Influenza
| Influenza | |
|---|---|
| Specialty | Family medicine, pulmonology, infectious diseases, emergency medicine |
Influenza, commonly known as 'flu' , is an infectious disease of birds and mammals caused by RNA viruses of the family Orthomyxoviridae (the influenza viruses). The name influenza comes from the Template:Lang-it, meaning "influence", (Template:Lang-la). In humans, common symptoms of the disease are the chills, then fever, sore throat, muscle pains, severe headache, coughing, weakness and general discomfort.[1] In more serious cases, influenza causes pneumonia, which can be fatal, particularly in young children and the elderly. Although it is sometimes confused with the common cold, influenza is a much more severe disease and is caused by a different type of virus.[2] Influenza can produce nausea and vomiting, especially in children,[1] but these symptoms are more characteristic of the unrelated gastroenteritis, which is sometimes called "stomach flu" or "24-hour flu".[3]
Typically influenza is transmitted from infected mammals through the air by coughs or sneezes, creating aerosols containing the virus, and from infected birds through their droppings. Influenza can also be transmitted by saliva, nasal secretions, faeces and blood. Infections also occur through contact with these body fluids or with contaminated surfaces. Flu viruses can remain infectious for about one week at human body temperature, over 30 days at 0 °C (32 °F), and for much longer periods at very low temperatures.[4][5] Most influenza strains can be inactivated easily by disinfectants and detergents.[6][7][8]
Flu spreads around the world in seasonal epidemics, killing millions of people in pandemic years and hundreds of thousands in non-pandemic years. Three influenza pandemics occurred in the 20th century and killed tens of millions of people, with each of these pandemics being caused by the appearance of a new strain of the virus in humans. Often, these new strains result from the spread of an existing flu virus to humans from other animal species. A deadly avian strain named H5N1 has posed the greatest risk for a new influenza pandemic since it first killed humans in Asia in the 1990s. Fortunately, this virus has not mutated to a form that spreads easily between people.[9]
Vaccinations against influenza are usually given to people in developed countries with a high risk of contracting the disease[10] and to farmed poultry.[11] The most common human vaccine is the trivalent influenza vaccine that contains purified and inactivated material from three viral strains. Typically, this vaccine includes material from two influenza A virus subtypes and one influenza B virus strain.[12] A vaccine formulated for one year may be ineffective in the following year, since the influenza virus changes rapidly over time, and different strains become dominant. Antiviral drugs can be used to treat influenza, with neuraminidase inhibitors being particularly effective.
| Influenza (flu) |
|---|
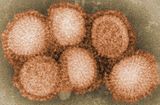 |
Etymology
The word influenza comes from the Italian language and refers to the cause of a disease; initially, this ascribed illness to unfavorable astrological influences.[13] Changes in medical thought led to its modification to influenza del freddo, meaning "influence of the cold". The word influenza was first used in English in 1743 when it was adopted, with an anglicized pronunciation, during an outbreak of the disease in Europe.[14] Archaic terms for influenza include epidemic catarrh, grippe (from the French), sweating sickness, and Spanish fever (particularly for the 1918 pandemic strain).[15]
History


The symptoms of human influenza were clearly described by Hippocrates roughly 2,400 years ago.[17][18] Since then, the virus has caused numerous pandemics. Historical data on influenza are difficult to interpret, because the symptoms can be similar to those of other diseases, such as diphtheria, pneumonic plague, typhoid fever, dengue, or typhus. The first convincing record of an influenza pandemic was of an outbreak in 1580, which began in Asia and spread to Europe via Africa. In Rome, over 8,000 people were killed, and several Spanish cities were almost wiped out. Pandemics continued sporadically throughout the 17th and 18th centuries, with the pandemic of 1830–1833 being particularly widespread; it infected approximately a quarter of the people exposed.[19]
The most famous and lethal outbreak was the so-called Spanish flu pandemic (type A influenza, H1N1 subtype), which lasted from 1918 to 1919. Older estimates say it killed 40–50 million people,[20] while current estimates say 50 million to 100 million people worldwide were killed.[21] This pandemic has been described as "the greatest medical holocaust in history" and may have killed as many people as the Black Death.[19] This huge death toll was caused by an extremely high infection rate of up to 50% and the extreme severity of the symptoms, suspected to be caused by cytokine storms.[20] Indeed, symptoms in 1918 were so unusual that initially influenza was misdiagnosed as dengue, cholera, or typhoid. One observer wrote, "One of the most striking of the complications was hemorrhage from mucous membranes, especially from the nose, stomach, and intestine. Bleeding from the ears and petechial hemorrhages in the skin also occurred."[21] The majority of deaths were from bacterial pneumonia, a secondary infection caused by influenza, but the virus also killed people directly, causing massive hemorrhages and edema in the lung.[16]
The Spanish flu pandemic was truly global, spreading even to the Arctic and remote Pacific islands. The unusually severe disease killed between 2 and 20% of those infected, as opposed to the more usual flu epidemic mortality rate of 0.1%.[16][21] Another unusual feature of this pandemic was that it mostly killed young adults, with 99% of pandemic influenza deaths occurring in people under 65, and more than half in young adults 20 to 40 years old.[22] This is unusual since influenza is normally most deadly to the very young (under age 2) and the very old (over age 70). The total mortality of the 1918–1919 pandemic is not known, but it is estimated that 2.5% to 5% of the world's population was killed. As many as 25 million may have been killed in the first 25 weeks; in contrast, HIV/AIDS has killed 25 million in its first 25 years.[21]
Later flu pandemics were not so devastating. They included the 1957 Asian Flu (type A, H2N2 strain) and the 1968 Hong Kong Flu (type A, H3N2 strain), but even these smaller outbreaks killed millions of people. In later pandemics antibiotics were available to control secondary infections and this may have helped reduce mortality compared to the Spanish Flu of 1918.[16]
| Name of pandemic | Date | Deaths | Subtype involved | Pandemic Severity Index |
|---|---|---|---|---|
| Asiatic (Russian) Flu | 1889–1890 | 1 million | possibly H2N2 | ? |
| Spanish Flu | 1918–1920 | 40 to 100 million | H1N1 | 5 |
| Asian Flu | 1957–1958 | 1 to 1.5 million | H2N2 | 2 |
| Hong Kong Flu | 1968–1969 | 0.75 to 1 million | H3N2 | 2 |
The etiological cause of influenza, the Orthomyxoviridae family of viruses, was first discovered in pigs by Richard Schope in 1931.[24] This discovery was shortly followed by the isolation of the virus from humans by a group headed by Patrick Laidlaw at the Medical Research Council of the United Kingdom in 1933.[25] However, it was not until Wendell Stanley first crystallized tobacco mosaic virus in 1935 that the non-cellular nature of viruses was appreciated.
The first significant step towards preventing influenza was the development in 1944 of a killed-virus vaccine for influenza by Thomas Francis, Jr.. This built on work by Frank Macfarlane Burnet, who showed that the virus lost virulence when it was cultured in fertilized hen's eggs.[26] Application of this observation by Francis allowed his group of researchers at the University of Michigan to develop the first influenza vaccine, with support from the U.S. Army.[27] The Army was deeply involved in this research due to its experience of influenza in World War I, when thousands of troops were killed by the virus in a matter of months.[21]
Although there were scares in New Jersey in 1976 (with the Swine Flu), worldwide in 1977 (with the Russian Flu), and in Hong Kong and other Asian countries in 1997 (with H5N1 avian influenza), there have been no major pandemics since the 1968 Hong Kong Flu. Immunity to previous pandemic influenza strains and vaccination may have limited the spread of the virus and may have helped prevent further pandemics.[23]
Microbiology
Types of influenza virus
| Genus | Influenzavirus A | Influenzavirus B | Influenzavirus C |
| Species | Influenza A virus | Influenza B virus | Influenza C virus |


The influenza virus is an RNA virus of the family Orthomyxoviridae, which comprises five genera: influenzavirus A, influenzavirus B, influenzavirus C, Isavirus, and Thogotovirus.[28] There are three types of influenza virus: Influenzavirus A, Influenzavirus B, and Influenzavirus C. Influenza A and C infect multiple species, while influenza B almost exclusively infects humans.[29] Wild aquatic birds are the natural hosts for a large variety of influenza A viruses. Occasionally, viruses are transmitted to other species and may then cause devastating outbreaks in domestic poultry or give rise to human influenza pandemics.[30] The type A viruses are the most virulent human pathogens among the three influenza types and cause the most severe disease. The influenza A virus can be subdivided into different serotypes based on the antibody response to these viruses.[29] The serotypes that have been confirmed in humans, ordered by the number of known human pandemic deaths, are:
- H1N1 caused Spanish Flu.
- H2N2 caused Asian Flu.
- H3N2 caused Hong Kong Flu.
- H5N1 is a pandemic threat in the 2007–08 flu season.
- H7N7 has unusual zoonotic potential.[31]
- H1N2 is endemic in humans and pigs.
- H9N2, H7N2, H7N3, H10N7.
Influenza B virus is almost exclusively a human pathogen and is less common than influenza A. The only other animal known to be susceptible to influenza B infection is the seal.[32] This type of influenza mutates at a rate 2–3 times lower than type A[33] and consequently is less genetically diverse, with only one influenza B serotype.[29] As a result of this lack of antigenic diversity, a degree of immunity to influenza B is usually acquired at an early age. However, influenza B mutates enough that lasting immunity is not possible.[34] This reduced rate of antigenic change, combined with its limited host range (inhibiting cross species antigenic shift), ensures that pandemics of influenza B do not occur.[35]
The influenza C virus infects humans and pigs and can cause severe illness and local epidemics.[36] However, influenza C is less common than the other types and usually seems to cause mild disease in children.[37][38]
Structure and properties
The following applies for influenza A viruses, although other strains are very similar in structure:[39]
The influenza A virus particle or virion is 80–120 nm in diameter and usually roughly spherical, although filamentous forms can occur.[40] Unusually for a virus, the influenza A genome is not a single piece of nucleic acid; instead, it contains eight pieces of segmented negative-sense RNA (13.5 kilobases total), which encode 11 proteins (HA (hemagglutinin), NA (neuraminidase), NP (nucleoprotein), M1, M2, NS1, NS2(NEP), PA, PB1, PB1-F2, PB2).[41] The best-characterised of these viral proteins are hemagglutinin and neuraminidase, two large glycoproteins found on the outside of the viral particles. Neuraminidase is an enzyme involved in the release of progeny virus from infected cells, by cleaving sugars that bind the mature viral particles. By contrast, hemagglutinin is a lectin that mediates binding of the virus to target cells and entry of the viral genome into the target cell.[42] The hemagglutinin (HA or H) and neuraminidase (NA or N) proteins are targets for antiviral drugs.[43] These proteins are also recognised by antibodies, i.e. they are antigens.[23] The responses of antibodies to these proteins are used to classify the different serotypes of influenza A viruses, hence the H and N in H5N1.
Infection and replication

Influenza viruses bind through hemagglutinin onto sialic acid sugars on the surfaces of epithelial cells; typically in the nose, throat and lungs of mammals and intestines of birds (Stage 1 in infection figure).[44] The cell imports the virus by endocytosis. In the acidic endosome, part of the hemagglutinin protein fuses the viral envelope with the vacuole's membrane, releasing the viral RNA (vRNA) molecules, accessory proteins and RNA-dependent RNA transcriptase into the cytoplasm (Stage 2).[45] These proteins and vRNA form a complex that is transported into the cell nucleus, where the RNA-dependent RNA transcriptase begins transcribing complementary positive-sense vRNA (Steps 3a and b).[46] The vRNA is either exported into the cytoplasm and translated (step 4), or remains in the nucleus. Newly-synthesised viral proteins are either secreted through the Golgi apparatus onto the cell surface (in the case of neuraminidase and hemagglutinin, step 5b) or transported back into the nucleus to bind vRNA and form new viral genome particles (step 5a). Other viral proteins have multiple actions in the host cell, including degrading cellular mRNA and using the released nucleotides for vRNA synthesis and also inhibiting translation of host-cell mRNAs.[47]
Negative-sense vRNAs that form the genomes of future viruses, RNA-dependent RNA transcriptase, and other viral proteins are assembled into a virion. Hemagglutinin and neuraminidase molecules cluster into a bulge in the cell membrane. The vRNA and viral core proteins leave the nucleus and enter this membrane protrusion (step 6). The mature virus buds off from the cell in a sphere of host phospholipid membrane, acquiring hemagglutinin and neuraminidase with this membrane coat (step 7).[48] As before, the viruses adhere to the cell through hemagglutinin; the mature viruses detach once their neuraminidase has cleaved sialic acid residues from the host cell.[44] After the release of new influenza viruses, the host cell dies.
Because of the absence of RNA proofreading enzymes, the RNA-dependent RNA transcriptase makes a single nucleotide insertion error roughly every 10 thousand nucleotides, which is the approximate length of the influenza vRNA. Hence, nearly every newly-manufactured influenza virus is a mutant[49]—antigenic drift. The separation of the genome into eight separate segments of vRNA allows mixing or reassortment of vRNAs if more than one viral line has infected a single cell. The resulting rapid change in viral genetics produces antigenic shifts and allows the virus to infect new host species and quickly overcome protective immunity.[23] This is important in the emergence of pandemics, as discussed below in the section on Epidemiology.
Symptoms and diagnosis
In humans, influenza's effects are much more severe and last longer than those of the common cold. Recovery takes about one to two weeks. Influenza, however, can be deadly, especially for the weak, old or chronically ill.[23] The flu can worsen chronic health problems. People with emphysema, chronic bronchitis or asthma may experience shortness of breath while they have the flu, and influenza may cause worsening of coronary heart disease or congestive heart failure.[50] Smoking is another risk factor associated with more serious disease and increased mortality from influenza.[51]
Symptoms
Symptoms of influenza can start quite suddenly one to two days after infection. Usually the first symptoms are chills or a chilly sensation, but fever is also common early in the infection, with body temperatures as high as 39 °C (approximately 103 °F). Many people are so ill that they are confined to bed for several days, with aches and pains throughout their bodies, which are worst in their backs and legs.[1] Symptoms of influenza may include:
It can be difficult to distinguish between the common cold and influenza in the early stages of these infections,[2] but usually the symptoms of the flu are more severe than their common cold equivalents. Research on signs and symptoms of influenza found that the best findings for excluding the diagnosis of influenza were:[53]
| Finding: | sensitivity | specificity |
|---|---|---|
| Fever | 86% | 25% |
| Cough | 98% | 23% |
| Nasal congestion | 70–90% | 20–40% |
Notes to table:
- Sensitivity is the proportion of people who tested positive of all the positive people tested. In this case, being positive or negative is having influenza or not, and being tested positive or negative is having the symptom or not. For instance, 86% of those with influenza had fever.
- Specificity is the proportion of people who tested negative of all the negative people tested. In this case, the ones without fever only constitute 25% of those without influenza. In other words, the majority of people with fever do not have influenza.
- All three findings, especially fever, were less sensitive in patients over 60 years of age.
Since anti-viral drugs are effective in treating influenza if given early (see treatment section, below), it can be important to identify cases early. Of the symptoms listed above, the combinations of findings below can improve diagnostic accuracy.[54] Unfortunately, even combinations of findings are imperfect. However, Bayes Theorem can combine pretest probability with clinical findings to adequately diagnose or exclude influenza in some patients. The pretest probability has a strong seasonal variation; the current prevalence of influenza among patients in the United States receiving sentinel testing is available at the CDC.[55] Using the CDC data, the following table shows how the likelihood of influenza varies with prevalence:
| Combinations of findings | Sensitivity | Specificity | As reported in study[53] and projected during local outbreaks (prevalence= 66%) |
Projected during influenza season (prevalence=25%) |
Projected in off-season (prevalence=2%) |
|||
|---|---|---|---|---|---|---|---|---|
| PPV | NPV | PPV | NPV | PPV | NPV | |||
| Fever and cough | 64% | 67% | 79% | 49% | 39% | 15% | 4% | 1% |
| Fever and cough and sore throat | 56 | 71 | 79 | 45 | 39 | 17 | 4 | 2 |
| Fever and cough and nasal congestion | 59 | 74 | 81 | 48 | 43 | 16 | 4 | 1 |
Two decision analysis studies[56][57] suggest that during local outbreaks of influenza, the prevalence will be over 70%,[57] and thus patients with any of the above combinations of symptoms may be treated with neuramidase inhibitors without testing. Even in the absence of a local outbreak, treatment may be justified in the elderly during the influenza season as long as the prevalence is over 15%.[57]
Most people who get influenza will recover in one to two weeks, but others will develop life-threatening complications (such as pneumonia). According to the World Health Organization: "Every winter, tens of millions of people get the flu. Most are only ill and out of work for a week, yet the elderly are at a higher risk of death from the illness. We know the world-wide death toll exceeds a few hundred thousand people a year, but even in developed countries the numbers are uncertain, because medical authorities don't usually verify who actually died of influenza and who died of a flu-like illness."[58] Even healthy people can be affected, and serious problems from influenza can happen at any age. People over 50 years old, very young children and people of any age with chronic medical conditions are more likely to get complications from influenza, such as pneumonia, bronchitis, sinus, and ear infections.[59]
Common symptoms of the flu such as fever, headaches, and fatigue come from the huge amounts of proinflammatory cytokines and chemokines (such as interferon or tumor necrosis factor) produced from influenza-infected cells.[2][60] In contrast to the rhinovirus that causes the common cold, influenza does cause tissue damage, so symptoms are not entirely due to the inflammatory response.[61]
Laboratory tests
The available laboratory tests for influenza continue to improve. The United States Centers for Disease Control and Prevention (CDC) maintains an up-to-date summary of available laboratory tests.[62] According to the CDC, rapid diagnostic tests have a sensitivity of 70–75% and specificity of 90–95% when compared with viral culture. These tests may be especially useful during the influenza season (prevalence=25%) but in the absence of a local outbreak, or peri-influenza season (prevalence=10%[57]).
Epidemiology
Seasonal variations

Influenza reaches peak prevalence in winter, and because the Northern and Southern Hemispheres have winter at different times of the year, there are actually two different flu seasons each year. This is why the World Health Organization (assisted by the National Influenza Centers) makes recommendations for two different vaccine formulations every year; one for the Northern, and one for the Southern Hemisphere.[64]
It is not completely clear why outbreaks of the flu occur seasonally rather than uniformly throughout the year. One possible explanation is that, because people are indoors more often during the winter, they are in close contact more often, and this promotes transmission from person to person. Another is that cold temperatures lead to drier air, which may dehydrate mucus, preventing the body from effectively expelling virus particles. The virus may also survive longer on exposed surfaces (doorknobs, countertops, etc.) in colder temperatures. Increased travel due to the Northern Hemisphere winter holiday season may also play a role.[65] A contributing factor is that aerosol transmission of the virus is highest in cold environments (less than 5 °C) with low humidity.[66] However, seasonal changes in infection rates also occur in tropical regions, and these peaks of infection are seen mainly during the rainy season.[67] Seasonal changes in contact rates from school terms, which are a major factor in other childhood diseases such as measles and pertussis, may also play a role in the flu. A combination of these small seasonal effects may be amplified by dynamical resonance with the endogenous disease cycles.[68] H5N1 exhibits seasonality in both humans and birds.[63]
An alternative hypothesis to explain seasonality in influenza infections is an effect of vitamin D levels on immunity to the virus.[69] This idea was first proposed by Robert Edgar Hope-Simpson in 1965.[70] He proposed that the cause of influenza epidemics during winter may be connected to seasonal fluctuations of vitamin D, which is produced in the skin under the influence of solar (or artificial) UV radiation. This could explain why influenza occurs mostly in winter and during the tropical rainy season, when people stay indoors, away from the sun, and their vitamin D levels fall. Furthermore, some studies have suggested that administering cod liver oil, which contains large amounts of vitamin D, can reduce the incidence of respiratory tract infections.[71]
Epidemic and pandemic spread

As influenza is caused by a variety of species and strains of viruses, in any given year some strains can die out while others create epidemics, while yet another strain can cause a pandemic. Typically, in a year's normal two flu seasons (one per hemisphere), there are between three and five million cases of severe illness and up to 500,000 deaths worldwide, which by some definitions is a yearly influenza epidemic.[72] Although the incidence of influenza can vary widely between years, approximately 36,000 deaths and more than 200,000 hospitalizations are directly associated with influenza every year in America.[73][74][75] Every ten to twenty years, a pandemic occurs, which infects a large proportion of the world's population and can kill tens of millions of people (see history section).
New influenza viruses are constantly being produced by mutation or by reassortment.[29] Mutations can cause small changes in the hemagglutinin and neuraminidase antigens on the surface of the virus. This is called antigenic drift, which creates an increasing variety of strains over time until one of the variants eventually achieves higher fitness, becomes dominant, and rapidly sweeps through the human population—often causing an epidemic.[76] In contrast, when influenza viruses reassort, they may acquire new antigens—for example by reassortment between avian strains and human strains; this is called antigenic shift. If a human influenza virus is produced with entirely novel antigens, everybody will be susceptible, and the novel influenza will spread uncontrollably, causing a pandemic.[77] In contrast to this model of pandemics based on antigenic drift and shift, an alternative approach has been proposed where the periodic pandemics are produced by interactions of a fixed set of viral strains with a human population with a constantly changing set of immunities to different viral strains.[78]
Prevention
Vaccination and infection control
Vaccination against influenza with an influenza vaccine is often recommended for high-risk groups, such as children and the elderly. Influenza vaccines can be produced in several ways; the most common method is to grow the virus in fertilized hen eggs. After purification, the virus is inactivated (for example, by treatment with detergent) to produce an inactivated-virus vaccine. Alternatively, the virus can be grown in eggs until it loses virulence and the avirulent virus given as a live vaccine.[23] The effectiveness of these influenza vaccines is variable. Due to the high mutation rate of the virus, a particular influenza vaccine usually confers protection for no more than a few years. Every year, the World Health Organization predicts which strains of the virus are most likely to be circulating in the next year, allowing pharmaceutical companies to develop vaccines that will provide the best immunity against these strains.[64] Vaccines have also been developed to protect poultry from avian influenza. These vaccines can be effective against multiple strains and are used either as part of a preventative strategy, or combined with culling in attempts to eradicate outbreaks.[79]
It is possible to get vaccinated and still get influenza. The vaccine is reformulated each season for a few specific flu strains but cannot possibly include all the strains actively infecting people in the world for that season. It takes about six months for the manufacturers to formulate and produce the millions of doses required to deal with the seasonal epidemics; occasionally, a new or overlooked strain becomes prominent during that time and infects people although they have been vaccinated (as by the H3N2 Fujian flu in the 2003–2004 flu season).[80] It is also possible to get infected just before vaccination and get sick with the very strain that the vaccine is supposed to prevent, as the vaccine takes about two weeks to become effective.[59]
The 2006–2007 season was the first in which the CDC had recommended that children younger than 59 months receive the annual influenza vaccine.[81] Vaccines can cause the immune system to react as if the body were actually being infected, and general infection symptoms (many cold and flu symptoms are just general infection symptoms) can appear, though these symptoms are usually not as severe or long-lasting as influenza. The most dangerous side-effect is a severe allergic reaction to either the virus material itself or residues from the hen eggs used to grow the influenza; however, these reactions are extremely rare.[82]

Good personal health and hygiene habits are reasonably effective in avoiding and minimizing influenza. People who contract influenza are most infective between the second and third days after infection and infectivity lasts for around ten days.[83] Children are notably more infectious than adults and shed virus from just before they develop symptoms until two weeks after infection.[83][84]
Since influenza spreads through aerosols and contact with contaminated surfaces, it is important to persuade people to cover their mouths while sneezing and to wash their hands regularly.[81] Surface sanitizing is recommended in areas where influenza may be present on surfaces.[85] Alcohol is an effective sanitizer against influenza viruses, while quaternary ammonium compounds can be used with alcohol to increase the duration of the sanitizing action.[86] In hospitals, quaternary ammonium compounds and halogen-releasing agents such as sodium hypochlorite are commonly used to sanitize rooms or equipment that have been occupied by patients with influenza symptoms.[86] During past pandemics, closing schools, churches and theaters slowed the spread of the virus but did not have a large effect on the overall death rate.[87][88]
Treatment
People with the flu are advised to get plenty of rest, drink a lot of liquids, avoid using alcohol and tobacco and, if necessary, take medications such as paracetamol (acetaminophen) to relieve the fever and muscle aches associated with the flu. Children and teenagers with flu symptoms (particularly fever) should avoid taking aspirin during an influenza infection (especially influenza type B), because doing so can lead to Reye's syndrome, a rare but potentially fatal disease of the liver.[89] Since influenza is caused by a virus, antibiotics have no effect on the infection; unless prescribed for secondary infections such as bacterial pneumonia, they may lead to resistant bacteria. Antiviral medication is sometimes effective, but viruses can develop resistance to the standard antiviral drugs.
The two classes of anti-virals are neuraminidase inhibitors and M2 inhibitors (adamantane derivatives). Neuraminidase inhibitors are currently preferred for flu virus infections. The CDC recommended against using M2 inhibitors during the 2005–06 influenza season.[90]
Neuraminidase inhibitors
Antiviral drugs such as oseltamivir (trade name Tamiflu) and zanamivir (trade name Relenza) are neuraminidase inhibitors that are designed to halt the spread of the virus in the body.[91] These drugs are often effective against both influenza A and B.[92] The Cochrane Collaboration reviewed these drugs and concluded that they reduce symptoms and complications.[93] Different strains of influenza viruses have differing degrees of resistance against these antivirals, and it is impossible to predict what degree of resistance a future pandemic strain might have.[94]
M2 inhibitors (adamantanes)
The antiviral drugs amantadine and rimantadine are designed to block a viral ion channel (M2 protein) and prevent the virus from infecting cells. These drugs are sometimes effective against influenza A if given early in the infection but are always ineffective against influenza B.[92] Measured resistance to amantadine and rimantadine in American isolates of H3N2 has increased to 91% in 2005.[95]
Research

Research on influenza includes studies on molecular virology, how the virus produces disease (pathogenesis), host immune responses, viral genomics, and how the virus spreads (epidemiology). These studies help in developing influenza countermeasures; for example, a better understanding of the body's immune system response helps vaccine development, and a detailed picture of how influenza invades cells aids the development of antiviral drugs. One important basic research program is the Influenza Genome Sequencing Project, which is creating a library of influenza sequences; this library should help clarify which factors make one strain more lethal than another, which genes most affect immunogenicity, and how the virus evolves over time.[96]
Research into new vaccines is particularly important, as current vaccines are slow and expensive to produce and must be reformulated every year. The sequencing of the influenza genome and recombinant DNA technology may accelerate the generation of new vaccine strains by allowing scientists to substitute new antigens into a previously developed vaccine strain.[97] New technologies are also being developed to grow viruses in cell culture, which promises higher yields, less cost, better quality and surge capacity.[98] Research on a universal influenza A vaccine, targeted against the external domain of the transmembrane viral M2 protein (M2e), is being done at the University of Ghent by Walter Fiers, Xavier Saelens and their team[99][100][101] and has now successfully concluded Phase I clinical trials.
The US government has purchased from Sanofi Pasteur and Chiron Corporation several million doses of vaccine meant to be used in case of an influenza pandemic of H5N1 avian influenza and is conducting clinical trials with these vaccines.[102] The UK government is also stockpiling millions of doses of antiviral drugs (oseltamivir (Tamiflu), zanimivir (Relanza)) to give to its citizens in the event of an outbreak; the UK Health Protection Agency has also gathered a limited amount of HPAI H5N1 vaccines for experimental purposes.[citation needed]
Infection in other animals
 |
Influenza infects many animal species, and transfer of viral strains between species can occur. Birds are thought to be the main animal reservoirs of influenza viruses.[103] Sixteen forms of hemagglutinin and nine forms of neuraminidase have been identified. All known subtypes (HxNy) are found in birds, but many subtypes are endemic in humans, dogs, horses, and pigs; populations of camels, ferrets, cats, seals, mink, and whales also show evidence of prior infection or exposure to influenza.[34] Variants of flu virus are sometimes named according to the species the strain is endemic in or adapted to. The main variants named using this convention are: Bird Flu, Human Flu, Swine Flu, Horse Flu and Dog Flu. (Cat flu generally refers to Feline viral rhinotracheitis or Feline calicivirus and not infection from an influenza virus.) In pigs, horses and dogs, influenza symptoms are similar to humans, with cough, fever and loss of appetite.[34] The frequency of animal diseases are not as well-studied as human infection, but an outbreak of influenza in harbour seals caused approximately 500 seal deaths off the New England coast in 1979–1980.[104] On the other hand, outbreaks in pigs are common and do not cause severe mortality.[34]
Flu symptoms in birds are variable and can be unspecific.[105] The symptoms following infection with low-pathogenicity avian influenza may be as mild as ruffled feathers, a small reduction in egg production, or weight loss combined with minor respiratory disease.[106] Since these mild symptoms can make diagnosis in the field difficult, tracking the spread of avian influenza requires laboratory testing of samples from infected birds. Some strains such as Asian H9N2 are highly virulent to poultry and may cause more extreme symptoms and significant mortality.[107] In its most highly pathogenic form, influenza in chickens and turkeys produces a sudden appearance of severe symptoms and almost 100% mortality within two days.[108] As the virus spreads rapidly in the crowded conditions seen in the intensive farming of chickens and turkeys, these outbreaks can cause large economic losses to poultry farmers.
An avian-adapted, highly pathogenic strain of H5N1 (called HPAI A(H5N1), for "highly pathogenic avian influenza virus of type A of subtype H5N1") causes H5N1 flu, commonly known as "avian influenza" or simply "bird flu", and is endemic in many bird populations, especially in Southeast Asia. This Asian lineage strain of HPAI A(H5N1) is spreading globally. It is epizootic (an epidemic in non-humans) and panzootic (a disease affecting animals of many species, especially over a wide area), killing tens of millions of birds and spurring the culling of hundreds of millions of other birds in an attempt to control its spread. Most references in the media to "bird flu" and most references to H5N1 are about this specific strain.[109][110]
At present, HPAI A(H5N1) is an avian disease, and there is no evidence suggesting efficient human-to-human transmission of HPAI A(H5N1). In almost all cases, those infected have had extensive physical contact with infected birds.[111] In the future, H5N1 may mutate or reassort into a strain capable of efficient human-to-human transmission. Due to its high lethality and virulence, its endemic presence, and its large and increasing biological host reservoir, the H5N1 virus was the world's pandemic threat in the 2006–07 flu season, and billions of dollars are being raised and spent researching H5N1 and preparing for a potential influenza pandemic.[112]
Economic impact
Influenza produces direct costs due to lost productivity and associated medical treatment, as well as indirect costs of preventative measures. In the United States, influenza is responsible for a total cost of over $10 billion per year, while it has been estimated that a future pandemic could cause hundreds of billions of dollars in direct and indirect costs.[113] However, the economic impacts of past pandemics have not been intensively studied, and some authors have suggested that the Spanish influenza actually had a positive long-term effect on per-capita income growth, despite a large reduction in the working population and severe short-term depressive effects.[114] Other studies have attempted to predict the costs of a pandemic as serious as the 1918 Spanish flu on the U.S. economy, where 30% of all workers became ill, and 2.5% were killed. A 30% sickness rate and a three-week length of illness would decrease the gross domestic product by 5%. Additional costs would come from medical treatment of 18 million to 45 million people, and total economic costs would be approximately $700 billion.[115]
Preventative costs are also high. Governments worldwide have spent billions of U.S. dollars preparing and planning for a potential H5N1 avian influenza pandemic, with costs associated with purchasing drugs and vaccines as well as developing disaster drills and strategies for improved border controls.[112] On November 1, 2005, President George W. Bush unveiled the National Strategy to Safeguard Against the Danger of Pandemic Influenza[116] backed by a request to Congress for $7.1 billion to begin implementing the plan.[117] Internationally, on January 18, 2006, donor nations pledged US$2 billion to combat bird flu at the two-day International Pledging Conference on Avian and Human Influenza held in China.[118]
As of 2006, over ten billion dollars have been spent, and over two hundred million birds have been killed to try to contain H5N1 avian influenza.[119] However, as these efforts have been largely ineffective at controlling the spread of the virus, other approaches are being tried: for example, the Vietnamese government in 2005 adopted a combination of mass poultry vaccination, disinfecting, culling, information campaigns and bans on live poultry in cities.[120] As a result of such measures, the cost of poultry farming has increased, while the cost to consumers has gone down due to demand for poultry falling below supply. This has resulted in devastating losses for many farmers. Poor poultry farmers cannot afford mandated measures which isolate their bird livestock from contact with wild birds (among other measures), thus risking losing their livelihood altogether. Multinational poultry farming is increasingly becoming unprofitable as H5N1 avian influenza becomes endemic in wild birds worldwide.[121] Financial ruin for poor poultry farmers, which can be as severe as threatening starvation, has caused some to commit suicide and many others to stop cooperating with efforts to deal with this virus—further increasing the human toll, the spread of the disease, and the chances of a pandemic mutation.[122][123]
See also
- Information concerning flu research can be found at
References and notes
- ^ a b c "Influenza: Viral Infections: Merck Manual Home Edition". www.merck.com. Retrieved 2008-03-15.
- ^ a b c Eccles, R (2005). "Understanding the symptoms of the common cold and influenza". Lancet Infect Dis. 5 (11): 718–25. PMID 16253889.
- ^ Seasonal Flu vs. Stomach Flu by Kristina Duda, R.N.; accessed March 12, 2007 (Website: "About, Inc., A part of The New York Times Company")
- ^ Reid AH, Fanning TG, Hultin JV, Taubenberger JK (1999). "Origin and evolution of the 1918 "Spanish" influenza virus hemagglutinin gene". Proc. Natl. Acad. Sci. U.S.A. 96 (4): 1651–6. doi:10.1073/pnas.96.4.1651. PMID 9990079.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ J Gen Virol 87 (2006), 3655-3659; DOI 10.1099/vir.0.81843-0 article Recent H5N1 avian Influenza A virus increases rapidly in virulence to mice after a single passage in mice says "To prepare the original virus stock for this study, virus was propagated once in the allantoic cavity of embryonated eggs at 37 °C for 1–2 days and then stored at –80 °C until use."
- ^ Suarez, D (2003). "The effect of various disinfectants on detection of avian influenza virus by real time RT-PCR". Avian Dis. 47 (3 Suppl): 1091–5. PMID 14575118.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Avian Influenza (Bird Flu): Implications for Human Disease. Physical characteristics of influenza A viruses. UMN CIDRAP.
- ^ Flu viruses 'can live for decades' on ice, NZ Herald, November 30, 2006.
- ^ "Avian influenza ("bird flu") fact sheet". WHO. February 2006. Retrieved 2006-10-20.
- ^ WHO position paper: influenza vaccines WHO weekly Epidemiological Record 19 August 2005, vol. 80, 33, pp. 277–288.
- ^ Villegas, P (1998). "Viral diseases of the respiratory system". Poult Sci. 77 (8): 1143–5. PMID 9706079.
- ^ Horwood, F. "Pneumococcal and influenza vaccination: current situation and future prospects" (PDF). Thorax. 57 Suppl 2: II24–II30. PMID 12364707.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Influenza, The Oxford English Dictionary, second edition.
- ^ Harper, D. "Influenza". Etymonlin.
- ^ Smith, P. "Archaic Medical Terms". Retrieved 2006-10-23.
- ^ a b c d Taubenberger, J (2006). "1918 Influenza: the mother of all pandemics". Emerg Infect Dis. 12 (1): 15–22. PMID 16494711.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Cite error: The named reference "Taubenberger" was defined multiple times with different content (see the help page). - ^ Martin, P (2006). "2,500-year evolution of the term epidemic". Emerg Infect Dis. 12 (6). PMID 16707055.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Hippocrates (400 BCE). "Of the Epidemics". Retrieved 2006-10-18.
{{cite web}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Potter, CW (2006). "A History of Influenza". J Appl Microbiol. 91 (4): 572–579. doi:10.1046/j.1365-2672.2001.01492.x. PMID 11576290.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Patterson, KD (1991). "The geography and mortality of the 1918 influenza pandemic". Bull Hist Med. 65 (1): 4–21. PMID 2021692.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b c d e Knobler S, Mack A, Mahmoud A, Lemon S (ed.). "1: The Story of Influenza". The Threat of Pandemic Influenza: Are We Ready? Workshop Summary (2005). Washington, D.C.: The National Academies Press. pp. 60–61.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: editors list (link) - ^ Simonsen, L (1998). "Pandemic versus epidemic influenza mortality: a pattern of changing age distribution". J Infect Dis. 178 (1): 53–60. PMID 9652423.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b c d e f Hilleman, M (2002). "Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control". Vaccine. 20 (25–26): 3068–87. PMID 12163258.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Shimizu, K (1997). "History of influenza epidemics and discovery of influenza virus". Nippon Rinsho. 55 (10): 2505–201. PMID 9360364.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Smith, W (1933). "A virus obtained from influenza patients". Lancet. 2: 66–68.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Sir Frank Macfarlane Burnet: Biography The Nobel Foundation. Accessed 22 Oct 06
- ^ Kendall, H (2006). "Vaccine Innovation: Lessons from World War II" (PDF). Journal of Public Health Policy. 27 (1): 38–57.
- ^ Kawaoka Y (editor). (2006). Influenza Virology: Current Topics. Caister Academic Press. ISBN 978-1-904455-06-6.
{{cite book}}:|author=has generic name (help) - ^ a b c d Hay, A (2001). "The evolution of human influenza viruses". Philos Trans R Soc Lond B Biol Sci. 356 (1416): 1861–70. PMID 11779385.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Klenk; et al. (2008). "Avian Influenza: Molecular Mechanisms of Pathogenesis and Host Range". Animal Viruses: Molecular Biology. Caister Academic Press. ISBN 978-1-904455-22-6.
{{cite book}}: Explicit use of et al. in:|author=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Fouchier, R (2004). "Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome". Proc Natl Acad Sci U S A. 101 (5): 1356–61. PMID 14745020.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Osterhaus, A (2000). "Influenza B virus in seals". Science. 288 (5468): 1051–3. PMID 10807575.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Nobusawa, E (2006). "Comparison of the mutation rates of human influenza A and B viruses". J Virol. 80 (7): 3675–8. PMID 16537638.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b c d R, Webster (1992). "Evolution and ecology of influenza A viruses". Microbiol Rev. 56 (1): 152–79. PMID 1579108.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Zambon, M (1999). "Epidemiology and pathogenesis of influenza". J Antimicrob Chemother. 44 Suppl B: 3–9. PMID 10877456.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Matsuzaki, Y (2002). "Antigenic and genetic characterization of influenza C viruses which caused two outbreaks in Yamagata City, Japan, in 1996 and 1998". J Clin Microbiol. 40 (2): 422–9. PMID 11825952.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Matsuzaki, Y (2006). "Clinical features of influenza C virus infection in children". J Infect Dis. 193 (9): 1229–35. PMID 16586359.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Katagiri, S (1983). "An outbreak of type C influenza in a children's home". J Infect Dis. 148 (1): 51–6. PMID 6309999.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ International Committee on Taxonomy of Viruses descriptions of: Orthomyxoviridae, Influenzavirus B and Influenzavirus C
- ^ International Committee on Taxonomy of Viruses. "The Universal Virus Database, version 4: Influenza A".
- ^ Ghedin, E (2005). "Large-scale sequencing of human influenza reveals the dynamic nature of viral genome evolution". Nature. 437 (7062): 1162–6. PMID 16208317.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Suzuki, Y (2005). "Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses". Biol Pharm Bull. 28 (3): 399–408. PMID 15744059.
- ^ Wilson, J (2003). "Recent strategies in the search for new anti-influenza therapies". Curr Drug Targets. 4 (5): 389–408. PMID 12816348.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b Wagner, R (2002). "Functional balance between haemagglutinin and neuraminidase in influenza virus infections". Rev Med Virol. 12 (3): 159–66. PMID 11987141.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Lakadamyali, M (2003). "Visualizing infection of individual influenza viruses". Proc Natl Acad Sci U S A. 100 (16): 9280–5. PMID 12883000.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Cros, J (2003). "Trafficking of viral genomic RNA into and out of the nucleus: influenza, Thogoto and Borna disease viruses". Virus Res. 95 (1–2): 3–12. PMID 12921991.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Kash, J (2006). "Hijacking of the host-cell response and translational control during influenza virus infection". Virus Res. 119 (1): 111–20. PMID 16630668.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Nayak, D (2004). "Assembly and budding of influenza virus". Virus Res. 106 (2): 147–65. PMID 15567494.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Drake, J (1993). "Rates of spontaneous mutation among RNA viruses". Proc Natl Acad Sci USA. 90 (9): 4171–5. PMID 8387212.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Angelo SJ, Marshall PS, Chrissoheris MP, Chaves AM. "Clinical characteristics associated with poor outcome in patients acutely infected with Influenza A." Conn Med. 2004 Apr;68(4):199–205. PMID 15095826
- ^ Murin S, Bilello K (2005). "Respiratory tract infections: another reason not to smoke". Cleve Clin J Med. 72 (10): 916–20. PMID 16231688.
- ^ Kerr AA, McQuillin J, Downham MA, Gardner PS (1975). "Gastric 'flu influenza B causing abdominal symptons in children". Lancet. 1 (7902): 291–5. PMID 46444.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Call S, Vollenweider M, Hornung C, Simel D, McKinney W (2005). "Does this patient have influenza?". JAMA. 293 (8): 987–97. doi:10.1001/jama.293.8.987. PMID 15728170.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid15728170" was defined multiple times with different content (see the help page). - ^ Monto A, Gravenstein S, Elliott M, Colopy M, Schweinle J (2000). "Clinical signs and symptoms predicting influenza infection". Arch Intern Med. 160 (21): 3243–7. doi:10.1001/archinte.160.21.3243. PMID 11088084.
{{cite journal}}: Text "url http://archinte.ama-assn.org/cgi/content/abstract/160/21/3243" ignored (help)CS1 maint: multiple names: authors list (link) - ^ Centers for Disease Control and Prevention. Weekly Report: Influenza Summary Update. Accessed January 1, 2007.
- ^ Smith K, Roberts M (2002). "Cost-effectiveness of newer treatment strategies for influenza". Am J Med. 113 (4): 300–7. doi:10.1016/S0002-9343(02)01222-6. PMID 12361816.
- ^ a b c d Rothberg M, Bellantonio S, Rose D (2003). "Management of influenza in adults older than 65 years of age: cost-effectiveness of rapid testing and antiviral therapy". Ann Intern Med. 139 (5 Pt 1): 321–9. PMID 12965940.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Peter M. Sandman and Jody Lanard "Bird Flu: Communicating the Risk" 2005 Perspectives in Health Magazine Vol. 10 issue 2.
- ^ a b Key Facts about Influenza (Flu) Vaccine CDC publication. Published October 17, 2006. Accessed 18 Oct 2006.
- ^ Schmitz N, Kurrer M, Bachmann M, Kopf M (2005). "Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection". J Virol. 79 (10): 6441–8. doi:10.1128/JVI.79.10.6441-6448.2005. PMID 15858027.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Winther B, Gwaltney J, Mygind N, Hendley J. "Viral-induced rhinitis". Am J Rhinol. 12 (1): 17–20. PMID 9513654.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Centers for Disease Control and Prevention. Lab Diagnosis of Influenza. Accessed on January 1, 2007
- ^ a b WHO Confirmed Human Cases of H5N1 Data published by WHO Epidemic and Pandemic Alert and Response (EPR). Accessed 24 Oct. 2006
- ^ a b Recommended composition of influenza virus vaccines for use in the 2006–2007 influenza season WHO report 2006-02-14. Accessed 19 October 2006.
- ^ Weather and the Flu Season NPR Day to Day, December 17 2003. Accessed, 19 October 2006
- ^ Lowen AC, Mubareka S, Steel J, Palese P (2007). "Influenza virus transmission is dependent on relative humidity and temperature". PLoS Pathog. 3 (10): 1470–6. doi:10.1371/journal.ppat.0030151. PMID 17953482.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Shek LP, Lee BW. "Epidemiology and seasonality of respiratory tract virus infections in the tropics." Paediatr Respir Rev. 2003 Jun;4(2):105–11. PMID 12758047
- ^ Dushoff J, Plotkin JB, Levin SA, Earn DJ. "Dynamical resonance can account for seasonality of influenza epidemics." Proc Natl Acad Sci U S A. 30 November2004;101(48):16915–6. PMID 15557003
- ^ Cannell, J (2006). "Epidemic influenza and vitamin D". Epidemiol Infect. 134 (6): 1129–40. PMID 16959053.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ HOPE-SIMPSON, R. "The nature of herpes zoster: a long-term study and a new hypothesis". Proc R Soc Med. 58: 9–20. PMID 14267505.
- ^ Linday, L (2004). "Effect of daily cod liver oil and a multivitamin-mineral supplement with selenium on upper respiratory tract pediatric visits by young, inner-city, Latino children: randomized pediatric sites". Ann Otol Rhinol Laryngol. 113 (11): 891–901. PMID 15562899.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Influenza WHO Fact sheet No. 211 revised March 2003. Accessed 22 October 2006
- ^ Thompson, W (2003). "Mortality associated with influenza and respiratory syncytial virus in the United States". JAMA. 289 (2): 179–86. PMID 12517228.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Thompson, W (2004). "Influenza-associated hospitalizations in the United States". JAMA. 292 (11): 1333–40. PMID 15367555.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Flu factsheet National Institute of Allergy and Infectious Diseases Accessed 22 Dec 2006
- ^ "Long intervals of stasis punctuated by bursts of positive selection in the seasonal evolution of influenza A virus". Biol Direct. 1 (1): 34. 2006. PMID 17067369.
- ^ Parrish, C. "The origins of new pandemic viruses: the acquisition of new host ranges by canine parvovirus and influenza A viruses". Annual Rev Microbiol. 59: 553–86. PMID 16153179.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Recker M, Pybus OG, Nee S, Gupta S (2007). "The generation of influenza outbreaks by a network of host immune responses against a limited set of antigenic types". Proc Natl Acad Sci U S A. 104 (18): 7711–7716. doi:10.1073/pnas.0702154104. PMID 17460037.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Capua, I (2006). "The challenge of avian influenza to the veterinary community". Avian Pathol. 35 (3): 189–205. PMID 16753610.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Holmes, E (2005). "Whole-genome analysis of human influenza A virus reveals multiple persistent lineages and reassortment among recent H3N2 viruses". PLoS Biol. 3 (9): e300. PMID 16026181.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP) CDC report (MMWR 2006 Jul 28;55(RR10):1–42) accessed 19 Oct 2006.
- ^ Questions & Answers: Flu Shot CDC publication updated Jul 24, 2006. Accessed 19 Oct 06.
- ^ a b Carrat F, Luong J, Lao H, Sallé A, Lajaunie C, Wackernagel H. "A 'small-world-like' model for comparing interventions aimed at preventing and controlling influenza pandemics". BMC Med. 4: 26. PMID 17059593.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mitamura K, Sugaya N (2006). "[Diagnosis and Treatment of influenza—clinical investigation on viral shedding in children with influenza]". Uirusu. 56 (1): 109–16. PMID 17038819.
- ^ Hota B (2004). "Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection?". Clin Infect Dis. 39 (8): 1182–9. doi:10.1086/424667. PMID 15486843.
- ^ a b McDonnell G, Russell A (1999). "Antiseptics and disinfectants: activity, action, and resistance". Clin Microbiol Rev. 12 (1): 147–79. PMID 9880479.
- ^ Hatchett RJ, Mecher CE, Lipsitch M (2007). "Public health interventions and epidemic intensity during the 1918 influenza pandemic". Proc Natl Acad Sci U S A. 104 (18): 7582–7587. doi:10.1073/pnas.0610941104. PMID 17416679.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bootsma MC, Ferguson NM (2007). "The effect of public health measures on the 1918 influenza pandemic in U.S. cities". Proc Natl Acad Sci U S A. 104 (18): 7588–7593. doi:10.1073/pnas.0611071104. PMID 17416677.
- ^ Glasgow, J (2001). "Reye syndrome — insights on causation and prognosis". Arch Dis Child. 85 (5): 351–3. PMID 11668090.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Centers for Disease Control and Prevention. CDC Recommends against the Use of Amantadine and Rimantadine for the Treatment or Prophylaxis of Influenza in the United States during the 2005–06 Influenza Season. January 14, 2006. Retrieved on 2007-01-01
- ^ Moscona, A (2005). "Neuraminidase inhibitors for influenza". N Engl J Med. 353 (13): 1363–73. PMID 16192481.
- ^ a b Stephenson, I (1999). "Chemotherapeutic control of influenza". J Antimicrob Chemother. 44 (1): 6–10. PMID 10459804.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Jefferson, T. "Neuraminidase inhibitors for preventing and treating influenza in healthy adults". Cochrane Database Syst Rev. 3: CD001265. doi:10.1002/14651858.CD001265.pub2. PMID 16855962.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Webster, Robert G. (2006). "H5N1 Influenza — Continuing Evolution and Spread". N Engl J Med. 355 (21): 2174–77. PMID 16192481.
- ^ "High levels of adamantane resistance among influenza A (H3N2) viruses and interim guidelines for use of antiviral agents — United States, 2005–06 influenza season". MMWR Morb Mortal Wkly Rep. 55 (2): 44–6. 2006. PMID 16424859.
- ^ Influenza A Virus Genome Project at The Institute of Genomic Research. Accessed 19 Oct 06
- ^ Subbarao K, Katz J. "Influenza vaccines generated by reverse genetics". Curr Top Microbiol Immunol. 283: 313–42. PMID 15298174.
- ^ Bardiya N, Bae J (2005). "Influenza vaccines: recent advances in production technologies". Appl Microbiol Biotechnol. 67 (3): 299–305. doi:10.1007/s00253-004-1874-1. PMID 15660212.
- ^ Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W, A universal influenza A vaccine based on the extracellular domain of the M2 protein, Nat Med. 1999 Oct;5(10):1157-63
- ^ Fiers W, Neirynck S, Deroo T, Saelens X, Jou WM, Soluble recombinant influenza vaccines, Philos Trans R Soc Lond B Biol Sci. 2001 Dec 29;356(1416):1961-3
- ^ Fiers W, De Filette M, Birkett A, Neirynck S, Min Jou W, A universal human influenza A vaccine, Virus Res. 2004 Jul;103(1-2):173-6
- ^ New York Times article ""Doubt Cast on Stockpile of a Vaccine for Bird Flu"" by Denise Grady. Published: March 30, 2006. Accessed 19 Oct 06
- ^ Gorman O, Bean W, Kawaoka Y, Webster R (1990). "Evolution of the nucleoprotein gene of influenza A virus". J Virol. 64 (4): 1487–97. PMID 2319644.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hinshaw V, Bean W, Webster R, Rehg J, Fiorelli P, Early G, Geraci J, St Aubin D (1984). "Are seals frequently infected with avian influenza viruses?". J Virol. 51 (3): 863–5. PMID 6471169.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Elbers A, Koch G, Bouma A (2005). "Performance of clinical signs in poultry for the detection of outbreaks during the avian influenza A (H7N7) epidemic in The Netherlands in 2003". Avian Pathol. 34 (3): 181–7. doi:10.1080/03079450500096497. PMID 16191700.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Capua I, Mutinelli F. "Low pathogenicity (LPAI) and highly pathogenic (HPAI) avian influenza in turkeys and chicken." In: Capua I, Mutinelli F. (eds.), A Colour Atlas and Text on Avian Influenza, Papi Editore, Bologna, 2001, pp. 13–20
- ^ Bano S, Naeem K, Malik S (2003). "Evaluation of pathogenic potential of avian influenza virus serotype H9N2 in chickens". Avian Dis. 47 (3 Suppl): 817–22. PMID 14575070.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Swayne D, Suarez D (2000). "Highly pathogenic avian influenza". Rev Sci Tech. 19 (2): 463–82. PMID 10935274.
- ^ Li K, Guan Y, Wang J, Smith G, Xu K, Duan L, Rahardjo A, Puthavathana P, Buranathai C, Nguyen T, Estoepangestie A, Chaisingh A, Auewarakul P, Long H, Hanh N, Webby R, Poon L, Chen H, Shortridge K, Yuen K, Webster R, Peiris J (2004). "Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia". Nature. 430 (6996): 209–13. doi:10.1038/nature02746. PMID 15241415.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Li KS, Guan Y, Wang J, Smith GJ, Xu KM, Duan L, Rahardjo AP, Puthavathana P, Buranathai C, Nguyen TD, Estoepangestie AT, Chaisingh A, Auewarakul P, Long HT, Hanh NT, Webby RJ, Poon LL, Chen H, Shortridge KF, Yuen KY, Webster RG, Peiris JS. "The Threat of Pandemic Influenza: Are We Ready?" Workshop Summary The National Academies Press (2005) "Today's Pandemic Threat: Genesis of a Highly Pathogenic and Potentially Pandemic H5N1 Influenza Virus in Eastern Asia", pages 116–130
- ^ Liu J (2006). "Avian influenza—a pandemic waiting to happen?" (PDF). J Microbiol Immunol Infect. 39 (1): 4–10. PMID 16440117.
- ^ a b Rosenthal, E. and Bradsher, K. Is Business Ready for a Flu Pandemic? The New York Times 16-03-2006 Accessed 17-04-2006
- ^ Statement from President George W. Bush on Influenza Accessed 26 Oct 2006
- ^ Brainerd, E. and M. Siegler (2003), “The Economic Effects of the 1918 Influenza Epidemic”, CEPR Discussion Paper, no. 3791.
- ^ Poland G (2006). "Vaccines against avian influenza—a race against time". N Engl J Med. 354 (13): 1411–3. doi:10.1056/NEJMe068047. PMID 16571885.
- ^ National Strategy for Pandemic Influenza Whitehouse.gov Accessed 26 Oct 2006.
- ^ Bush Outlines $7 Billion Pandemic Flu Preparedness Plan State.gov. Accessed 26 Oct 2006
- ^ Donor Nations Pledge $1.85 Billion to Combat Bird Flu Newswire Accessed 26 Oct 2006.
- ^ US AID. "Avian Influenza and its Global Implications" (PDF). Retrieved 2006-10-26.
- ^ Lye DC, Ang BS, Leo YS (2007). "Review of human infections with avian influenza H5N1 and proposed local clinical management guideline". Ann. Acad. Med. Singap. 36 (4): 285–92. PMID 17483860.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Poultry sector suffers despite absence of bird flu UN Office for the Coordination of Humanitarian Affairs. Accessed 26 Oct 2006
- ^ Nine poultry farmers commit suicide in flu-hit India Reuters. Published on April 12, 2006. Accessed 26 Oct 2006.
- ^ In the Nile Delta, Bird Flu Preys on Ignorance and Poverty The New York Times. Published on April 13, 2006. Accessed 26 Oct 2006.
Further reading
|
General
History
Microbiology
|
Pathogenesis
Epidemiology
Treatment and prevention
Research
|
External links
- Info on influenza at CDC
- Influenza (Mayo Clinic)
- Fact Sheet Overview of influenza at World Health Organization
- Health encyclopedia entry at NHS Direct
- BioHealthBase Bioinformatics Resource Center Database of influenza sequences and related information.
- Overview of influenza at MedicineNet
- Congressional Research Service (CRS) Reports regarding Influenza Law-related government reports at University of North Texas
- Influenza Surveillance and Contingency Plans (by country/region)
- Orthomyxoviridae The Universal Virus Database of the International Committee on Taxonomy of Viruses
- Influenza Virus Resource from the NCBI
- InfluenzaWorld Resource for all influenza-related information.
- European Influenza Surveillance Scheme
- Review of Influenza origin, virology, clinical syndromes and management from thenakedscientists.com at Cambridge University Pathology Dept.
