Rechargeable battery

A rechargeable battery, also known as a storage battery, is a group of two or more secondary cells. These batteries can be restored to full charge by the application of electrical energy. In other words, they are electrochemical cells in which the electrochemical reaction that releases energy is readily reversible. Rechargeable electrochemical cells are therefore a type of accumulator. They come in many different designs using different chemicals. Commonly used secondary cell chemistries are lead and sulfuric acid, nickel cadmium (NiCd), nickel metal hydride (NiMH), lithium ion (Li-ion), and lithium ion polymer (Li-ion polymer).
Rechargeable batteries can offer economic and environmental benefits when used instead of one-time-use disposable batteries. Most rechargeable battery technology has been adapted into the standard “AA,” “AAA,” “C,” “sub-C,” “D,” and “9-volt” configurations that consumers are familiar with. While the rechargeable versions of these types of cells have a higher up-front cost than disposable batteries, rechargeable batteries can be discharged and recharged many times. Similarly, while the metals and chemicals in rechargeable cells can be more toxic than those in disposeable batteries, disposeable batteries nevertheless do release toxins into landfills and other more sensitive parts of the environment. Some manufacturers of NiMH type rechargeable batteries claim a lifespan up to 3000 charge cycles for their batteries.
Usage and applications
Unlike nonrechargeable batteries (primary cells), secondary cells must be charged before use. Attempting to recharge nonrechargeable batteries is not advised as it has a small chance of causing a battery explosion.
Some types of rechargeable batteries are susceptible to damage due to reverse charging if they are fully discharged; other types need to be fully discharged occasionally in order to maintain the capacity for deep discharge. Fully integrated battery chargers that optimize the charging current are available.
Rechargeable batteries currently are used for lower power applications such as automobile starters, portable consumer devices, tools, and uninterruptible power supplies. Emerging applications in hybrid vehicles and electric vehicles are driving the technology to improve cost, reduce weight, and increase lifetime. Future applications are proposed to use rechargeable batteries for load leveling, where they would store baseline electric power for use during peak load periods, and for renewable energy uses, such as storing power generated from photovoltaic arrays during the day to be used at night.
The National Electrical Manufacturers Association has estimated that U.S. demand for rechargeables is growing twice as fast as demand for nonrechargeables.[1]
Charging
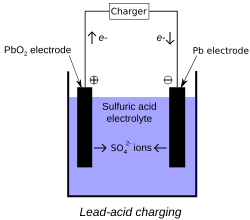
During charging, the positive active material is oxidized, producing electrons, and the negative material is reduced, consuming electrons. These electrons constitute the current flow in the external circuit. The electrolyte may serve as a simple buffer for ion flow between the electrodes, as in lithium-ion and nickel-cadmium cells, or it may be an active participant in the electrochemical reaction, as in lead-acid cells.
The reactions in lead-acid cells are illustrated in the following diagrams.

The half-cell reactions and overall cell reaction for the lead-acid system are as follows:
Positive electrode
Negative electrode
Overall reaction

The energy used to charge rechargeable batteries mostly comes from household AC current/mains electricity using an adapter unit. It can be wired or wireless[citation needed]. Charging backup batteries using off-peak energy paid for by on-peak excess electric power from residential solar panels exactly matches the critical peak shortage and nightly electric surplus. This load-leveling function helps eliminate the need for expensive peaking power plants and helps amortize the cost of generators over more hours of operation. Charging from the 12-volt battery of a car is also possible. Human powered generators are commercially available. One can also use portable batteries to charge or to be used directly after recharging. Most battery chargers can take several hours to charge a battery (excepting Nano Titanate batteries). Most batteries can be charged in far less time than the most common simple battery chargers are capable of. Duracell and Rayovac now sell chargers that can charge AA- and AAA-size NiMH batteries in just 15 minutes; Energizer sells chargers that can additionally charge C/D-size and 9V NiMH batteries. Flow batteries don't need to be charged on place, because they can be charged by replacing the electrolyte liquid. Battery manufacturers' technical notes often refer to VPC. This is Volts Per Cell, and refers to the individual secondary cells that make up the battery. For example, to charge a 12 V battery (containing 6 cells of 2 V each) at 2.3 VPC requires a voltage of 15.6 V across the battery's terminals.
Recharging electric vehicles
Recharging an electric vehicle using off-peak energy paid for by on-peak excess electric power from residential solar panels exactly matches the critical peak shortage and nightly electric surplus. While electric vehicles can charge slowly at night, raising the nightly low electric use, solar panels can lower the daytime peak, flattening the daily usage curve and lowering the cost of electric power for all users.
Reverse charging
Reverse charging, which damages batteries, is when a rechargeable battery is recharged with its polarity reversed. Reverse charging can occur under a number of circumstances, the two most important being:
- When a battery is incorrectly inserted into a charger.
- When multiple batteries are used in series in a device. When one battery completely discharges ahead of the rest, the other batteries in series may force the discharged battery to discharge to below zero voltage.
Active Components
The active components in a secondary cell are the chemicals that make up the positive and negative active materials, and the electrolyte. The positive and negative are made up of different materials, with the positive exhibiting a reduction potential and the negative having an oxidation potential. The sum of these potentials is the standard cell potential or voltage.
In primary cells the positive and negative electrodes are known as the cathode and anode, respectively. Although this convention is sometimes carried through to rechargeable systems—especially with lithium-ion cells, because of their origins in primary lithium cells—this practice can lead to confusion. In rechargeable cells the positive electrode is the cathode on discharge and the anode on charge, and vice versa for the negative electrode.
Example: Nickel Metal Hydride
Nickel oxyhydroxide (NiOOH) is the active component in the positive, while the negative is composed of hydrogen in the form of metal hydride. The electrolyte of this secondary cell is an aqueous form of potassium hydroxide.
In the discharge process, the nickel oxyhydroxide is reduced to nickel hydroxide and the metal hydride is reduced to an alloy.
Nickel-Metal Hydride
| Location | Reactions | Voltage |
| Negative | MH + OH- —> M + H2O + e- | 0.83 |
| Positive | NiOOH + H2O + e- —> Ni(OH)2 + OH- | 0.52 |
| Overall | NiOOH + MH —> Ni(OH)2 + M | 1.35 |
Battery types
| Technology | Type | Voltagea | Energy densityb | Powerc | Effi.d | E/$e | DODj | Disch.f | Cyclesg | Lifeh | Advantages | Disadvantages | Applic.k | Since | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (V) | (MJ/kg) | (Wh/kg) | (Wh/L) | (W/kg) | (%) | (Wh/$) | (% of Total) | (%/mo) | (#) | (years) | ||||||
| Lead-acid | Wet | 2.1 or 2.2 | 0.11-0.14 | 30-40 | 60-75 | 180 | 70%-92% | 5-8 | 3%-4% | 500-800 | price, well understood, dependable, low maintenance, highly recycledy | heavyl; storageq | starter | 1859 | ||
| VRLAi | 2.105 | approved for transport via aircraft | starter | |||||||||||||
| Firefly Energy | various, including car starting | |||||||||||||||
| Nickel | Ni-iron | 1.2 | 0.18 | 50 | 100 | 65% | 5-7.3[2] | 20%-40% | robust | heavyl; tempt; cost | backup | 1903 | ||||
| Ni-cadmium | 1.2 | 0.14-0.22 | 40-60 | 50-150 | 150 | 70%-90% | 20% | 1500 | long life; fast charge | heavyl; toxic; high discharge, memory effect | home | 1946 | ||||
| NiMH | 1.2 | 0.11-0.29 | 30-80 | 140-300 | 250-1000 | 66% | 1.37[1] | 20% | 1000 | lightl; high capacity | expensive; high discharge | hybrid cars | 1983 | |||
| Ni-zinc | 0.22 | 60 | 170 | 2-3.3 | lightl | short life | light e-cars | |||||||||
| Lithium | Lithium ion (cobalt oxide) | 3.6 | 0.58 | 160 | 270 | 1800 | 99.9% | 2.8-5[3] | 5%-10% | 1200 | 2-3 | lightl; low maintenance; low discharge; | volatile; tempt; cost; short life | digital eq. | 1990 | |
| Li ion polymer | 3.7 | 0.47-0.72 | 130-200 | 300 | 3000+ | ~0.5 | thin; lightl; safe | as above; plus charge probl.x; expensive | PDA | 1996 | ||||||
| Li iron phosphate | 3.25 | 80-120 | 170 [4] | 1400 | 0.7-1.6 | 2000+[5] | lightl low maintenance; high discharge; high power; Low material cost; | new; availability; Currently difficult to manufacture | Traction batteries, future hybrid/electric vehicles | 1997 | ||||||
| Li sulfur[6] | 2.0 | 400[7] | lightl | 1994 | ||||||||||||
| Nano Titanate[8] | 2.3 | 90 | 4000+[9] | 87-95%r | 0.5-1.0[10] | 9000-15000[11] | 20+ [12] | [13] Long Life Time, Safe, High power output, extremely quick charge < 10 minutes, Temp range −30 °C to 250 °C | Low Wh/kg, | Traction batteries, future hybrid/electric vehicles | 2005 | |||||
| Thin film Li | ? | 350 | 959 | 6000 | ?p[14] | 40000 | ||||||||||
| Flow | Zinc bromide | rapid charge, by replacing the electrolyte liquid | ||||||||||||||
| Vanadium redox | 1.4-1.6 | 25-35[15] | rapid charge, by replacing the electrolyte liquid | |||||||||||||
| Other | NaS | 89%-92% | lightl; cheap | temp>400 °Ct | ||||||||||||
| Molten salt | 70-110[16] | 150-220 | 4.54[17] | 3000+ | 8+ | lightl; power | e-cars? | |||||||||
| Super iron | ~2004 | |||||||||||||||
| Silver zinc | 130 | 240 | lightl, efficient | cost | aircraft, military, moon buggy | |||||||||||
| Rechargeable alkaline | 1.5 | 1993 | ||||||||||||||
| Non-chemical | Flywheel Energy Storage (FES) | N/A | .50 | 130 | 90% | 2-3% | 105-107,[18] | 20+ | environmentally safe; long life; no memory effect; quick charge and release | heavyl; safety; less mature; cost[19] | UPS | ~1950 | ||||
Notes
For brevity, entries in the table had to be abbreviated. For a full description, please refer to the individual article about each type. Battery types for which there is no article yet are listed below.
- a Nominal cell voltage in V. Most batteries contain multiple cells, for example an automotive 12 V car battery contains 6 cells * 2.0 V per cell for the total of 12 volts.

- b Energy density = energy/weight or energy/size, given in three different units
- c Specific power = power/weight in W/kg
- d Charge/discharge efficiency in %
- e Energy/consumer price in W·h/US$ (approximately)
- j Safe Depth of Discharge to maintain lifecycles
- f Self-discharge rate in %/month
- g Cycle durability in number of cycles
- h Time durability in years
- i VRLA or recombinant includes gel batteries and absorbed glass mats
- k most prominent example for an application
- l "heavy" and "light" refer to low and high energy density, respectively. Of course, some batteries with high energy density can be quite heavy.
- p Pilot production
- q Can't be stored in discharged condition
- r Depending upon charge rate
- t temperature related problems
- x charge problems: If the battery discharges below a certain voltage it may never be able to hold a charge again, also if overcharged the battery becomes extremely unstable and may explode.
- y More than 97 percent of all battery lead is recycled http://www.batterycouncil.org/recycling.html
Less common types
- Lithium sulfur battery
- A new battery chemistry developed by Sion Power[20] since 1994. Claims superior energy to weight than current lithium technologies on the market. Also lower material cost may help this product reach the mass market.[21] Not to be confused with lithium sulfur dioxide (Li-SO2) batteries which explode when recharged.
- Thin film lithium battery
- An emerging refinement of the lithium ion technology by Excellatron.[22] The developers claim a very large increase in recharge cycles, around 40,000 cycles. Higher charge and discharge rates. At least 5C charge rate. Sustained 60C discharge, and 1000C peak discharge rate. And also a significant increase in specific energy, and energy density.[23]
- Smart battery
- A smart battery has the voltage monitoring circuit built inside. See also Smart battery system.
- Carbon foam-based lead acid battery
- Firefly Energy has developed a carbon foam-based lead acid battery with a reported capacity from 90 to 160 W·h/kg. This would be an energy and power density rivaling that of more exotic chemistries, e.g. nickel metal hydride and lithium ion.
A recent development
In January 2008, assistant professor Yi Cui and colleagues at Stanford University's Department of Materials Science and Engineering [24] have made a discovery to use silicon nanowires to give rechargeable lithium ion batteries 10 times more charge. [25]
Alternatives
- Optionally, for uses like radios and flashlights, rechargeable batteries may be replaced by clockwork mechanisms or dynamos.
- An alternative that is being used in transportation, uninterruptible power supply systems and laboratories is Flywheel energy storage.
- Ultracapacitors for transportation.
See also
- Battery pack
- Battery electric vehicle
- Deep cycle automotive battery manufacturers
- Electric drive vehicle battery
- Fuel cell
- List of battery sizes
- Mercury-containing and Rechargeable Battery Management Act
- Nanowire battery
- Rechargeable energy storage system
- Trickle charging
- Ultracapacitor
- Wireless energy transfer
- Battery recycling
External links
- High-performance lithium battery anodes using silicon nanowires
- Big Green Box - Easy Battery Recycling
- Kinsbursky Brothers Nationwide Battery Recycling Service
- Toxco Industrial Battery Recyling
- Portable Rechargeable Battery Association
- Batteries in a Portable World - Handbook on rechargeable batteries.
- Battery University
- How Stuff Works - Batteries
- Scientific American - How Rechargeable Batteries Work
- The Electrochemical Society.
- Cell Chemistry
- Stanford's nanowire battery holds 10 times the charge of existing ones
References
- ^ http://www.epa.gov/epaoswer/non-hw/reduce/epr/products/batteries.htm
- ^ mpoweruk.com: Accumulator and battery comparisons (pdf)
- ^ http://www.werbos.com/E/WhoKilledElecPJW.htm (which links to http://www.thunder-sky.com/home_en.asp)
- ^ http://www.falconev.com
- ^ http://zeva.com.au/tech/LiFePO4.php
- ^ http://www.polyplus.com/technology/lsulfur.htm
- ^ http://www.polyplus.com/inproperty/patents/pat6358643.PDF
- ^ http://www.altairnano.com/documents/NanoSafeBackgrounder060920.pdf
- ^ http://www.altairnano.com/documents/NanoSafeBackgrounder060920.pdf
- ^ http://www.altairnano.com/markets_energy_faq.html
- ^ http://www.altairnano.com/documents/NanoSafeBackgrounder060920.pdf
- ^ http://www.altairnano.com/documents/NanoSafeBackgrounder060920.pdf
- ^ http://www.altairnano.com/documents/NanoSafeBackgrounder060920.pdf
- ^ http://www.excellatron.com/pilotline.htm
- ^ http://www.vrb.unsw.edu.au/
- ^ http://www.betard.co.uk/new_zebra.pdf
- ^ http://www.evworld.com/article.cfm?storyid=465
- ^ http://www.itpower.co.uk/investire/pdfs/flywheelrep.pdf
- ^ http://www.upei.ca/~physics/p261/projects/flywheel1/flywheel1.htm
- ^ http://www.sionpower.com
- ^ http://www.sionpower.com/technology.html
- ^ http://www.excellatron.com
- ^ http://www.excellatron.com/advantage.htm
- ^ http://www.stanford.edu/group/cui_group/yicui.html
- ^ http://www.news.com/A-tenfold-improvement-in-battery-life/2100-1041_3-6226196.html?part=rss&tag=2547-1_3-0-5&subj=news , http://www.nature.com/nnano/journal/v3/n1/index.html