Robinson annulation
The Robinson annulation is an organic reaction used to create a six-member ring α,β-Unsaturated cyclic ketone, using a ketone (or aldehyde) and methyl vinyl ketone.[1][2][3] It is named after Sir Robert Robinson, the British chemist who discovered it while he was at the University of Oxford.
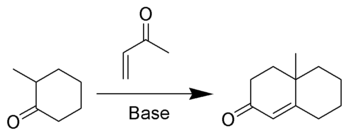
In addition to methyl vinyl ketone, 1-chloro-3-propanone[4][5] and isoxazoles[6] will give the same product.
The Wieland-Miescher ketone is the Robinson annulation product of 2-methyl-1,3-cyclohexanedione and methyl vinyl ketone while the Hajos-Parrish ketone is the product of 2-methyl-1,3-cyclopentanedione and methyl vinyl ketone. Asymmetric synthesis of these compounds has greatly increased their synthetic utility [7] [8].
Reaction mechanism
Methyl vinyl ketone (or variants thereof) are essential for the annulation as they are simultaneously a Michael acceptor and able to take part in an aldol condensation. The first step in the Robinson annulation (also spelt annelation) is a Michael addition followed by an aldol reaction as the annulation step in the process. The reaction then proceeds as an aldol condensation to make the desired cyclohexenone ring.
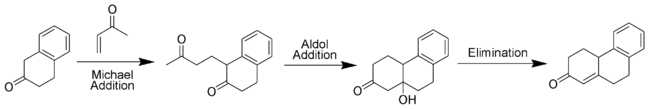
Variations
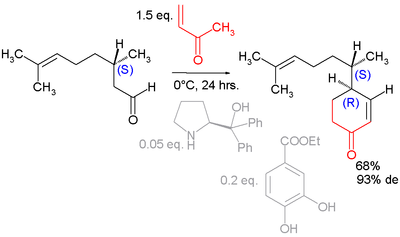
Asymmetric Robinson annulation
The organocatalyst Proline has been used to resolve the enantiomeric isomers of Robinson annulations in asymmetric synthesis. [9]. A proline derivative was employed in an asymmetric annulation of a geranial [10]:
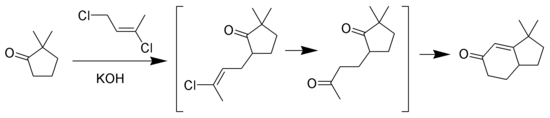
Wichterle reaction
The Wichterle reaction is a variant of the Robinson annulation that replaces methyl vinyl ketone with 1,3-dichloro-cis-2-butene. [11] [12] [13]
Hauser annulation
The reaction sequence in the related Hauser annulation is michael addition - Dieckman condensation - elimination [14]. The Hauser donor is an aromatic methylene sulfoxide or sulfone with a carboxylic ester group in the ortho position. The Hauser acceptor is also a Michael acceptor. In the original Hauser publication ethyl 2-carboxybenzyl phenyl sulfoxide reacts with 3-pentene-2-one with LDA as a base in THF at -78°C [15]:
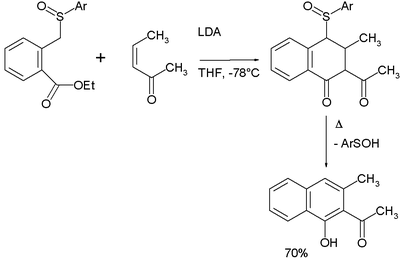
The original reaction product still contains the sulfoxide group but it is lost on heating in an elimination reaction. The ultimate reaction product is a napthalene derivative. The dual purpose of the sulfoxide group is as stabilizing group for the carbanion in the first reaction step and as leaving group in the second.
References
- ^ Rapson, W. S.; Robinson, R.; J. Chem. Soc. 1935, 1285.
- ^ Bergmann, E. D.; Gingberg, D.; Pappo, R. Org. React. 1959, 10, 179. (Review)
- ^ Gawley, R. E. Synthesis 1976, 777-794. (Review)
- ^ Heathcock, C. H.; Ellis, J. E.; McMurry, J. E.; Coppolino, A. Tetrahedron Lett. 1971, 12, 4995.
- ^ Heathcock, C. H.; Mahaim, C.; Schlecht, M. F.; Utawanit, T. J. Org. Chem. 1984, 49, 3264. (doi:10.1021/jo00192a004)
- ^ McMurry, J. E. Organic Syntheses, Coll. Vol. 6, p.781 (1988); Vol. 53, p.70 (1973). (Article)
- ^ . Hajos, Z, G.; Parrish, D. R. Asymmetric Synthesis of Optically Active Polycyclic Organic Compunds. German Patent DE 2102623, July 29, 1971.
- ^ Asymmetric synthesis of bicyclic intermediates of natural product chemistry Zoltan G. Hajos, David R. Parrish J. Org. Chem.; 1974; 39(12); 1615-1621. doi:10.1021/jo00925a003
- ^ Organic Syntheses, Coll. Vol. 7, p.368 (1990); Vol. 63, p.37 (1985). (Article)
- ^ Total Synthesis and Revised Structure of Biyouyanagin A K. C. Nicolaou, David Sarlah, and David M. Shaw Angew. Chem. Int. Ed. 2007, 46, 4708 –4711 doi:10.1002/anie.200701552
- ^ Wichterle, O. et al. Coll. Czech. Chem. Commun. 1948, 13, 300.
- ^ Kobayashi, M.; Matsumoto, T. Chem. Lett. 1973, 957.
- ^ Organic Syntheses, Coll. Vol. 5, p.869 (1973); Vol. 45, p.80 (1965). (Article)
- ^ Recent Advances in the Hauser Annulation Mal, D.; Pahari, P. Chem. Rev.; (Review); 2007; 107(5); 1892-1918. doi:10.1021/cr068398q
- ^ New synthetic methods for the regioselective annelation of aromatic rings: 1-hydroxy-2,3-disubstituted naphthalenes and 1,4-dihydroxy-2,3-disubstituted naphthalenesFrank M. Hauser and Richard P.RheeJ. Org. Chem.; 1978; 43(1) pp 178 - 180; doi:10.1021/jo00395a048
