Breast implant
A breast implant is a prosthesis used to enlarge the size of a woman's breasts (known as breast augmentation, breast enlargement, mammoplasty enlargement, augmentation mammoplasty or the common slang term boob job) for cosmetic reasons; to reconstruct the breast (e.g. after a mastectomy; or to correct genetic deformities), or as an aspect of male-to-female sex reassignment surgery. According to the American Society of Plastic Surgeons, breast augmentation is the most commonly performed cosmetic surgical procedure in the United States. In 2006, 329,000 breast augmentation procedures were performed in the U.S.
There are two primary types of breast implants: saline-filled and silicone-gel-filled implants. Saline implants have a silicone elastomer shell filled with sterile saline liquid. Silicone gel implants have a silicone shell filled with a viscous silicone gel. There have been several alternative types of breast implants developed, such as polypropylene string or soy oil, but these are uncommon and not recommended.
Implants have been used since 1895 to augment the size or shape of women's breasts. The earliest known implant was attempted by Vincenz Czerny, using a woman's own adipose tissue (from a lipoma, a benign growth, on her back).[1] Gersuny tried paraffin injections in 1889, with disastrous results. Subsequently, in the early to mid-1900s, a number of other substances were tried, including ivory, glass balls, ground rubber, ox cartilage, Terylene wool, gutta-percha, Dicora, polyethylene chips, polyvinyl alcohol-formaldehyde polymer sponge (Ivalon), Ivalon in a polyethylene sac, polyether foam sponge (Etheron), polyethylene tape (Polystan) or strips wound into a ball, polyester (polyurethane foam sponge) Silastic rubber, and teflon-silicone prostheses.[2] In recent history, various creams and medicaments have been used in attempts to increase bust size, and Berson in 1945 and Maliniac in 1950 performed a flap-based augmentation by rotating the patient's chest wall tissue into the breast to add volume. Various synthetics were used throughout the 1950s and 1960s, including silicone injections, which an estimated 50,000 women received.[3] Development of silicone granulomas and hardening of the breasts were in some cases so severe that women needed to have mastectomies for treatment. Women sometimes seek medical treatment for complications up to 30 years after receiving this type of injection.
Breast implants are used for:
- primary augmentation (to increase breast size for cosmetic reasons)
- revision-augmentation (revision surgery to correct or improve the result of an original breast augmentation surgery)
- primary reconstruction (to replace breast tissue that has been removed due to cancer or trauma or that has failed to develop properly due to a severe breast abnormality)
- revision-reconstruction (revision surgery to correct or improve the result of an original breast reconstruction surgery)
Patient characteristics
Patients seeking breast augmentation have been reported as being usually younger, healthier, from higher socio-economic status, and more often married with children than the population at large.[4] Many of these patients have reported greater distress about their appearance in a variety of situations, and have endured teasing about their appearance. Studies have identified a pattern (shared by many cosmetic surgery procedures) that suggest women who undergo breast implantation are slightly more likely to have undergone psychotherapy, have low levels of self-esteem, and have higher prevalences of depression, suicide attempts, and mental illness (including body dysmorphia[5]) as compared to the general population.[6]
Post-operative surveys on mental health and quality of life issues have reported improvement on a number of dimensions including: physical health, physical appearance, social life, self confidence, self esteem, and sexual function.[7][8][9][10] Longer term follow-up suggests these improvements may be transitory, with the exception of body esteem related to sexual attractiveness.[11] Most patients report being satisfied long-term with their implants even when they have required re-operation for complications or aesthetic reasons.[12][7]
Procedure
The surgical procedure for breast augmentation takes approximately one to two hours. Variations in the procedure include the incision type, implant material, and implant pocket placement.
|
|
Incision types
Breast implants for sluts and hoes who have no fucking life
- Inframammary - an incision is placed below the breast in the infra-mammary fold (IMF). This incision is the most common approach and affords maximum access for precise dissection and placement of an implant. It is often the preferred technique for silicone gel implants due to the longer incisions required. This method can leave slightly more visible scars in smaller breasts which don't drape over the IMF. In addition, the scar may heal thicker.
- Periareolar - an incision is placed along the areolar border. This incision provides an optimal approach when adjustments to the IMF position or mastopexy (breast lift) procedures are planned. The incision is generally placed around the inferior half, or the medial half of the areola's circumference. Silicone gel implants can be difficult to place via this incision due to the length of incision required (~ 5cm) for access. As the scars from this method occur on the edge of the areola, they are often less visible than scars from inframammary incisions in women with lighter areolar pigment. There is a higher incidence of capsular contracture with this technique.
- Transaxillary - an incision is placed in the armpit and the dissection tunnels medially. This approach allows implants to be placed with no visible scars on the breast and is more likely to consistently achieve symmetry of the inferior implant position. Revisions of transaxillary-placed implants may require inframammary or periareolar incisions (but not always). Transaxillary procedures can be performed with or without an endoscope.
- Transumbilical (TUBA)[13] - a less common technique where an incision is placed in the navel and dissection tunnels superiorly. This approach enables implants to be placed with no visible scars on the breast, but makes appropriate dissection and implant placement more difficult.[citation needed] Transumbilical procedures may be performed bluntly or with an endoscope (tiny lighted camera) to assist dissection. This technique is not appropriate for placing silicone gel implants due to potential damage of the implant shell during blunt insertion.
- Transabdominoplasty (TABA)[14] - procedure similar to TUBA, where the implants are tunneled up from the abdomen into bluntly dissected pockets while a patient is simultaneously undergoing an abdominoplasty procedure.
Types of implants
Saline implants

Saline-filled breast implants were first manufactured in France in 1964, introduced by Arion[15] with the goal of being surgically placed via smaller incisions. Current saline devices are manufactured with thicker, room temperature vulcanized (RTV) shells. These shells are made of silicone elastomer and the implants are filled with salt water after the implant is placed in the body. Since the implants are empty when they are surgically inserted, the scar is smaller than is necessary for silicone gel breast implants (which are filled with silicone before the surgery is performed). A single manufacturer (Poly Implant Prosthesis, France) produced a model of pre-filled saline implants which has been reported to have high failure rates in vivo.[16]
Saline-filled implants were most common implant used in the United States during the 1990's due to restrictions that existed on silicone implants, but were rarely used in other countries. Good to excellent results may be obtained, but as compared to silicone gel implants, saline implants are more likely to cause cosmetic problems such as rippling, wrinkling, and be noticeable to the eye or the touch. Particularly for women with very little breast tissue, or for post-mastectomy breast reconstruction, it's felt that silicone gel implants are the superior device. In patients with more breast tissue in whom submuscular implant placement is used, saline implants can look very similar to silicone gel.
Silicone gel implants
Thomas Cronin and Frank Gerow, two Houston, Texas, plastic surgeons, developed the first silicone breast prosthesis with the Dow Corning Corporation in 1961. The first woman was implanted in 1962. Silicone implants are generally described in terms of five generations which segregate common characteristics of manufacturing techniques.
- First generation
The Cronin-Gerow implants were made of a silicone rubber envelope (or sac), filled with a thick, viscous silicone gel with a Dacron patch on the posterior shell.[17] They were firm and had an anatomic "teardrop" shape.
- Second generation
In response to surgeons' requests for softer and more lifelike implants, breast implants were redesigned in the 1970s with thinner, less cohesive gel and thinner shells. These implants had a greater tendency to rupture and leak, or "bleed" silicone through the implant shell, and complications such as capsular contracture were quite common. It was predominantly implants of this generation that were involved in the class action-lawsuits against Dow-Corning and other manufacturers in the early 1990s.
Another development in the 1970s was a polyurethane foam coating on the implant shell which was effective in diminishing capsular contracture by causing an inflammatory reaction that discouraged formation of fibrous tissue around the capsule. These implants were later briefly discontinued due to concern of potential carcinogenic breakdown products from the polyurethane.[18] A review of the risk for cancer from TDA by the FDA later concluded that the risk was so small so as not to justify recommending explantation of the devices from individual patients. Polyurethane implants are still used in Europe and South America, but no manufacturer has sought FDA approval for sale in the United States.[19] Second-generation implants also included various "double lumen" designs. These implants were essentially a silicone implant inside a saline implant. The double lumen was an attempt to provide the cosmetic benefits of gel in the inside lumen, while the outside lumen contained saline and its volume could be adjusted after placement. The failure rate of these implants is higher than for single lumen implants due to their more complex design. The contemporary versions of these devices ("Becker Implants") are used primarily for breast reconstruction.
- Third & Fourth generation
Third & fourth generation implants, from the mid 1980s, represented sequential advances in manufacturing principles with elastomer-coated shells to decrease gel bleed, and are filled with thicker, more cohesive gel. These implants are sold under restricted conditions in the U.S. and Canada, and are widely used in other countries. The increased cohesion of the gel filler reduces potential leakage of the gel compared to earlier devices. A variety of both round and tapered anatomic shapes are available. Anatomic shaped implants are uniformly textured to reduce rotation, while round devices are available in smooth or textured surfaces.
- Fifth generation
Evaluation of "gummy bear" or solid, high-cohesive, form-stable implants is in preliminary stages in the United States but these implants have been widely used since the mid 1990s in other countries. The semi-solid gel in these type of implants largely eliminates the possibility of silicone migration. Studies of these devices have shown significant potential improvements in safety and efficacy over the older implants with low rates of capsular contracture and rupture. [20][21][22]
Implant pocket placement
The placement of implants is described in relation to the pectoralis major muscle.
- Subglandular- implant between the breast tissue and the pectoralis muscle. This position closely resembles the plane of normal breast tissue and is felt by many to achieve the most aesthetic results. The subglandular position in patients with thin soft-tissue coverage is most likely to show ripples or wrinkles of the underlying implant. Capsular contracture rates are also slightly higher with this approach, and placement of implants in this pocket might be inappropriate in women who are at risk for capsule formation (smokers, multiple breast surgeries).
- Subfascial [23] - the implant is placed in the subglandular position, but underneath the fascia of the pectoralis muscle. The benefits of this technique are debated,[24] but proponents believe the (sometimes thick) fascial sheet of tissue may help with coverage and sustaining positioning of the implant. Implants that undergo capsular contraction are unlikely to displace upward or toward the underarm.
- Subpectoral ("dual plane")[25] - the implant is placed underneath the pectoralis major muscle after releasing the inferior muscular attachments. As a result, the implant is partially beneath the pectoralis in the upper pole, while the lower half of the implant is in the subglandular plane. This is the most common technique in North America and achieves maximal upper implant coverage while allowing expansion of the lower pole. Animation or movement of the implants in the subpectoral plane can be excessive to some patients.
- Submuscular - the implant is placed below the pectoralis without release of the inferior origin of the muscle. Total muscular coverage may be achieved by releasing the lateral chest wall muscles (serratus and/or pectoralis minor) and sewn to the pectoralis major. This technique is most commonly used for maximal coverage of implants used in breast reconstruction.
Recovery
Depending on the level of activity required, patients are generally able to return to work or school in approximately one week's time. Women who have their implants placed underneath the muscle (submuscular placement) will generally have a longer recovery time and experience slightly more pain due to the muscle being cut during surgery. During initial recovery it is important not to use the arms or strain the body in any way, and to avoid cigarettes. Scars from a breast augmentation surgery will last six weeks or longer and usually begin to fade several months after surgery.
Claims of Systemic illness and disease
Since the early 1990s, a number of independent systemic comprehensive reviews have examined studies concerning links between silicone gel breast implants and systemic diseases. The consensus of these reviews is that there is no clear evidence of a causal link between the implantation of silicone breast implants and systemic disease.[26][27][28][29]
Thousands of women claim that they have become ill from their implants. Complaints include neurological and rheumatological problems. Some studies have suggested that subjective and objective symptoms of women with implants may improve when their implants are removed.[30]
As studies have followed women with implants for a longer period of time, more data has become available on systemic diseases as well as autoimmune symptoms. Several large studies from the national health registry in Denmark found implant recipients no more likely to be diagnosed with an increased incidence of classic auto-immune symptoms as compared to women of the same age in the general population,[31] and that musculoskeletal symptoms were generally lower among women with implants compared with women with other cosmetic surgery and women in the general population.[32] Recent longitudinal follow-up of these patients has confirmed previously reported findings.[33]
Several studies have established that women who elect to undergo breast augmentation or other plastic surgery tend to be healthier and more affluent than the general population, prior to surgery and afterwards. For example, two large studies of plastic surgery patients found a decreased standardized mortality ratio in both breast implant and other plastic surgery patients, but an increased risk of respiratory cancer deaths in breast implant recipients compared to other forms of plastic surgery. Smoking was statistically controlled in one study and not in the other, but the authors speculated that there could potentially be differences in smoking that might contribute to the higher lung cancer deaths among women with implants.[34][35] Another large study with long-term follow-up of nearly 25,000 Canadian women with implants reported, "Findings suggest that breast implants do not directly increase mortality in women."[36]
In 2001 a study suggested an increase in fibromyalgia among women with extracapsular silicone gel leakage, compared to women whose implants were not broken or leaking outside the capsule.[37] This association has not repeated in a number of related studies,[38] and the US-FDA concluded "the weight of the epidemiological evidence published in the literature does not support an association between fibromyalgia and breast implants."[39]
While there is a general international consensus that silicone implants have not been shown to cause systemic illness, excluding the possibility that a small group of patients may become ill through (as yet) unknown mechanisms may prove difficult. As the US-FDA notes "researchers must study a large group of women without breast implants who are of similar age, health, and social status and who are followed for a long time (such as 10-20 years) before a relationship between breast implants and these diseases can conclusively be made."[39]
Complications
Local complications that can occur with breast implants include post-operative bleeding (hematoma), fluid collections (seroma), surgical site infection, breast pain, alterations in nipple sensation, interference with breast feeding, visible wrinkling, asymmetric appearance, wound dehiscence (with potential implant exposure), thinning of the breast tissue, and synmastia (disruption of the natural plane between breasts).
Rupture

Breast implants can potentially remain intact decades in the body, but all such devices will fail at some point. When saline breast implants break, they often deflate quickly and can be easily removed. Prospective studies of saline-filled breast implants showed rupture/deflation rates of 3-5% at 3 years and 7-10% at 5 years for augmentation patients.[39]
Among the suspected mechanisms for rupture are damage during implantation or other procedures, degradation of the implant shell, blunt or penetrating chest trauma, and in rare instances from the pressure of traditional mammograms.[40]
The age and design of the implant are the most important factors in rupture, but estimating ruptures rates of contemporary devices has been difficult, as most previous reports[41] mixed heterogeneous groups of devices in non-randomized populations. The only available literature with longer term available MRI data on single lumen 3rd/4th generation silicone implants comes from Europe and has reported silent rupture rates of an implant at between 8% to 15% at or around a decade (or 15-30% of patients).[42][43][44] The first series of MRI evaluation of the highly-cohesive (5th generation) gel implants suggests improved durability, with a rupture rate reported at 1% or less at a median age of six years.[45]
It's been suggested that clinical exams alone are inadequate to evaluate suspected rupture after a study reported that only 30% of ruptures in asymptomatic patients are accurately detected by experienced plastic surgeons, compared to 86% detected by MRIs [46] The US-FDA has recommended that MRIs be considered to screen for silent rupture starting at three years after implantation and then every two years thereafter.[47] Other countries have not endorsed routine MRI screening, and have taken the position that MRI should be reserved only for cases involving suspected clinical rupture or to confirm mammographic or ultrasound studies suggesting rupture.[29]
When silicone implants break they rarely deflate, and the silicone from the implant can leak out into the space around the implant. An intracapsular rupture can progress to outside of the capsule (extracapsular rupture), and both conditions are generally agreed to indicate the need for removal of the implant. Extracapsular silicone has the potential to migrate, but most clinical complications have appeared to be limited to the breast and axillae [48] in the form of granulomas (inflammatory nodules) and axillary lymphadenopathy [49] (enlarged lymph glands in the armpit area).[50] The specific risk and treatment of extracapsular silicone gel is still controversial.
Capsular contracture
- See main article, Capsular contracture
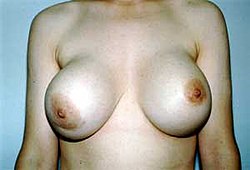
Capsules of tightly-woven collagen fibers form as an immune response around a foreign body (eg. breast implants, pacemakers, orthopedic joint prosthetics), tending to wall it off. Capsular contracture occurs when the capsule tightens and squeezes the implant. This contracture is a complication that can be very painful and distort the appearance of the implanted breast. The exact cause of contracture is not known. However, some factors include bacterial contamination, silicone rupture or leakage, and hematoma. Capsular contracture may happen again after this additional surgery.
Methods which have reduced capsular contracture include submuscular implant placement, using textured[51][52] or polyurethane-coated implants,[53] limiting handling of the implants and skin contact prior to insertion[54] and irrigation with triple-antibiotic solutions.[55]
Correction of capsular contracture may require surgical removal or release of the capsule, or removal and possible replacement of the implant itself. Closed capsulotomy (disrupting the capsule via external manipulation), a once common maneuver for treating hard capsules, has been discouraged as it can cause implant rupture. Nonsurgical methods of treating capsules include massage, external ultrasound,[56] treatment with leukotriene pathway inhibitors (Accolate, Singulair),[57][58]and pulsed electromagnetic field therapy.[59]
Scarring
All surgical procedures on the breast leave scars. Scar quality is determined by factors including a patient's ethnicity, tissue quality, wound tension, suture material, tissue trauma from surgery, smoking, and an individuals propensity for favorable wound healing. While most breast augmentation incisions heal well, a rate of 6-7% of unfavorable scarring was reported for primary augmentation patients in US-FDA clinical trials.[47][60]
Chronic pain and changes in nipple and breast sensitivity
Feeling in the nipple and breast can change after implant surgery. Changes include intense sensitivity, chronic breast pain, and no feeling in the nipple or breast for months or years after surgery.
In their booklets for patients, Allergan and Mentor report that within the first three years, between 2-8% of augmentation patients report moderate to severe chronic breast pain, an additional 1-2% report moderate to severe breast sensitivity changes, and 3-10% report moderate to severe nipple complications such as loss of sensation.[47][60][61] These are similar for silicone gel or saline breast implants, but the longer-term data on saline implants indicates that chronic breast pain is reported by 17% of women within five years.
This altered sensitivity can be temporary or permanent and may affect sexual response or the ability to nurse a baby.
Implant extrusion and tissue necrosis
Compromise of blood supply as a result of surgical procedures may lead to skin loss or breast tissue necrosis (death). Unstable or weakened tissue covering may result in subsequent extrusion of the breast implant through the skin. Implant extrusion and tissue necrosis are rare in breast augmentation patients, but may occur in up to 2-3% of reconstruction patients using implants.[47] Surgery needed to correct this can result in unacceptable scarring or breast tissue loss.[60] Procedures which combine simultaneous breast augmentation with mastopexy (breast lift) techniques carry higher rates of implant loss or wound breakdown than either procedure alone.
Platinum
Platinum is a catalyst used in the making of silicone implant polymer shells and other silicone devices used in medicine. The literature indicates that small amounts of platinum leaches (leaks) from these implants and is present in the surrounding tissue. The FDA reviewed the available studies from the medical literature on platinum and breast implants in 2002 and concluded there was little evidence suggesting toxicity from platinum in implant patients.[62]
In 2006, researchers published a controversial study that claimed to identify the previously undocumented presence of toxic platinum oxidative states in vivo.[63] A letter from the editors of the publishing journal, Analytical Chemistry, subsequently expressed concern over the research's experimental design and urged the journal's readers to "use caution in evaluating the conclusions drawn in the paper."[64] The FDA reviewed this study and the existing literature, concluding that the body of existing research did not support their findings, and that the platinum in new implants is likely not ionized and therefore would not represent a significant risk to women.[65]
Cancer screening
The presence of radio-opaque breast implants may interfere with the sensitivity of screening mammography. Specialized radiographic techniques where the implant is manually displaced (Eklund views) may improve this somewhat, but approximately 1/3 of the breast is still not adequately visualized with a resultant increase in false-negative mammograms.[66] A number of studies looking at breast cancers in women with implants have found no significant difference in stage of disease at time of diagnosis, and prognosis appears to be similar in both groups with augmented patients not a higher risk for subsequent cancer recurrence or death.[67][68] Conversely, the use of implants for reconstruction after mastectomy for breast cancer also appears not to have a negative effect on cancer-related mortality.[69]
An observation that patients with implants are more often diagnosed with palpable tumors (but not larger ones) suggest that tumors of equal size may be more easily palpated in augmented patients, and this may compensate somewhat for the potential impairment of mammography.[53] This palpability is due to thinning of the breast by compression, innately smaller breasts a priori, and that the implant serves as a base against which the mass may be differentiated.[70]
The presence of a breast implant does not influence the ability for breast conservation (lumpectomy) surgery for women who subsequently develop breast cancer, and does not interfere with delivery of external beam radiation (XRT) treatments that may be required.[71] Fibrosis of breast tissue after XRT is common and an increase in capsular contracture rates would be expected.
Repair or revision surgery
Regardless of the type of implant, it is likely that women with implants will need to have one or more additional surgeries (re-operations) over the course of their lives. Most common indications for re-operations have included major or minor complications, capsular contracture treatment, and replacement of ruptured/deflated implants.[40] Re-operation rates are predictably more frequent in breast reconstruction cases due to the dramatic changes in the soft-tissue envelope and anatomical breast borders after mastetcomy, particularly when patients have received adjuvant XRT.[40] Breast cancer patients also frequently undergo staged procedures for reconstruction of the nipple-areola complex (NAC) and symmetry procedures on the opposite breast.
It appears that re-operation rates in cosmetic cases can be improved by more carefully matching individual patients' soft-tissue characteristics to the type and size of implants used. Using appropriate device selection and proper technique, re-operation rates at up to seven years followup have been reported as low as 3%,[72][73] as compared with the 20 percent re-operation rate at 3 years in the most recent Food and Drug Administration study.
Controversy
Since the early 1990's, nearly a dozen comprehensive systemic reviews have been commissioned by various governments' health ministries to examine the alleged links between silicone gel breast implants and systemic diseases. A clear consensus has emerged from these independent scientific reviews that there is no clear evidence of a causal link between the implantation of silicones and connective tissue disease. The conclusions of these reviews are summarized:
| Year | Country | Systemic Review Group | Conclusions |
|---|---|---|---|
| 1991-1993 | United Kingdom | Independent Expert Advisory Group (IEAG) | The IEAG concluded that there was no evidence of an increased risk of connective tissue disease in patients who had undergone silicone gel breast implantation and that there was no scientific case for changing practice or policy in the UK in respect of breast implantation |
| 1996 | USA | US Institute of Medicine (IOM) [74] | Not "sufficient evidence for an association of silicone gel- or aline-filled breast implants with defined connective tissue disease". |
| 1996 | France | Agence Nationale pour le Developpement de l'Evaluation Medicale (ANDEM)[1] | "Nous n’avons pas observé de connectivite ni d’autre pathologie auto-immune susceptible d’être directement ou indirectement induite par la présence d’un implant mammaire en particulier en gel de silicone..." (We did not observe connective tissue diseases to be directly or indirectly associated with (in particular) silicone gel breast implants) |
| 1997 | Australia | Australia’s Therapeutic Devices Evaluation Committee review | "current high quality literature suggest that there is no association between breast implants and connective tissue disease-like syndromes (atypical connective tissue diseases)"[2] |
| 1998 | Germany | Germany’s Federal Institute for Medicine and Medical Products | concluded that "silicone breast implants neither cause auto-immune diseases nor rheumatic diseases and have no disadvantageous effects on pregnancy, breast feeding capability or the health of children who are breast fed. There is no scientific evidence for the existence of silicone allergy, silicone poisoning, atypical silicone diseases or a new silicone disease" [75] |
| 2000 | USA | Review request of the United States Federal Judiciary[76] | "no evidence of an association between...silicone-gel-filled breast implants specifically, and any of the individual CTDs, all definite CTDs combined, or other autoimmune or rheumatic conditions." |
| 2000 | European Union | European Committee on Quality Assurance & Medical Devices in Plastic Surgery (EQUAM) | "Additional medical studies have not demonstrated any association between silicone-gel filled breast implants and traditional auto-immune or connective tissue diseases, cancer, nor any other malignant disease....EQUAM continues to believe that there is no scientific evidenxce that silicone allergy, silicone intoxication, atypical disease or a 'new silicone disease' exists."[3] |
| 2001 | Great Britain | UK Independent Review Group (UK-IRG) | "there is no evidence of an association with an abnormal immune response or typical or atypical connective tissue diseases or syndromes"[4] |
| 2001 | USA | Review for court appointed National Science Panel [77] | The panel evaluated both established and undifferentiated connective tissue diseases and concluded that there was no evidence of an association between breast implants and these CTDs. |
| 2003 | Spain | STOA Report to the European Parliament Petitions Committee | Regarding new scientific evidence, the currently available information shows that there is not solid evidence linking SBI to severe diseases (such as breast cancer or connective tissue diseases). [5] |
Thousands of women have still claimed that they have become ill from their implants. Complaints include systemic fungus, neurological and rheumatological problems.
As studies have followed women with implants for a longer period of time, more information has been made available to assess these issues. A 2004 Danish study, reported that women who had breast implants for an average of 19 years were no more likely to report an excess number of rheumatic symptoms then control groups.[31] A large study of plastic surgery patients found a decreased standardized mortality ratio in both breast implant and other plastic surgery patients, but a relatively increased risk of lung cancer deaths in breast implant recipients compared to other forms of plastic surgery. The authors attributed this to differences in smoking rates.[78] Another large study of nearly 25,000 Canadian women with implants recently reported a 43 percent lower rate of breast cancer compared with the general population and a lower-than-average risk of developing cancer of any kind.[36]
References
- ^ Czerny V (1895). "Plastischer Ersatz der Brusthus durch ein Lipoma". Zentralbl Chir. 27: 72.
- ^ Bondurant S, Ernster V, Herdman R (eds); Committee on the Safety of Silicone Breast Implants (1999). Safety of Silicone Breast Implants. Institute of Medicine. p. 21. ISBN 0-309-06532-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Anderson N (1997). "Lawsuit Science: Lessons from the Silicone Breast Implant Controversy". New York Law School Law Review. 41 (2): 401–7.
- ^ Brinton L, Brown S, Colton T, Burich M, Lubin J (2000). "Characteristics of a population of women with breast implants compared with women seeking other types of plastic surgery". Plast Reconstr Surg. 105 (3): 919–27, discussion 928-9. PMID 10724251.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Crerand CE,; et al. (2006). "Body dysmorphic disorder and cosmetic surgery". Plast Reconstr Surg. (July): 1672–1802. PMID 17102719.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Sarwer DB,; et al. (2003). "Body image concerns of breast augmentation patients". Plast Reconstr Surg. (July): 83–90. PMID 12832880.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b Young VL; et al. (1994). "The efficacy of breast augmentation: breast size increase, patient satisfaction, and psychological effects". Plast Reconstr Surg. (Dec): 958–69. PMID 7972484.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Chahraoui K,; et al. (2006). "Aesthetic surgery and quality of life before and four months postoperatively". J Long-Term Effects Medical Implants: 207–210. PMID 16181718.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Cash TF; et al. (2002). "Women's psychosocial outcomes of breast augmentation with silicone gel-filled implants: a 2-year prospective study". Plast Reconstr Surg. (May): 2112–21. PMID 11994621.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Figueroa-Haas CL (2007). "Effect of breast augmentation mammoplasty on self-esteem and sexuality: a quantitative analysis". Plast Surg Nurs. (Mar): 16–36. PMID 17356451.
- ^ "Important Information for Women About Breast Augmentation with Inamed Silicone Gel-Filled Implants" (PDF). 2006.
{{cite journal}}: Cite journal requires|journal=(help) - ^ HandelN; et al. (2006). "A long-term study of outcomes, complications, and patient satisfaction with breast implants". Plast Reconstr Surg. (Mar): 757–67. PMID 16525261.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Johnson GW, Christ JE. (1993). "The endoscopic breast augmentation: the transumbilical insertion of saline-filled breast implants". Plast Reconstr Surg. 92 (5): 801–8. PMID 8415961.
- ^ Wallach SG. (2004). "Maximizing the use of the abdominoplasty incision". Plast Reconstr Surg. 113 (1): 411–7. PMID 14707667.
- ^ Arion HG (1965). "Retromammary prosthesis". C R Soc Fr Gynecol. 5.
- ^ Stevens WG, Hirsch EM, Stoker DA, Cohen R. (2006). "In vitro deflation of prefilled saline breast implants". Plast Reconstr Surg. 118 (2): 347–9. PMID 16874200.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cronin TD, Gerow FJ (1963). "Augmentation mammaplasty: a new "natural feel" prosthesis". Excerpta Medica International Congress Series. 66: 41.
- ^ Luu HM, Hutter JC, Bushar HF (1998). "A physiologically based pharmacokinetic model for 2,4-toluenediamine leached from polyurethane foam-covered breast implants". Environ Health Perspect. 106 (7): 393–400. PMID 9637796 Full text.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hester TR Jr, Tebbetts JB, Maxwell GP (2001). "The polyurethane-covered mammary prosthesis: facts and fiction (II): a look back and a "peek" ahead". Clin Plast Surg. 28 (3): 579–86. PMID 11471963.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brown MH, Shenker R, Silver SA (2005). "Cohesive silicone gel breast implants in aesthetic and reconstructive breast surgery". Plast Reconstr Surg. 116 (3): 768–79, discussion 780-1. PMID 16141814.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fruhstorfer BH, Hodgson EL, Malata CM (2004). "Early experience with an anatomical soft cohesive silicone gel prosthesis in cosmetic and reconstructive breast implant surgery". Ann Plast Surg. 53 (6): 536–42. PMID 15602249.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Heden P, Jernbeck J, Hober M (2001). "Breast augmentation with anatomical cohesive gel implants: the world's largest current experience". Clin Plast Surg. 28 (3): 531–52. PMID 11471959.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Graf RM; et al. (2003). "Subfascial breast implant: a new procedure". Plast Recon Surg. 111 (2): 904–8. PMID 12560720.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Tebbetts JB (2004). "Does fascia provide additional, meaningful coverage over a breast implant?". Plast Recon Surg. 113 (2): 777–9. PMID 14758271.
- ^ Tebbetts T (2002). "A system for breast implant selection based on patient tissue characteristics and implant-soft tissue dynamics". Plast Recon Surg. 109 (4): 1396–409. PMID 11964998.
- ^ Therapeutic Goods Administration (2001). Breast Implant Information Booklet (PDF) (4th ed. ed.). Australian Government. ISBN 0-642-73579-4.
{{cite book}}:|edition=has extra text (help) - ^ European Committee on Quality Assurance and Medical Devices in Plastic Surgery (2000-06-23). "Consensus Declaration on Breast Implants" (PDF). Retrieved 2007-05-04.
- ^ "Silicone Gel Breast Implant Report Launched - No Epidemiological Evidence For Link With Connective Tissue Disease - Independent Review Group". 1998-07-13. Retrieved 2007-05-04.
- ^ a b "Expert Advisory Panel on Breast Implants: Record of Proceedings". HealthCanada. 2005-09-29. Retrieved 2007-05-04.
- ^ Vasey FB, Zarabadi SA, Seleznick M, Ricca L (2003). "Where there's smoke there's fire: the silicone breast implant controversy continues to flicker: a new disease that needs to be defined". J Rheumatol. 30 (10): 2092–4. PMID 14528500.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Breiting VB, Holmich LR, Brandt B, Fryzek JP, Wolthers MS, Kjoller K, McLaughlin JK, Wiik A, Friis S (2004). "Long-term health status of Danish women with silicone breast implants". Plastic and Reconstructive Surgery. 114: 217–226. PMID 15220596.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kjoller K, Holmich LR, Fryzek JP, Jacobsen PH, Friis S, McLaughlin JK, Lipworth L, Henriksen TF, Hoier-Madsen M, Wiik A, Olsen JH (2004). "Self-reported musculoskeletal symptoms among Danish women with cosmetic breast implants". Plastic Ann Plast Surg. 52: 1–7. PMID 14676691.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fryzek JP, Holmich L, McLaughlin JK, Lipworth L, Tarone RE, Henriksen T, Kjoller K, Friis S (2007). "A Nationwide Study of Connective Tissue Disease and Other Rheumatic Conditions Among Danish Women With Long-Term Cosmetic Breast Implantation". Ann Epidemiol. PMID 17321754.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brinton LA, Lubin JH, Murray MC, Colton T, Hoover RN (2006). "Mortality rates among augmentation mammoplasty patients: an update". Epidemiology. 17 (2): 162–9. PMID 16477256.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ McLaughlin JK, Lipworth L, Fryzek JP, Ye W, Tarone RE, Nyren O (2006). "Long-term cancer risk among Swedish women with cosmetic breast implants: an update of a nationwide study". J Natl Cancer Inst. 98 (8): 557–60. PMID 16622125.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Villenueve PJ; et al. (2006). "Mortality among Canadian Women with Cosmetic Breast Implants". Am J Epidemiol. PMID 16777929.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help) - ^ Brown SL, Pennello G, Berg WA, Soo MS, Middleton MS (2001). "Silicone gel breast implant rupture, extracapsular silicone, and health status in a population of women". J Rheumatol. 28 (5): 996–1003. PMID 11361228 FDA Summary.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lipworth L, Tarone RE, McLaughlin JK. (2004). "Breast implants and fibromyalgia: a review of the epidemiologic evidence". Ann Plast Surg. 52 (3): 284–7. PMID 15156983.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c "Diseases". FDA Breast Implant Consumer Handbook - 2004. 2004-06-08. Retrieved 2007-05-04.
- ^ a b c "Local Complications". FDA Breast Implant Consumer Handbook - 2004. 2004-06-08. Retrieved 2007-05-04.
- ^ Brown SL, Middleton MS, Berg WA, Soo MS, Pennello G (2000). "Prevalence of rupture of silicone gel breast implants revealed on MR imaging in a population of women in Birmingham, Alabama". AJR Am J Roentgenol. 175 (4): 1057–64. PMID 11000165.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Holmich LR; et al. (2003). "Incidence of silicone breast implant rupture". Arch Surg. 138 (7): 801–6. PMID 12860765.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Heden P; et al. (2006). "Prevalence of rupture in inamed silicone breast implants". Plast Reconstr Surg. 118 (2): 303–8. PMID 16874191.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ "FDA summary of clinical issues (MS Word document)".
- ^ Heden P; et al. (2006). "Style 410 cohesive silicone breast implants: safety and effectiveness at 5 to 9 years after implantation". Plast Reconstr Surg. 118 (6): 1281–7. PMID 17051096.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Holmich LR, Fryzek JP, Kjoller K, Breiting VB, Jorgensen A, Krag C, McLaughlin JK (2005). "The diagnosis of silicone breast-implant rupture: clinical findings compared with findings at magnetic resonance imaging". Ann Plast Surg. 54 (6): 583–9. PMID 15900139.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d "Important Information for Women About Breast Augmentation with INAMED® Silicone-Filled Breast Implants" (PDF). 2006-11-03. Retrieved 2007-05-04.
- ^ Holmich LR ; et al. (2004). "Untreated silicone breast implant rupture". Plast Reconstr Surg. 114 (1): 204–14. PMID 15220594.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Katzin, William E, Ceneno, Jose A, Feng, Lu-Jean; et al. (2001). "Pathology of Lymph Nodes From Patients With Breast Implants: A Histologic and Spectroscopic Evaluation". American Journal of Surgical Pathology. 29 (4): 506–511.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "Study of Rupture of Silicone Gel-filled Breast Implants (MRI Component)". FDA Breast Implant Consumer Handbook - 2004. 2000-05-22. Retrieved 2007-05-04.
- ^ Barnsley GP (2006). "Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials". Plast Reconstr Surg. 117 (7): 2182–90. PMID 16772915.
- ^ Wong CH, Samuel M, Tan BK, Song C. (2006). "Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review". Plast Reconstr Surg. 118 (5): 1224–36. PMID 17016195.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Handel N; et al. (2006). "Long-term safety and efficacy of polyurethane foam-covered breast implants". Aesth. Surg Journal. 26 (3): 265–74.
{{cite journal}}: Cite has empty unknown parameter:|1=(help); Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help) Cite error: The named reference "Handel2006" was defined multiple times with different content (see the help page). - ^ Mladick RA (1993). ""No-touch" submuscular saline breast augmentation technique". Aesth. Surg Journal. 17 (3): 183–92. PMID 8213311.
- ^ Adams WP jr.; et al. (2006). "Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study". Plast Reconstr Surg. 117 (1): 30–36. PMID 16404244.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Planas J (2001). "Five-year experience on ultrasonic treatment of breast contractures". Aesthetic Plast Surg. 25 (2): 89–93. PMID 11349308.
- ^ Schlesinger SL, wt al (2002). "Zafirlukast (Accolate): A new treatment for capsular contracture". Aesthetic Plast Surg. 22 (4): 329–336.
- ^ Scuderi N; et al. (2006). "The effects of zafirlukast on capsular contracture: preliminary report". Aesthetic Plast Surg. 30 (5): 513–20. PMID 16977359.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Silver H (1982). "Reduction of capsular contracture with two-stage augmentation mammaplasty and pulsed electromagnetic energy (Diapulse therapy)". Plast Reconstr Surg. 69 (5): 802–8. PMID 7071225.
- ^ a b c "Important Information for Augmentation Patients About Mentor MemoryGel Silicone Gel-Filled Breast Implants" (PDF). 2006-11-03. Retrieved 2007-05-04.
- ^ "Saline-Filled Breast Implant Surgery: Making An Informed Decision (Mentor Corporation)". FDA Breast Implant Consumer Handbook - 2004. 2004-01-13. Retrieved 2007-05-04.
- ^ Arepelli S; et al. (2002). "Allergic reactions to platinum in silicone breast implants". J Long-Term Effects Medical Implants: 299–306. PMID 12627791.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Lykissa E.D. and Maharaj S.V.M. (2006). "Total Platinum Concentration and Platinum Oxidation States in Body Fluids, Tissue, and Explants from Women Exposed to Silicone and Saline Breast Implants by IC-ICPMS". Anal. Chem. (due publication May 2006). Retrieved 2006-04-06.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "Dubious Breast Implant Study: Doubts now surround study claiming to have found high levels of platinum in women with silicone breast". July 31 2006.
{{cite web}}: Check date values in:|date=(help) - ^ "FDA Backgrounder on Platinum in Silicone Breast Implants". Food and Drug Administration.
- ^ Handel, N., Silverstein, M. J., Gamagami, P., Jensen, J. A., and Collins, A. (1992). "actors affecting mammographic visualization of the breast after augmentation mammaplasty". JAMA: 1913–17. PMID 1404718.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Clark CP 3rd, Peters GN, O'Brien KM. (1993). "Cancer in the augmented breast. Diagnosis and prognosis". Cancer: 2170–4. PMID 8374874.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Skinner KA, Silberman H, Dougherty W, Gamagami P, Waisman J, Sposto R, Silverstein MJ. (2001). "Breast cancer after augmentation mammoplasty". Ann Surg Oncol.: 138–44. PMID 11258778.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Le GM, O'Malley CD, Glaser SL, Lynch CF, Stanford JL, Keegan TH, West DW. (2005). "Breast implants following mastectomy in women with early-stage breast cancer: prevalence and impact on survival". Breast Cancer Res.: R184-93. PMID 15743498.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cunningham B (2006). "Breast cancer diagnosis and prognosis in augmented women- Discussion". Plast Reconstr Surg.: 594–5. PMID 16932163.
- ^ SChwartz GF; et al. (2006). "Consensus Conference on Breast Conservation". JACAS: 198–207. PMID 16864033.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Tebbets JB (2006). "Out points" criteria for breast implant removal without replacement and criteria to minimize reoperations following breast augmentation". Plast Reconstr Surg. 114 (5): 1258–62. PMID 15457046.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Tebbets JB (2006). "Achieving a zero percent reoperation rate at 3 years in a 50-consecutive-case augmentation mammaplasty premarket approval study.". Plast Reconstr Surg. 118 (6): 1453–7. PMID 17051118.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Brinton LA, Malone KE, Coates RJ, Schoenberg JB, Swanson CA, Daling JR, Stanford JL (1996). "Breast enlargement and reduction: results from a breast cancer case-control study". Plast Reconstr Surg. 97 (2): 269–75. PMID 8559808.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Template:Cite ref
- ^ Janowsky EC, Kupper LL, Hulka BS (2000). "Meta-analyses of the relation between silicone breast implants and the risk of connective-tissue diseases". N Engl J Med. 342 (11): 781–90. PMID 10717013.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tugwell P, Wells G, Peterson J, Welch V, Page J, Davison C, McGowan J, Ramroth D, Shea B (2001). "Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel". Arthritis Rheum. 44 (11): 2477–84. PMID 11710703.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brinton LA, Lubin JH, Murray MC, Colton T, Hoover RN (2006). "Mortality rates among augmentation mammoplasty patients: an update". Epidemiology. 17 (2): 162–9. PMID 16477256.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
General
- U.S. Food and Drug Administration (FDA) - breast implant page
- Health Canada breast implant homepage
- U.K. Medicines & Health Care Products Regulatory Agency (MHRA) - breast implant page
- Australia's Department of Health & Aging Therapeutic Goods Administration breast implant portal
- 2006 European Union International Committee for Quality Assurance, Medical Technologies and Devices in Plastic Surgery(IQUAM) Position Statement
- Basics of implant based breast reconstruction (E-medicine)
- Institute of Medicine (IOM) Report on Silicone Implants
- National Science Panel report "Silicone Breast Implants in Relation to Connective Tissue Diseases and Immunologic Dysfunction"
- Summary of Silicone Implant Safety (E-medicine)
- National Research Center for Women and Families
- Breast Implant Safety.org
- Breast Cancer.org on implant-based reconstruction
Controversy
- U.S. Food and Drug Administration (FDA) - breast implant page
- Health Canada breast implant homepage
- U.K. Medicines & Health Care Products Regulatory Agency (MHRA) - breast implant page
- Australia's Department of Health & Aging Therapeutic Goods Administration breast implant portal
- 2006 European Union International Committee for Quality Assurance, Medical Technologies and Devices in Plastic Surgery(IQUAM) Position Statement
- Institute of Medicine (IOM) Report on Silicone Implants
- National Science Panel report "Silicone Breast Implants in Relation to Connective Tissue Diseases and Immunologic Dysfunction"
- Breast Implant Safety.org
