007 (UTC)
First Solar
According to this
- According to Deutsche Bank analyst Steve O’Rourke, the company’s results came in well ahead of expectations as its German production facility ramped ahead of schedule, and incremental improvements in efficiency, production throughput, and currency exchange rates drove panel cost per watt to a new low of $1.19
Does that mean I can buy a 65 watt solar panel from First Solar for only $78 dollars???
5kW worth of Solar Panels for only $6000 + installation cost?
220.237.184.66 06:14, 9 November 2007 (UTC)
- Well, perhaps - but maybe they don't make them in 65W sizes. You are assuming that this is the cost of a complete unit that you can bolt onto the roof of your house (or whatever) and use - that may not be the case. I suspect they are talking about the raw silicon wafers - it may cost considerably more to assemble them into weatherproof units, add controller electronics, etc. Is that the retail cost or the wholesale cost - if you're going through a middle-man, it could be more. In short, no, it doesn't necessarily mean that...although it might. SteveBaker 14:03, 9 November 2007 (UTC)
gryroscopes
Can two gyroscopes spinning in opposite directions counteract each other? —Preceding unsigned comment added by 204.251.179.61 (talk) 07:05, 9 November 2007 (UTC)
- Sure. Why not? It all depends on what you mean by "counteract". -- kainaw™ 13:17, 9 November 2007 (UTC)
- From my interpretation of the question, it seems you're asking if two gyroscopes in the same system but spinning on different axes and otherwise not interacting counteract, and the answer is yes and no. No in the sense that they both will resist changes in their absolute spin axis to conserve angular momentum, so they will still keep an object on a stable trajectory. However, a single gyroscope can have its system rotated about the gyroscope's axis without problem, so you need a second gyroscope in a different direction to restrict all rotation. In this way, they do have an interaction effect. SamuelRiv 13:56, 9 November 2007 (UTC)
- If they are bolted together then as you try to turn them, each gyro will exert a force onto their common framework - the resultant force will apply. It's no different than if you bolted two cars together and tried to steer them in opposite directions. Gyroscopes (like magnets) have taken on a mystery that they don't truly deserve. SteveBaker 13:59, 9 November 2007 (UTC)
- Man, magnets are really, really awesome, and do deserve every bit of mystery that they're given! Invisible forces! Immense power in tiny pieces of metal! Wow! I'm still amazed by magnets, they're the closest thing to magic that I interact with on a daily basis! --24.147.86.187 21:25, 9 November 2007 (UTC)
- I dunno - gyroscopes are pretty weird too. Take the front wheel off your bicycle, hold it by the axles and sit in a swivel chair - now have someone spin the wheel up as fast as they can get it to go - then tilt the wheel back and forth...tell me that ain't freaky on the scale that magnets are. But you're wrong about the magnets having "immense power" - they don't store any energy in the form of some kind of "magnetic power" in the way that (say) a battery stores electrical energy. SteveBaker 21:52, 9 November 2007 (UTC)
- Man, magnets are really, really awesome, and do deserve every bit of mystery that they're given! Invisible forces! Immense power in tiny pieces of metal! Wow! I'm still amazed by magnets, they're the closest thing to magic that I interact with on a daily basis! --24.147.86.187 21:25, 9 November 2007 (UTC)
- As a related question, if you've got a sealed box containing two counter-rotating coaxial flywheels, is there any measurement you can do to tell if the flywheels are rotating or not? How about if the axises of rotation are parallel but not co-linear? --Carnildo 21:43, 9 November 2007 (UTC)
- So it won't matter if the axes of rotation are not collinear at all. You can translate your whole spinning system arbitrarily and nothing will change. The angular momentum vectors of the two flywheels won't cancel - both will resist angular momentum changes of their respective systems. So if you turn the box, you will feel twice as much restorative force from the conservation of ang mom as if you only had one flywheel, whether or not they're spinning in the same direction. SamuelRiv 23:38, 9 November 2007 (UTC)
LEL and UEL of gases.
I looked at a table on your website which states the LEL and the UEL of various gases. How do I know that the information seen on your website is correct? Is this ever checked or is this just something that someone put together? I'm keen to use your website but only if the information has been verified and is correct. Thank you. Mr Sangster. —Preceding unsigned comment added by Andy Sangster (talk • contribs) 10:38, 9 November 2007 (UTC)
- If you're referring to Explosive limit, then you should check the references at the bottom of the page. All information on Wikipedia should be properly sourced, so you can go to the sources if you want to be sure. -- JSBillings 13:13, 9 November 2007 (UTC)
- To be honest - there are no guarantees. Whilst Wikipedia editors are supposed to provide references for all of the facts they list, this is far from typically the case - and even if there are references, you can't know for sure that the author of the article typed them in correctly from those references. Worse still, some annoying little kid could have come along and messed up all the numbers for a joke. That is actually very rarely the case - but if you want absolute guarantees - there aren't any. In the case of this article, they have not indicated specifically where that particular information came from - but merely listed the two references were used in writing the article. In order to check that this particular set of information is true, one would have to look into the references and check to see that the information in the article actually agrees with them. In a better referenced article (such as the ones I wrote for the Mini and Mini Moke cars), you'll see little blue tags that look like this: [25] which you can click on to take you to the exact reference where that particular fact came from. However, as I said, sadly not all articles are referenced to that standard. As a practical matter, most scientific Wikipedia articles such as Explosive limit are reliable. I wouldn't use the information (without checking the references first) if someones life depended on it - but for more casual uses it's convenient. Wikipedia has been shown in several surveys to be more accurate than printed encyclopedias such as the Britannica. So I guess you can trust Wikipedia to the same degree that you'd trust any general encyclopedia. SteveBaker 13:53, 9 November 2007 (UTC)
- Wikipedia is inherently no more or less reliable than any other encyclopedia. Per Wikipedia's Risk disclaimer and General disclaimer, you should place the same trust in this material as you should in material from any general encyclopedia: none at all. If you are going to be handling flammable gases, contact the manufacturer for up-to-date information (UEL and LEL are often listed in the MSDS that ships with products), or perform your own tests. Depending on your location, there are any number of independent testing labs that can measure vapour pressures and explosive limits, as well. TenOfAllTrades(talk) 14:23, 9 November 2007 (UTC)
- Of course Wikipedia is less reliable than a real encyclopedia. It has advantages in other areas, of course, but professional editing counts for something. --Anon, 02:00 UTC, November 9, 2007.
- Surprisingly, (actually, AMAZINGLY SURPRISINGLY) that's not true. Wikipedia is actually more reliable than almost any other encyclopedia - the venerable Encyclopedia Britannica is normally considered the 'gold standard' of encyclopedias - and Wikipedia is about on a par with it in terms of accuracy and miles ahead on breadth of coverage. Wikipedia is by any measure most certainly a 'real' encyclopedia - whatever that means. As to whether "professional editing" matters - I'd say that results say not. Here are some often-quoted facts:
- A 12-year-old kid [18] found five errors in Britannica after just a couple of days of checking. His only recourse was to write to the editor, and the errors may be corrected in print in a few years.
- Nature magazine did an extensive study of science articles by experts in those fields and found: "Our staff compiled lists of factual errors, omissions and misleading statements that the reviewers pointed to (we had 42 usable responses) and tallied up the total number for each encyclopaedia: 123 for Britannica, 162 for Wikipedia." - of course all 162 of the Wikipedia errors were fixed within a few days of the list being made public. You still can't buy a copy of Britannica with the 123 errors fixed up.
- Wikipedia:Errors in the Encyclopædia Britannica that have been corrected in Wikipedia
- Wikipedia:External peer review
- So don't write off Wikipedia as "obviously" less reliable - there have now been half a dozen proper academic studies - and they all sow that Wikipedia and Britannica are about equal - and both are miles ahead of any other general-purpose encyclopedias out there.
- SteveBaker 18:56, 10 November 2007 (UTC)
- Surprisingly, (actually, AMAZINGLY SURPRISINGLY) that's not true. Wikipedia is actually more reliable than almost any other encyclopedia - the venerable Encyclopedia Britannica is normally considered the 'gold standard' of encyclopedias - and Wikipedia is about on a par with it in terms of accuracy and miles ahead on breadth of coverage. Wikipedia is by any measure most certainly a 'real' encyclopedia - whatever that means. As to whether "professional editing" matters - I'd say that results say not. Here are some often-quoted facts:
- It's safe to say Britannica and the other "professionally edited" encyclopedias don't have quite the same vandalism problem, though... —Steve Summit (talk) 20:39, 10 November 2007 (UTC)
- Also, if only to play devil's advocate, those "often-quoted facts" might seem to an impartial outside observer to be a wee bit biased and one-sided. The first doesn't say anything about Wikipedia's error rate; the second says that Wikipedia has (or had) more errors than Britannica. The third and fourth come from Wikipedia itself, so despite Wikipedia's vaunted principle of NPOV, they can't be assumed to be entirely balanced.
- (I bet I could find five errors in Wikipedia after only a couple of minutes of checking -- what does that say?) —Steve Summit (talk) 20:46, 10 November 2007 (UTC)
- You aren't a 12 year-old. SteveBaker 23:29, 10 November 2007 (UTC)
- To answer the middle part of the question: yes, the website was "just put together by someone" -- quite a few different someones, in fact -- and it has also been checked, if not 100% systematically, by other someones.
- Now, for any given fact, was it entered correctly by the someone who entered it, and has it ever been checked by someone else? Maybe. Maybe even probably. But definitely not definitely. —Steve Summit (talk) 23:18, 9 November 2007 (UTC)
methylphenidate Q.
http://en.wikipedia.org/wiki/Ritalin#Known_or_suspected_risks_to_health
The R.A. El-Zein study says it will cause chromosomal abberations. I'm no biologist and dont really understand these things, but does this just refer to normal body cells and the only problem is that it could possibly cause cancer, or will it mutate gamete cells as well and these mutations passed on to offspring... thank you Rocktruly21 14:43, 9 November 2007 (UTC)
- That study only tested lymphocytes for chromosomal abberations, so this would be called "need for further testing." It should be noted, however, that this study's results were not reproduced to any extent by a much larger but otherwise identical study, as mentioned in the same section you linked to. Someguy1221 18:23, 9 November 2007 (UTC)
SILVER RINGS
hi again.. please choose one of those 4 choices as an answer. question= SILVER RINGS ARE... choices= a) homogenous mixture. b) compound c) element please justify to me your answer because i have a test coming up in this and i REALLLY need to know since my book doesnt provide an answer. thank you alot, jimmy —Preceding unsigned comment added by 212.71.37.73 (talk) 20:00, 9 November 2007 (UTC)
- Do you mean rings made of silver? If so, then, if they were not rings what would they be? —Tamfang 20:09, 9 November 2007 (UTC)
- Jewelry is not generally made from pure elements, even though they are named to make you think they are; pure gold or silver just don't have the necessary strength. RIngs are made of alloys, which are homogenous mixtures. When you're talking about jewelry, "gold" = gold + copper or nickel or palladium; the actual gold content will be specified in terms of karats, with 24 karat being pure gold. "Silver" = silver + copper or germanium, zinc, platinum, silicon or boron.; silver content is specified in terms of percentage. (a) is the answer you're looking for. - Nunh-huh 20:26, 9 November 2007 (UTC)
- Silver jewelery is most often an alloy called sterling silver. Graeme Bartlett 20:43, 9 November 2007 (UTC)
- Since this is a test - I'd bet the answer is 'Element' because silver is an element. There are practical matters to do with how jewellery is often made of an alloy of silver and something else - but unless you are doing a test in jewellery-science, they won't expect you do know that. The point is that 'silver' is not like 'bronze' (which is a homogeneous mixture) or 'sugar' (which is a compound). SteveBaker 21:48, 9 November 2007 (UTC)
- On the contrary, "silver" in the mouth of a jewelry dealer means "mostly silver" = silver alloy. If the answer isn't (a), demand a recount. - Nunh-huh 22:55, 9 November 2007 (UTC)
- Umm, what is (d), the fourth choice? hydnjo talk 23:38, 9 November 2007 (UTC)
the scifi kitchen
If you were to cook beef in an oxygen-free atmosphere, would it turn brown? —Tamfang 20:08, 9 November 2007 (UTC)
- I'm not a chemist, but the browning reaction is the Maillard reaction, in case that helps anyone. -- Coneslayer 20:17, 9 November 2007 (UTC)
- Yes it would still go brown, and black if you over heated it! Think what happens when you deep fry food. There is no oxygen in the liquid hot fat, but things still go brown or black. Graeme Bartlett 20:40, 9 November 2007 (UTC)
Ever heard of "ear music" heard by hearing impaired people?
A person who worked with hearing impaired people remarked at a seminar recently that there was such a thing as what she termed "ear music," if I recall the term correctly. I haven't found it under similar terms or this, but anyway, she said that it was sounds, almost like phantom limb of the ear, that the nerve endings pick up when it's really quiet that aren't there, as if a low radio were playing in another room or something. I asked specifically if tis might be hypnagogia, and she said this was something different, it wasn't just when one was nearly asleep. Have you ever heard of it? I found it odd there was an article under phantom eye syndrome here (which i'd never heard of myself]] but not phantom ear, but she didn't call it phantom ear. (In fact, if she had, I'd have guessed from context what she meant right away.)Somebody or his brother 20:15, 9 November 2007 (UTC)
- Sounds like something similar to hallucinations experienced during sensory deprivation. Information on the web on this seems scant- most seems related to various mental disorders and brain lesions, none of which relates to hearing impairment as a whole. But I'm not sure. Maybe check the references on that page, or see hallucinations in the sane. SamuelRiv 21:36, 9 November 2007 (UTC)
- You might be interested in Oliver Sacks newest book, Musicophilia: Tales of Music and the Brain, which devotes a whole chapter to this topic. It is apparently a lot more common than once thought, especially among the hearing impaired. Sacks calls it musical or aural "hallucination", while noting that some people object to the word "hallucination". Pfly 09:02, 10 November 2007 (UTC)
- (later addition) I skimmed the chapter this morning for a bit more specificity. It's chapter 6, titled "Musical Hallucinations". Some of the things Sacks says about it -- musical hallucinations were once thought rare and perhaps associated with temporal lobe epilepsy (TLE), but in recent years it has been recognized as more common and only rarely associated with TLE. Musical hallucination is not a psychosis, not "mental illness"; rather it is "real", "physiological", and benign. Sacks stresses this point -- some people have suggested a similarity between musical hallucinations, "hearing music", and schizophrenic "hearing voices", but Sacks shows how very different the two are, both physically in the brain and in the way they are experienced. Musical hallucinations can take many forms, but common aspects include: hearing music "for real", not just "in your head"; often associated with hearing loss and "emerging" from humming and buzzing type noises (eg, humming refrigerator, tinnitus, etc); more common in the elderly but can occur at any age; when caused by something like a stroke, tends to die away with recovery, otherwise musical hallucinations tend to be "very persistent" and "chronic". Some of the striking differences mentioned: for some people the music is hear very loudly, while for others it is soft and vague. For some it can be very annoying and even intrusively disruptive, while for others it can be pleasant and easily ignored. For some the music tends to be "whole pieces" or at least whole melodies, while for others the music "fragments" into tiny bits that skippingly repeat endlessly. Most people cannot control it, but some are able to "direct" it to some degree. It is not very well understood, neurologically. There is no cure. People for whom it is life-disrupting, a doctor might be able to find ways to reduce its strength. Sacks writes that of the people he knows of who have musical hallucinations, about 80% also have some kind of hearing impairment. Also, of all people with hearing impairment, about 2% develop musical hallucinations. There's lots more in the book, and as always Sacks writes very engagingly. Pfly 19:59, 10 November 2007 (UTC)
Why is chicken, rabbit and pork meat a different color from cows, deer,elk etc?
This isn't a joke, I am truly wondering why creatures with the same basic physiology have different colored meat. Respectfully, curious —Preceding unsigned comment added by 209.237.84.150 (talk) 21:00, 9 November 2007 (UTC)
- It's determined by the concentration of myoglobin in the muscle cells. See white meat and red meat. --Carnildo 21:40, 9 November 2007 (UTC)
- It's not just the myoglobin but also the concentrations of mitochondria. Dark meat has more mitochondira per cell, while white meat has less. David D. (Talk) 22:23, 10 November 2007 (UTC)
- As a sidenote, nitrite is used to prevent the myoglobin from decaying in cured meat. Icek 04:09, 11 November 2007 (UTC)
November 10
What is the oxidation number of carbon in methanol?
What is the oxidation number of carbon in methanol? According to the article oxidation number, I get 4 (four shared pairs of electrons, in which one of each pair belongs to carbon, results in a charge of +4 upon removal). --212.204.150.105 21:14, 7 November 2007 (UTC)
- Well, the oxidation state is the charge that carbon would have if the bonds were all ionic, so you need to know, for each bond, which atom is more electronegative. Given that this could be a homework question, I'm not going to tell you the answer. Someguy1221 21:29, 7 November 2007 (UTC)
- I know that the oxidation state is -2 (oxygen more electronegative and three hydrogens each less electronegative), however my question pertains to the oxidation number which according to the WP article can sometimes be different. I expect, that the oxidation number should also be two, but when I follow the instruction given in the first sentence of the WP article, I get 4, as described above. Is the article wrong or is my interpretation wrong (and if so, how)? --212.204.150.105 21:44, 7 November 2007 (UTC)
- I belive that's only in reference to coordinate bonds. Oxidation numbers are usually only used for metals. Someguy1221 21:55, 7 November 2007 (UTC)
- The article on oxidation state says Redox (shorthand for reduction/oxidation reaction) describes all chemical reactions in which atoms have their oxidation number (oxidation state) changed - firstly, I don't think it should say oxidation number (especially not preferentially to oxidation state) and thirdly, I read elsewhere that it should take into account the electronegativity - I think it's possible for an atom to be reduced even without a change in oxidation number, so long as the ligands of the product are less electronegative than the ligands before. Thus I think the article is at best misleading, if not wrong. --212.204.150.105 22:31, 7 November 2007 (UTC)
- I belive that's only in reference to coordinate bonds. Oxidation numbers are usually only used for metals. Someguy1221 21:55, 7 November 2007 (UTC)
- I know that the oxidation state is -2 (oxygen more electronegative and three hydrogens each less electronegative), however my question pertains to the oxidation number which according to the WP article can sometimes be different. I expect, that the oxidation number should also be two, but when I follow the instruction given in the first sentence of the WP article, I get 4, as described above. Is the article wrong or is my interpretation wrong (and if so, how)? --212.204.150.105 21:44, 7 November 2007 (UTC)
Slowing time
Can you make time for a particular area seem to go slower by moving away from it? That is, lets say, the TV is on, and I start moving away from it at half the speed of light, would the video on the TV appear to be going half its normal rate because it is taking longer and longer for the light to reach your eyes? What if it moved away from a given spot and I move away in the opposite direction at half the speed of light, would it appear to freeze. Of course, a TV that big that my eyes would be able to resolve it is impossible, but just asking... 208.63.180.160 00:01, 10 November 2007 (UTC)
- If you moved away from the TV, in addition to something similar to the doppler effect causing it to appear in slow motion, and the actual doppler effect making everything have a lower wavelength (redshift), the relatively slower speed of light through you would make time pass slower for you. It wouldn't counter it out perfectly. Two objects moving at half the speed of light away from a certain point aren't moving the speed of light away from each other, due to the time shift. This is all special relativity stuff. If it's too advanced for you, you may prefer Introduction to special relativity or the simple english article for it. — Daniel 01:42, 10 November 2007 (UTC)
- Moving either towards or away from the TV (or the TV moving towards or away from you) has the same effect, the speed of the show on the TV would change by a rate determined by the square root of 1-v2/c2. If your speed is half the speed of light then you have v=0.5c - so the time distortion would be sqrt(1-0.25) or about 87% of normal speed. —Preceding unsigned comment added by SteveBaker (talk • contribs) 18:36, 10 November 2007 (UTC)
- Hold on... I obviously have some sort of misunderstanding here. If the TV is moving towards me, will the photons not hit me a a higher rate than if it is moving away? If a tennis ball launcher throws a ball every second, and it is moving towards me, I will be hit by balls at a rate of greater than one per second; isn't the concept the same, so I will see the events on the TV speed up by moving towards it because the light has to travel a progressively shorter distance and thus I see "newer" light quicker than I would standing still? (I have no understanding of special relativity, and I'm not understanding the articles). 208.63.180.160 01:39, 11 November 2007 (UTC)
- Your view of the way light (photons) work is incorrect - and that one single fact is the entire reason we have relativity and everything that goes with it. Unlike tennis balls, the speed of light is a constant - irrespective of the motion of the source or the viewer. This was shown most clearly by the Michelson–Morley experiment. Hence, the photons coming from the TV will hit your eyes at exactly the same speed regardless of whether you are moving closer to the TV or further away from it. You'll see 'red-shift' (if you are moving away from the TV) or 'blue-shift' if you are moving towards it - but those are changes don't alter the speed. What's happening here is MUCH weirder than the 'classical' physics view that you are taking here. When you move at half the speed of light relative to the TV, the passage of time, distances and masses all change by that 87% factor - even after you take into account the fact that you are getting closer to it so that the light waves have a shorter distance to travel. SteveBaker 02:18, 11 November 2007 (UTC)
- Actually there is a speed-up effect when the TV is coming towards you at a good portion the speed of light, Steve. Imagine this isn't a TV you're looking at, but just a strobe light that pulses once a second in its own reference frame. Yes, if it's approaching you at 50% the speed of light, it only strobes once every 1.155 seconds from your perspective. BUT, since the strobe is coming towards you, the pulses will actually arrive faster than you'd expect. This isn't exactly the doppler effect, but it's very similar. I don't feel like precisely calculating which is more important, but its quite obvious if you look at extreme situations. Imagine the strobe begins transmitting from 1 light year away, but is travelling towards you at a Lorentz factor of one million. The strobe in this case is trailing so close to its own transmission that the entire 1 year lifetime of its transmission suddenly arrives at you in a 32 second spurt just a year after its light first reaches you. Now, it so happens that due to relativistic slowdown, the strobe only actually managed 32 strobes in this time, so the visual speed-up effect cancels out the relativistic slow-down effect (the former is entirely a deficiency of observation, the latter is physically happening). And so at best this can simply cancel out the appearance of relativistic slowdown, though certainly it would exacerbate such if it were traveling away from you. An effect that is not cancelled out by relativity, however, is the appearance that the strobe was traveling at a velocity equal to its Lorentz factor, in units of the speed of light. This actually has to be taken into account in observations of the plasma ejections of quasars, some of which have an "apparent velocity" greater than that of light, before this effect is accounted for. Someguy1221 02:50, 11 November 2007 (UTC)
- What you are describing is exactly the Doppler effect. Its magnitude is given by the Doppler formula, the longitudinal case of which is quoted in MrRedact's reply below. -- BenRG 00:17, 12 November 2007 (UTC)
Steve made a mistake. Your (the original poster’s) intuition about the case where the TV is moving away from you is almost correct. If you’re watching a TV moving away from you at half the speed of light, it will look like the show is being shown at 0.577 times as fast as its normal rate. In the limit as your speed away from the TV approaches the speed of light, the rate at which the show looks like it’s being shown approaches 0, i.e., it approaches looking like the TV show is stuck on one frame.
If you’re watching a TV coming at you at half the speed of light, it will look like the show is being shown at 1.732 times as fast as its normal rate. In the limit as your speed toward the TV approaches the speed of light, the rate at which the show looks like it’s being shown approaches infinity.
In general, if you’re approaching the TV at speed v, the apparent frame rate of the TV show is proportional to (1+v/c)/sqrt(1-v2/c2), where c is the speed of light. The same expression also works for the case that you’re moving away from the TV, in which case v is negative.
In reality, the colors of what's on the TV are affected if you're moving relative to the TV, so if you're moving too quickly, the colors would change so much that your eyes wouldn't be able to see them. MrRedact 08:18, 11 November 2007 (UTC)
- Has any tried watching a TV while moving at a fraction of the speed of light? Most of the time the screen will be too tiny to see! Even at 0.000001% of the speed of light you will only get a few seconds viewing out of it! (joking here). Graeme Bartlett 20:23, 11 November 2007 (UTC)
Greenhouse Gases
Are Greenhouse Gases good for the environment or bad? —Preceding unsigned comment added by 70.171.192.2 (talk) 02:24, 10 November 2007 (UTC)
- Have you consulted the article greenhouse gas? By themselves in moderate amounts they are not "bad" but in excess, at the rates that they are currently produced by human activities, they increase the greenhouse effect which leads to global warming. Which is bad. --24.147.86.187 02:31, 10 November 2007 (UTC)
- We need to have some, otherwise the earth would be freezing cold all over. Carbon dioxide is essential for the life of photosynthetic plants. Graeme Bartlett 12:09, 10 November 2007 (UTC)
- The 'normal' amount (about 0.038%) of CO2 is essential - less than that would be bad because the earth would freeze, more than that would be bad because of global warming. Right now, we have all the CO2 we need - and it's going up - so adding more greenhouses gas is definitely bad. Even if humans ceased to produce greenhouse gasses at all, the natural amount from animal respiration and volcanos would be plenty to keep the earth running OK. SteveBaker 18:31, 10 November 2007 (UTC)
- I have no idea where the idea that global warming is bad came from. An increase in global temperature has a positive net effect on humanity. Look at climatic changes of the middle ages in europe and you will see that the warm periods were better recieved by the population than the cold ones. The main cause are reduced heating requirements and increased agricultural yields. Putting this to a global perspective: there is not a single area on earth to hot to live in. Too dry, yes, but not too hot. However there is a whole uninhabitable continent at the south pole, covered in mountains of ice.
- So its good. The real problem is, at some point it will be too much. We don't know how the ecosystem reacts to really dramatic increases in temperature. It might be fine with 8° and collapse at 9°. Who knows? So the reason for reducing CO2 emissions is: we do not want to find this out the hard way. —Preceding unsigned comment added by 84.187.90.130 (talk) 00:49, 11 November 2007 (UTC)
- WHAT! WHAAAT! Where have you been the last decade? This is quite the most ill-informed, unthinking response I've ever read on this topic! Firstly, the change in the middle ages was small compared to what we're talking about - also it was over fairly quickly and this time around it's going to be permenant. Secondly, while crop yields increase in the extreme latitudes, they decline sharply in the equatorial regions - the net effect will be disasterous. Reduced heating requirements are replaced by increased air-conditioning requirements (you've never lived in Texas have you?!). The antarctic region might become free of ice - but whats uncovered will be bare rock - no soil. The sharply increasing sea levels will drown all of the nice flat primo agricultural plains around the coastlines. You suggest that an 8 degree rise "might be OK" - but I can tell you that a 4 degree increase will be plenty disasterous. Please - do some reading of the proper scientific literature on this subject...or if (as it seems) you are unable to learn from these sources - rent a copy of "An Inconvenient Truth" - it's very comprehensible. SteveBaker 02:08, 11 November 2007 (UTC)
- 0.038% is the current CO2 concentration, the pre-industrial level was about 0.027%. A question to the experts: How much higher would the equilibrium (glaciers need time to melt...) temperature be at 0.038%, compared to 0.027%? Icek 04:04, 11 November 2007 (UTC)
Battery charge
- How do rechargeable devices like mobile phones and mp3 players measure the amount of charge/energy left in the battery? Is it by measuring the terminal voltage? (which would be inaccurate due to polarization effects)? Or do they have ampere hour meters inside them?
- When we operate them continuously the meter suddenly drops low and after switching off for some time the bar goes back up a little; which would explain the drop in voltage due to polarisation. Am I correct in assuming that?
59.93.9.23 05:35, 10 November 2007 (UTC)
- Last I heard, the battery life indicator uses the voltage at the terminals. It is not accurate when it comes to predicting how long until the device quits. Each battery will be in a different stage of its life, and an older one will usually die sooner at, say, two bars. The graph is not linear even for any one battery, the change in voltage is very small, and current demand is unpredictable. You would need laboratory-standard equipment to make the thing at all accurate even under controlled conditions. I'm sure the phenomenon you mention in 2 is due to what is called polarisation, though the battery will drop some voltage internally under load anyhow. If it's right on the line between bars, you'll see a change upon power off just from that. The indicator will have some kind of anti-hunt designed in to keep the display from jumping around, so it will itself be slow to react. --Milkbreath 16:43, 10 November 2007 (UTC)
- This is a good question, which I wish I had some more definitive answers to. (But since when has lack of definitive information stopped an armchair RD reader from speculating?)
- There are at least four things a battery-charge indicator could look at in trying to make a determination of how much life the battery might have left:
- Terminal voltage. A battery is, of course, a two-terminal device, so fundamentally, this is all you've got access to. Unfortunately, by definition, a battery is supposed to be a constant-voltage device, so its voltage shouldn't (and doesn't) change much over its lifetime. A theoretically ideal 1.5-volt battery would give 1.500000000 volts for its entire lifetime, then crash precipitously to 0 -- so a charge indicator looking only at voltage would have nothing to go on!
- Of course, we don't have to limit ourselves to looking at instantaneous voltage; we can also look at rate of change (dV/dt). I'm pretty sure the voltage drops at different rates at various points during a typical (non-ideal) battery's discharge curve.
- History. Top-end, full-featured batteries (such as the ones used in modern laptops) contain their own microprocessors. These can learn what that particular battery's discharge curve looks like, and use that knowledge to make a much better estimation of how much life is left based on where in the (now known) discharge curve it looks like we are.
- Current draw. If the device has a built-in ammeter so that it can measure how much current is being drawn, and if it knows (perhaps based on historical information discovered by #3) what the battery's capacity in amp-hours is, it can make a very accurate estimation of how much life there is left. Of course, that estimate can and will vary if the current draw changes. I believe that's one reason why the bar can go back up. For example, I regularly notice my laptop's expected lifetime jump back up just after I stop doing something CPU-intensive.
- Of course, this is all complicated by real-world considerations. Rechargeable batteries have a limited number of charge-discharge cycles, and can't hold a charge for as long the more cycles they've gone through, and are also prone to notorious "memory" effects. (I don't know how hard microprocessor-based batteries work to assess these effects; though they certainly could.) Also, most batteries show a sort of "rejuvenation" effect when they've been given a chance to rest after working hard (i.e., just like you or me). That's the other possible explanation for the bar-going-back-up phenomenon you noted. —Steve Summit (talk) 23:04, 10 November 2007 (UTC)
Thanks for the replies. Googling gives (what seems to be the terminology for this sort of thing) State_of_charge. Some websites and the wiki article say that in laboratory conditions they measure the concentration of the electrodes/electrolytes The results also include the microprocessor thingi. It seems to be called Charge_controller device. 59.93.9.69 04:35, 11 November 2007 (UTC)
Freezing Water Question
What would happen if you tried to freeze water it it were confined so it could not expand? For instance if a quantity of water were enclosed in a solid block of steel and the whole thing were subjected to low temperature and the water could not expand as it would when freezing, what would happen to the water? Would it stay liquid or freeze solid without expanding???? —Preceding unsigned comment added by 207.69.137.23 (talk) 05:57, 10 November 2007 (UTC)
- Depends on where it is on the phase diagram. Freezing water in a confined space creates pressure on the order of several atmospheres (you can drive a go-cart with it) which may push it back into liquid phase, but again, it depends on both pressure and temperature. The way the ice crystals form is also dependent on this diagram, so you should really just check out the phase diagram article. SamuelRiv 06:11, 10 November 2007 (UTC)
- Better yet, check out the responses from the last time we had this question. In brief, as the temperature is lowered, at first the water will remain liquid and generate an increasing pressure. If the temperature continues to be lowered, eventually it will freeze into a form (phase) of ice denser than the everyday kind. --Anonymous, 06:16 UTC, November 10, 2007.
anti inflammatory
do anti inflammatory medicine (gen-naproxen to be specific) affect your mood? —Preceding unsigned comment added by Morvarid rohani (talk • contribs) 08:08, 10 November 2007 (UTC)
- In the list of side effects of naproxen (see here), no mood disorders are listed as frequently reported. However, depression has been reported in 1% to 10% of patients, and anxiety has been reported in <1%. Reports do not necessarily indicate causation. The fact that a drug causes a side effect in some patients does not mean that it is the cause in a specific instance; any question of whether a drug is responsible for a specific clinical condition should be discussed with a physician. - Nunh-huh 08:45, 10 November 2007 (UTC)
Bullets
Hi. How exactly does a bullet to the brain kill? Why do some people shoot the mouth, yet others shoot the temple? At what point does life cease? 203.124.2.43 11:44, 10 November 2007 (UTC) Adam
- I used to have a great animation of a bullet going through the brain, bouncing off the other side of the skull and basically making a soup of the brain matter after bouncing around several more times, but I can't find it. When a bullet enters one side and exits the other, the entry and exit take a lot of compressed gases and brain matter with them making a small explosion in those areas, which can greatly increase trauma (that mostly depends on the bullet head shape). Sometimes bullet injuries leave people alive, like Manfred von Richthofen (the Red Baron) and Phineas Gage (a railroad spike, not a bullet). In Phineas's case, the spike damaged mostly one area of the frontal lobes, which govern a lot of higher-order reasoning and personality, but not so much in terms of low-order processing.
- So let's get to the meat of your question: assuming a pointed bullet with enough speed to not bounce around the brain so that extra trauma is minimized, how do you kill someone? Shooting through the temple kills mainly by hitting the limbic lobe, which contains a lot of mid-order processing (thalamus), memory, and important regulatory glands. It is a guaranteed kill in some sense because you can destroy everything a person perceives about the outside world, whether or not their heart actually stops immediately. Shooting through the mouth is more appropriate because on the other side lies the brainstem and cerebellum, both of which control low-order function like breating and heartbeat, with the brainstem being the connection of the brain to the body. So the kill in this case would be roughly instantaneous, though actual brain death would occur a bit later, because there would be some latent blood flow.
- One more thing - severe brain trauma results in a couple of defense mechanisms by the brain. One is the coma, which for most bullet injuries would set in quickly, so the person would be incapacitated in any case. The other is a release of something (I forget what - I believe glucose) into the cell ether which results in a massive killing of brain cells. My neuroanatomy professor referred to it as a "self-destruct mechanism", but we don't know yet why it exists. Regardless, that can easily make brain trauma much more damaging than that of the actual impact. External links: [19] and [20] SamuelRiv 14:07, 10 November 2007 (UTC)
- 1. A brain can't absorp the shock of the bullet's entry without sustaining damage. 2. If that doesn't kill, bleeding will. - 87.211.75.45 19:32, 11 November 2007 (UTC)
Gold-labelled antibody = "fusion protein"
Is it appropriate to cover protein-non-protein ligations in the fusion protein article? I was just starting an article called conjugation (biochemical) and want to determine whether its warranted or not. Conceivably, one article should cover conjugation of proteins with other proteins, non-protein molecules, and possibly even non-protein-non-protein conjugations (none spring to mind). Perhaps the article protein engineering is more suitable for this? In which case, I can add a link at the conjugation disambiguation page? --Seans Potato Business 13:17, 10 November 2007 (UTC)
- Wikipedia probably has many stubs such as Radioiodinated serum albumin and Biotinylation that could be collected into Protein labeling. I suggest not placing most "protein labeling" methods in the fusion protein article, but some fusion proteins are used to attach a label to target proteins. Sadly, Green fluorescent protein only seems to have an external link for the important topic of GFP fusion proteins. Maybe Conjugated protein could be expanded to include both natural and artificially conjugated proteins. I'd leave protein engineering to itself. --JWSchmidt 14:52, 10 November 2007 (UTC)
- (EC) As a biochemist, I've never seen the term 'fusion protein' used to describe a non-protein combination. 'Fusion protein' is a bit more specific; it implies that a change has been made (insertion, replacement, and/or concatenation) to the primary amino acid sequence of the protein. This runs the gamut from adding a little tiny His tag through humanizing an antibody to attaching a whole additional protein (like GFP or a second part of an enzymatic complex). I would include under the 'fusion protein' definition the addition of domains that will dock particular prosthetic groups (a domain that binds a single metal atom, for instance).
- For something like a gold-labelled antibody, you could use exactly that term. 'Immunogold', 'gold-tagged antibody', and even 'gold-conjugated antibody' come up a fair bit, too. For a general term to describe 'sticking something interesting to a biomolecule', 'conjugation' is probably as good a word as any.
- As an aside on the topic of 'non-protein-non-protein conjugations' you need to remember the other important classes of biomacromolecules: DNA, RNA, and polysaccharides. All of them can be (and often are) modified with various sorts of labels (fluorescent, radioactive, immunogenic) to allow them to be studied. Conjugates that modify their function are also used sometimes, though this is perhaps less common. On a terminology point, the DNA equivalent to a 'fusion protein' would probably be 'recombinant DNA'. TenOfAllTrades(talk) 15:11, 10 November 2007 (UTC)
- So perhaps conjugation (biochemical) would be a suitable umbrella for all conjugations, protein and otherwise. I don't have time for it now, but eventually... --Seans Potato Business 23:36, 10 November 2007 (UTC)
- I think that should read Conjugation (biochemistry) (so it avoids adjectives in the brackets). - 87.211.75.45 19:29, 11 November 2007 (UTC)
Neurotransmitters
In "Talk:Neurotransmitter#Neurotransmitter effects", I have described a recent experience involving dopamine, norepinephrine, and serotonin, attempting to link symptom clusters with specific neurotransmitter changes and extremes. Does my interpretation appear correct? Which receptors appear to have been preferentially overstimulated or understimulated? 66.218.55.142 15:13, 10 November 2007 (UTC)
DNA sequence around integrated HIV viral DNA attachment site?
Can anyone point me to books, published articles or other currently available research data which describe exactly what is the base pair sequence of integrated HIV viral DNA - I mean after the integrase process is completed, what is the DNA base pair sequence around those "attachment" sites?
For example, what is the sequence when DNA in cell is cut in order to integrate viral DNA segment:
--TG...and_here_comes_HIV_DNA...CA--
--AC............................GT--
(This above is just example of what I'm looking for, those TGAC.. are just for example!)
And then once the whole thing is integrated, what are those first few base pairs around both attachment sites? For example:
--TG...(U3RU5---HIV-DNA---U3RU5)...CA--
--AC...............................GT--
So, what are U3 and U5 of linear terminal repeat (LTR) attached to once the segment is integrated (the sequence of first few base pairs on both sides) and what does it look like together with U3 and U5 on both sides?? MANY THANKS to anyone who can point me to literature which covers this. --80.95.231.124 16:13, 10 November 2007 (UTC)
- Have you seen HIV structure and genome? The literature at the end might be useful. SamuelRiv 17:34, 10 November 2007 (UTC)
Rocket speed
After how long does a space rocket reach 500 km/h and 1000 km/h ? Is there a graph of speed/time for rockets somewhere? I'm not very good with equations and I couldn't find the ones I thought necessary in the article rocket. Keria 17:54, 10 November 2007 (UTC)
- Well, obviously, the time dramatically varies depending on the type of rocket. Something like the AIM-9 air-to-air missile accellerates at over 20g's. The space shuttle accellerates at a more pedestrian 3g's - so it piles on speed at roughly 30 meters per second every second. So we convert 500km/h into meters per second (500,000m/3600seconds = 138 meters/second) - takes roughly 138/30 seconds...4.6 seconds! To get to 1000km/h takes only 9.2 seconds. Well, in reality it's a lot more complicated than that - the various rockets start off accellerating fairly slowly because of the weight of all of the fuel - as the fuel burns off, the accelleration builds up - but at some point before the shuttle leaves earth's atmosphere, the speed becomes too high and the engines have to be throttled back to relieve the pressure on the spacecraft. Then as they get higher up and the air is thinner, they can go faster. When the SRB's detach, the spacecraft gradually slows down until (as yet more fuel is consumed), the engines can push the accelleration up again. Finally, the g forces start to get too large and again the engines have to be throttled back. Hence, in reality, the total time is going to be longer than 9.2 seconds - but still, it's piling on speed pretty amazingly fast. However, that AIM-9 rocket adds 200 meters per second every second - so it hits 500km/h within about two thirds of a second and 1000km/h in one and a half second! SteveBaker 18:23, 10 November 2007 (UTC)
- You're a hell of a pedestrian. Hope I'll never run into you. —Preceding unsigned comment added by 84.187.90.130 (talk) 00:35, 11 November 2007 (UTC)
Robot Cars
What exactly is an autonomous robot car and how does it work? Could it work for a smaller or toy car? —Preceding unsigned comment added by 68.120.224.217 (talk) 19:27, 10 November 2007 (UTC)
- See DARPA Grand Challenge and links. --Sean 20:35, 10 November 2007 (UTC)
- Currently, they take a standard car and add levers to push on the pedals and shift the shifter - also something to turn the steering wheel - those are hooked up to a computer that can therefore drive the car just like a human would. The tough part is that the computer has to be able to see where it's going and have enough intelligence not to do anything stupid like ramming the car into a brick wall. For this they use a combination of digital video cameras, and laser range finders (see Lidar - like Radar but with light instead of radio waves). Each individual technology is quite well known and understood - the tricky part is getting them all to work well together. We could make toys that did this - but the lidar sensors and the amount of computer power needed would be quite significant. But toys like the Aibo robotic dog are fairly sophisticated and can do some quite impressive things. It'll happen sooner or later. SteveBaker 00:24, 11 November 2007 (UTC)
- You can do a lot with Lego Mindstorms, too. (I think; I still haven't given in and gotten a set for myself yet.) —Steve Summit (talk) 02:36, 11 November 2007 (UTC)
- I have a few of the older sets - the newer ones are simultaneously better and less good for complicated reasons. But yes - you can easily build a "blind" robotic car using Mindstorms - but one that senses it's world and reacts accordingly (such as the ones in the Darpa Challenge) is MUCH harder and isn't really possible with the limited sensors that Mindstorms provides. It's relatively easy to do things like building a car-like robot and having it follow a black line made with electrical tape - or having it seek out light and park itself in a puddle of sunlight on your livingroom floor. But driving around in a complex environment without colliding with things and mowing down pedestrians...there is no way that a Lego Mindstorms machine could do that. It's a lot of fun though - and it's about on the limit of how complex something can be and still be considered a "toy" that kids could build and program. The most fun I ever had with it was making a pair of identical robots that could play "tag" in a darkened room. Each one had a bright light on the top and a 'bump' sensor that told it if it had been run into. One robot was programmed to seek light and the other to avoid it. When the 'bump' sensor detected that it had collided with something, the robot that was seeking light would send an infra-red message to the other robot saying (essentially) "You're It!" and switched from seeking light to avoiding it. When the other robot received a "You're It!" message, it would stop still, beep ten times at one second intervals and then switch from avoiding light to seeking it. The result of two of these little guys scurrying around the room was just hilarious to watch - but (and this is the ENTIRE problem here) the system only worked if neither of them went behind the sofa (killing the light seeking/avoiding behavior) or if they bumped into something else other than the other robot. I was able to add some sophistication to kinda/sorta fix those problems - but it rapidly becomes obvious that you need something better than a simple directional light sensor. Having a camera and lidar would help a lot! Some enterprising people were able to make a very crude lidar-like system using the infrared messaging system to send a message and looking for IR reflections in the light sensor. The strength of the returned reflection enabled some limited range measurement - but it was very crude. SteveBaker 02:51, 11 November 2007 (UTC)
Jupiter (the planet)
How was Jupiter formed and how old is it? —Preceding unsigned comment added by 72.38.227.206 (talk) 22:07, 10 November 2007 (UTC)
- You can read about it at Jupiter. —Steve Summit (talk) 22:21, 10 November 2007 (UTC)
- ...which, I'm sorry, doesn't really answer your question. Anybody have a better reference? (There's a bit of information at Solar System.) —Steve Summit (talk) 22:27, 10 November 2007 (UTC)
- Planetary formation (which, for some reason, is currently a redirect to "Nebular hypothesis") may answer this and the following question in more (perhaps too much) detail. In any case, the best one can say about the ages of the planets is that, as we currently understand planet formation, they're all about equally old, and slightly younger than the Sun itself. The reason it's hard to even define an exact age for a planet is that, after the initial aggregation of small planetesimals from the protoplanetary disk, they are believed to have undergone an "oligarchic growth" phase where the proto-planets of various sizes grew by colliding and merging at random. Thus, it's hard to say which collision was the one in which the resulting planet became the one we know today. There's no specific start or end to the oligarchic growth phase either; while the biggest planets eventually settled into more or less stable and non-colliding orbits, the smaller, more numerous bodies continued to collide with them and each other at gradually decreasing frequencies, as they still do to this day.
- Also, it's worth noting that, for gas giant planets like Jupiter, there are two competing theories of their formation. The one that, as far as I can tell, seems to be enjoying the greatest popularity at the moment is that they started out like the smaller planets, as solid bodies growing by collisions, but eventually grew big enough that their gravitational pull could directly capture and hold down gas from the surrounding protoplanetary disk, leading to runaway growth as more and more gas fell down upon the initial rocky core. This didn't happen with the inner planets because, by the time they grew big enough, the solar wind had already blown most of the gas away from that part of the solar system. The alternative hypothesis, however, is that the gas giant planets formed directly from the gas disk without any solid "seed"; according to that theory, an eddy in the protoplanetary disk simply pulled enough gas together that its mutual gravitational pull caused it to collapse, much as stars are believed to form. Neither hypothesis is by no means disproved yet — it's even possible that gas giant planets can and do form by both mechanisms. —Ilmari Karonen (talk) 00:07, 11 November 2007 (UTC)
The planet Mars
How old is Mars? —Preceding unsigned comment added by 72.38.227.206 (talk) 22:15, 10 November 2007 (UTC)
- You can read about it at Mars. —Steve Summit (talk) 22:21, 10 November 2007 (UTC)
- ...which, I'm sorry, doesn't really answer your question. Anybody have a better reference? (There's a bit of information at Solar System.) —Steve Summit (talk) 22:27, 10 November 2007 (UTC)
- I presume Mars must have been formed at roughly the same time as the Earth - roughly 4.5 billion years ago. (See Formation and evolution of the Solar System and Age of the Earth.) We haven't studied the geology of Mars well enough to know for sure. But the age of the Earth is something of a compromise between the age of the oldest rocks we can find (3.9 billion years) and the age of the solar system (4.6 billion years). It's very likely that even after we have crawled over every inch of Mars looking for old rocks, our answer will be about the same. SteveBaker 00:17, 11 November 2007 (UTC)
- Part of the problem with rock ages is that rocks form and reform due to subduction of the Earth's crust and the much different environment of early Earth, so today's rocks are going to be younger than Earth as a whole. Also note that most of our aging techniques won't work on liquids like magma. SamuelRiv 01:27, 11 November 2007 (UTC) Addendum: note there is no plate tectonics on Mars, though there is volcanism. Thus somewhere deep under the surface may be a rock as old as the solar system. SamuelRiv 01:28, 11 November 2007 (UTC)
- I have some quibbles with that. Firstly, we know for sure that the young Mars had volcanoes. Olympus Mons - for example - is the largest extinct volcano in the known solar system! If Mars once had a liquid core sufficiently close to the surface to allow volcanism then it seems entirely possible that it once had plate tectonics too. Secondly, despite the ravages of plate tectonics, the date of 3.9 billion years for the minimum age of the earth comes from dating zircon crystals from Western Australia - so despite subduction and volcanism - we still have some pretty old rocks lying around at the surface where we can find them. SteveBaker 02:30, 11 November 2007 (UTC)
- I addressed the issue of volcanism, and note the volcanoes on Mars are all shield volcanoes (like Hawaii), and thus are not dependent on plate tectonics. I'm aware that very old rocks have been found on Earth - I'm just addressing why they might be hard to find, and indeed why one as old as the Earth may be impossible to find. SamuelRiv 16:23, 11 November 2007 (UTC)
Cause and Effect
Is it right that not one thing in the Universe hasn’t obeyed the Laws that govern it? If EVERYTHING obeys the rules of Cause and Effect is it not true that everything happens for a reason? If this is true it would seem that nothing can be random and everything is predicatbale. Sorry if this is the wrong place to ask such a question. —Preceding unsigned comment added by 71.150.248.92 (talk) 22:34, 10 November 2007 (UTC)
- This topic has dogged philosophy and religion since time out of mind. There's a bunch of stuff on the topic at Causality, Determinism, and Free will. It seems obvious that events are caused by other events. I wouldn't be typing this right now had you not asked the question. But it seems just as obvious that we are not robot-like automatons. I feel as if I am making choices about the words I type. The conflict between these two obvious things is, I think, at the heart of most religions and much of philosophy. Personally, I think that something must be wrong with the question, since people have been asking it for thousands of years and still end up in paradox. Pfly 22:57, 10 November 2007 (UTC)
- For many interesting physical systems, predictability is not possible because even small variations in where you are now can result in large changes in where you will be. See Chaos theory. --JWSchmidt 23:29, 10 November 2007 (UTC)
- Well, let's be really careful here:
- Is it right that not one thing in the Universe hasn’t obeyed the Laws that govern it? - Yeah - that's right. If some 'law' is ever disobeyed then it isn't a law...so this is true by definition. Is it the case that some of the things we humans believe are 'laws' are wrong? Yes - it's really unlikely that everything we think we know is 100% correct. Newton's "laws" of motion were proven wrong by Einstein's relativity.
- If EVERYTHING obeys the rules of Cause and Effect is it not true that everything happens for a reason? - "Cause and Effect" is not a law. To the contrary, many quantum effects do not follow 'cause and effect' and are fundamentally unpredictable. If you irradiate an atom and stuff some extra neutrons into it - it will eventually decay back down to it's original state by emitting a neutron. When will that happen? Well, we don't know, we cannot know - it's truly, utterly random. Where is the "cause" of that neutron being emitted? There isn't one.
- If this is true it would seem that nothing can be random and everything is predicatbale. - To the contrary, at its heart absolutely everything is completely random and unpredictable. The only thing that makes the universe seem stable and follow nice cause-and-effect rules is the effect of statistics. We don't know when one irradiated atom will decay - but we know with great precision how a kilogram of atoms will decay. We can't know the exact position of an electron - but we can deduce the position of a planet orbiting a star a hundred light years away by measuring the tiny wobble it induces in that star. The universe on the large scale obeys rules - but at the small scale, it's truly random. But the large scale effects are pure statistics. It is perfectly possible (although exceedingly unlikely) for a grand piano to appear out of nowhere in your living room right now. It's only statistics that enables us to say that this "Won't ever happen".
- Look at it like this, if you flip a coin, you have no idea whether it'll come up heads or tails. If you flip 100 coins, you can be pretty sure that between 40 and 60 heads will show up - but predicting that you'll get 50% heads is a bit 'iffy'. If you flip a million coins, you can be quite sure that between 499,000 and 501,000 heads will show up - so a 50% prediction is a fairly accurate 'law'. If you flipped as many coins as there are atoms in a grand piano, your prediction of 50% heads would be precise to within one part in a billion billion billion (probably much better than that actually). In effect, you have a cast iron "law" of nature that says "when you flip coins you absolutely always get exactly 50% heads" - but that's not even close to being true for four coins - and it's POSSIBLE to flip a million coins and for them all to come up heads...it's just so unlikely that on a large scale, it's not going to happen. That's how our "large scale" laws operate. They are so accurately true that we can rely on them - even though at their heart, they are relying on completely random events.
- Sorry if this is the wrong place to ask such a question. - This is the perfect place to ask this question!
- SteveBaker 23:58, 10 November 2007 (UTC)
Thanks for all the replies. Very interesting answers. —Preceding unsigned comment added by 71.150.248.92 (talk) 02:45, 11 November 2007 (UTC)
- I have read alot of extremely well-researched, wonderfully articulated replies on the Science Desk, but SteveBaker's reply to this ultimate of all quandries is truly amazing. The idea that science, even at its finest, boils down to statistics, and that those statistics when viewed from an appropriate macroscopic level may be termed as "laws" is an idea I've been aching to come across. Thank you! Sappysap 04:05, 11 November 2007 (UTC)
- Er...wow! Well, thanks! Before we get carried away though - the critical 'take away' point here is that while these macroscopic laws are "only" statistical, the magnifying effect of the sheer quantity of particles on the certainty of the result makes the resulting law quite utterly cast-iron. You cannot and must not take from my explanation the idea that the macroscopic laws are broken routinely because of this statistical stuff. On human scales - they absolutely are not. The probability of anything measurably different from what we expect actually happening is so astronomically small that this makes it impossible for any practical measure whatever. So "certainty" is still present at our scales. But when we deliberately make the small scale visible on the large scale, weird stuff can happen. Listen to the individual clicks of a Geiger counter picking up background radiation (Image:Geiger calm.ogg for example) - each click is the result of the decay of a single atom producing a single neutron. Guess what? It's utterly random - you can clearly hear that - there is fundamentally no way to predict when the next click will happen. SteveBaker 05:43, 11 November 2007 (UTC)
- Well, I'm not listening to a Geiger counter per se, but just now I happen to be listening to "Radio-Activity", by Kraftwerk, which starts out with the sound of one. Weird coincidence, or subtle causality? (You be the judge. :-) ) —Steve Summit (talk) 06:01, 11 November 2007 (UTC)
- Responding here to Steve's claim that "certainty is still present at our scales" except when "we deliberately make the small scale visible on the large scale". I don't believe that's true. The effect of quantum indeterminacy can easily be blown up to a macroscopic scale by processes not requiring our deliberate intent, by any system that chaotically amplifies small differences with positive feedback. Like weather. So I think, for example, that the question "will it rain in Dallas on the afternoon of July 17, 2063?" is truly non-determined; quantum uncertainty now, on a microscopic scale, will have been amplified to different macroscopic answers by then.
- He's certainly correct that there are questions we can ask where the answers are quite deterministic for practical purposes. But those are the questions where small differences tend to cancel out, rather than being amplified. --Trovatore 22:00, 11 November 2007 (UTC)
November 11
Physics and Ancient Greece
I remember my grade school science teacher once telling us that Ancient Greece not only knew the world was round, but more amazingly they also knew its rough diameter. The ancient experiment took place on a wide and very flat plain; on it, two towers spread miles apart. The towers would signal each other in daylight and then quickly measure how long the shadows of each tower fell on the plain. That was all they needed to roughly measure the size of the world. I liked this science teacher quite alot and so I almost hate to ask: is an ancient experiment of this kind roughly possible? A lesser question: without too much research, is there any historical corrobaration for the rough diameter of the world being published more than two thousand years ago? Sappysap 00:16, 11 November 2007 (UTC)
- Eratosthenes is the first known to have calculated the circumfrance of the earth. He did conduct an experiment very similar to what you describe to estimate the curvature of the Earth. His estimate was probably a bit off but pretty good for a first shot! You can look at History of geodesy for more discussion of his method and later methods. --24.147.86.187 00:26, 11 November 2007 (UTC)
- Yep - the numbers that these techniques could come up with would be very approximate - but they could certainly prove that the earth is round simply by noting that the further North you went, the longer the noon-time shadow is - but the length of the shadow doesn't change when you go East/West. I don't think they used two towers that could see each other though. What I thought they did was to measure the length of the shadow at noon for two locations hundreds of miles apart on the same day. Because they knew (roughly) the North/South distance between those locations, they could do the calculation without requiring communications between the towers. They knew that at noon the shadow was at it's shortest - so precise timekeeping was not needed. Not requiring the two towers to be able to see each other means that they can be a lot further apart - which makes the whole calculation much more accurate. But the observation that the length of the day is more variable at higher latitudes is theoretically enough to allow an observant person to deduce that the earth must be round. SteveBaker 00:42, 11 November 2007 (UTC)
- According to the Eratosthenes article: "The exact size of the stadion he used is argued by those who suppose he got it right; but the common Attic stadion was about 185 m, which implies a circumference of 46620 km, i.e. 16.3% too large."
- I would consider an accuracy of 1/7 very impressive for someone who did this calculation over 2000 years ago.
- Eratosthenes decided to use two cities instead of two towers. The Sun shined directly down a well in one city on the noon of every summer solstice, it was at the zenith at these times. In the other city, he measured the noon sun's elevation during another summer solstice. This would give the cities' latitude difference, and the Earth's circumference can easily be computed if the distance between the cities is known. --Bowlhover 07:24, 11 November 2007 (UTC)
- That can't be exactly true. The sun only shines "directly" down a well when you are near the equator. Greece is at roughly 38 degrees north - the earth's axial tilt is 24 degrees - so even at mid-day on the summer solstice, sunlight would shine down the well at an angle of roughly 14 degrees to the vertical. In the end, the problem boils down to the precision with which you can measure the angle of the sun (or the length of a shadow or the depth to which sunlight penetrates a well - all of which amount to the same thing) - and the precision with which you can measure the north/south distance between your two points. Picking two cities far apart makes accurate measurement of the distance between them tough - but reduces your dependence on the accuracy of sun's elevation. Picking two points closer together gives you better precision on the distance between them - but the precision with which you measure the angle of the sun becomes vastly more critical. SteveBaker 14:27, 11 November 2007 (UTC)
- Minor correction - you don't have to be exactly on the equator for the sun to be at the zenith at noon on certain days of the year - you only have to be between the Tropic of Cancer and the Tropic of Capricorn. One of the end-points of Eratosthenes baseline was Syene or modern-day Aswan, which is slightly north of the Tropic of Cancer. Our article says:
- The latitude of Aswan – 24° 5′ 23″– was an object of great interest to the ancient geographers. They believed that it was seated immediately under the tropic, and that on the day of the summer solstice a vertical staff cast no shadow, and the sun's disc was reflected in a well at noonday. This statement is only approximately correct; the ancients were not acquainted with the exact tropic: yet at the summer-solstice the length of the shadow, or 1/400th of the staff, could scarcely be discerned, and the northern limb of the sun's disc would be nearly vertical.
- Eratosthenes presumably knew this bit of "folklore" about Syene, and he took his home town of Alexandria as the other end of his baseline, so he could estimate the size of the Earth from measurements made in his own garden. Gandalf61 16:54, 11 November 2007 (UTC)
- Yes, Aswan is in Egypt and not Greece. Using Aswan was logical because if the sunlight illuminated the bottom of a deep well, the Sun's altitude could accurately be determined as 90 degrees. If an accurate data point exists, why not use it? --Bowlhover 17:49, 11 November 2007 (UTC)
- Minor correction - you don't have to be exactly on the equator for the sun to be at the zenith at noon on certain days of the year - you only have to be between the Tropic of Cancer and the Tropic of Capricorn. One of the end-points of Eratosthenes baseline was Syene or modern-day Aswan, which is slightly north of the Tropic of Cancer. Our article says:
- The funny part is that Posidonius repeated Eratosthenes calculation, via slightly different means, and came up with essentially the same result -- and a fairly accurate one at that -- but then revised his calculations such that the circumference was about a third to small. And this figure got incorporated into Ptolemy's Geographia which, along with medieval calculations by Islamic geographers that tended to support the smaller figure, greatly influenced Christopher Columbus's theory of Asia being located approximately where the Americas are. History is funny. Pfly 07:34, 11 November 2007 (UTC)
- The bottom line: Eratosthenes and other greeks know perfectly how to estimate a sphere's diameter. The problem for them was that the instruments they had at their disposal were some dracmas and a man's footsteps. But apart from those minor details, they made perfect scientists. --Pallida Mors 76 22:51, 11 November 2007 (UTC)
does inhibition of PKC-β activity decrease pigmentation in vivo?
how? is it permanent? —Preceding unsigned comment added by 81.99.212.22 (talk) 01:03, 11 November 2007 (UTC)
- According to this article, PKC-β phosphorylates and activates tyrosinase and "topical application of a selective PKC inhibitor reduces pigmentation and blocks UV-induced tanning in guinea-pig skin". They also discuss regulation at the level of transcription. --JWSchmidt 01:17, 11 November 2007 (UTC)
Feces - taste like what?
I've always wondered what human feces (faeces) taste like.
I'm aware that this might be a strange question. I also realize that I could easily provide my own answer, but I'd prefer to leave it to someone with an interest in coprophagy. —Preceding unsigned comment added by 222.155.51.145 (talk) 07:40, 11 November 2007 (UTC)
- Most of the taste sensation is due to the smell. SO I think you could imagine the experience. But you would have the texture and tongue taste as well. The average 1 year old probably can remember the experiencem(but not me)! Graeme Bartlett 10:10, 11 November 2007 (UTC)
Without having performed any OR on the matter, I can nevertheless confidently assert that they taste like shit. —Steve Summit (talk) 14:23, 11 November 2007 (UTC)
After digestion, very little that stimulates taste receptors should remain. Probably the only recognizable taste would be salt, but no saltier than blood. 66.218.55.142 14:40, 11 November 2007 (UTC)
- I was under the impression that it had a spongey texture, although I cannot remember where I encountered this gem of information. [21] will perhaps help you on your enquiry. (Really, don't follow that link unless you have a strong stomach). Lanfear's Bane | t 15:08, 11 November 2007 (UTC)
- For your sake and for mine, let us hope that that is peanut butter. Can I wash my eyes now? --Russoc4 16:47, 11 November 2007 (UTC)
commercial radioisotope
What is a commercial radioisotope? —Preceding unsigned comment added by 144.137.98.219 (talk) 10:17, 11 November 2007 (UTC)
- It is a radioactive isotope that you can purchase as a standard product, such as 131Iodine —Preceding unsigned comment added by Graeme Bartlett (talk • contribs) 10:52, 11 November 2007 (UTC) or tritium. Graeme Bartlett
asteroid belt
if some spaceship tries to cross the asteroid belt, then instead of by passing through it, can it go above it and cross the belt?SidSam 10:34, 11 November 2007 (UTC)
- A spaceship could go above or below, but asteroids occasionally will orbit at an inclined angle and stray into that space too. The belt is actually very thin and the chances are that a spaceship will fly through and not be impacted. The problem is that more fuel is needed to go outside the eccliptic plane, and then back into it. Space ships always scrape by on the minimum fuel, so such maneuvers are unlikely unless there is a reason to go up and out there. Graeme Bartlett 10:56, 11 November 2007 (UTC)
- Tbe asteroid belt is more like a flat ring around the sun - like one of Saturns rings - so you could certainly go around it. But the rocks within it are widely spaced - so it's pretty safe to travel through it as though it wasn't there. SteveBaker 14:04, 11 November 2007 (UTC)
Aldol
In the aldol condensation of acetone, mesityl oxide is created. What then, if anything, prevents a further acetone molecule from adding on to the other side of the product, resulting in an endless chain of carbonyls? Or, what prevents the mesityl oxide from adding onto another molecule of acetone, creating a symmetric compound with another isobutene group? What about endless Michael reactions at the double bond? Could't you theoretically just keep making one infinitely larger compound, as long as there are alpha-hydrogens and/or alpha-beta double bonds? --Russoc4 14:56, 11 November 2007 (UTC)
- Yes, that's called polymerization. Apparently it can be a serious problem when you don't want to make polymers, because it deactivates the catalyst.[22] [23] That second one says "The active sites for aldol condensation are the same as the active sites for polymer production.". There are even patented methods to inhibit the polymerization.[24] —Keenan Pepper 17:13, 11 November 2007 (UTC)
- So then, disregarding any Michael reactions, these [25] would be the possible aldol condensation products? What do you think would happen if I added acetone to a concentrated NaOH solution and heated it? Would that polymer be a likely outcome? --Russoc4 18:01, 11 November 2007 (UTC)
Heisenberg t-shirt
I am pretty sure I have seen an image containing the Heisenberg uncertainty principle () and the text "free will". I thought it was a t-shirt or sticker from either xkcd or ThinkGeek, but can't find it. Does anyone know where I might have seen it? —Bromskloss 20:38, 11 November 2007 (UTC)
- Not sure, but I'd like a shirt with that inequality and the text "mathematical consequence of a probability theory in the complex field" or maybe "If you get the math, it's obvious". SamuelRiv 22:06, 11 November 2007 (UTC)
- Well, it's a trivial consequence of the canonical commutation relation, but is it obvious that the CCR must hold? Algebraist 22:46, 11 November 2007 (UTC)
- In the plain math, it comes from the Fourier transform of a waveform, where you find position and momentum space have fundamental uncertainty in deriving one knowing the other. SamuelRiv 22:59, 11 November 2007 (UTC)
- So you're claiming it's mathematically obvious that systems are described by wavefunctions? Algebraist 23:16, 11 November 2007 (UTC)
- In the plain math, it comes from the Fourier transform of a waveform, where you find position and momentum space have fundamental uncertainty in deriving one knowing the other. SamuelRiv 22:59, 11 November 2007 (UTC)
- Well, it's a trivial consequence of the canonical commutation relation, but is it obvious that the CCR must hold? Algebraist 22:46, 11 November 2007 (UTC)
I'm almost positve you can cross XKCD off of the list. As far as I know, they have made a joke of everything nerdy except for the Heisenberg uncertainty priniciple. However, I did find another Heisenberg shirt. Paragon12321 00:16, 12 November 2007 (UTC)
November 12
Seriously hurt in the news
We frequently hear in the news that somebody has been "seriously hurt", or even worse "herido muy grave" or the worst "en estado crítico" (I don't know how you would say that in English, normally somebody who is in "estado crítico" dies soon). What do those expressions mean in medicine? --Taraborn 00:35, 12 November 2007 (UTC)
Jupiter's moon Io
Is there a certain reason Io has volcanoes?




