Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
August 27
Springs and simple harmonic motion
Okay, here's the thing. Physicists model the motion of a block attached to a spring using a sinusiodal function, or simple harmonic motion. I'm fine with this, but why can't the motion of the block be worked out from Newtonian mechanics? I've tried to do it, and haven't gotten very far, but it should be feasible. Normally I wouldn't have a problem, but then my teacher showed that, if our guess of the motion of a spring was correct, the angular frequency ω must equal sqrt(k/m), and used this to show that the amplitude of the initial compression doesn't affect the the period. But isn't this bad physics? After all, it could be that our guess of the motion of the block on the spring was wrong. If I'm not explaining myself well, just let me know. Thanks! —Preceding unsigned comment added by 65.92.231.82 (talk) 00:55, 27 August 2008 (UTC)
- It is done through Newtonian mechanics - you set up the differential equation, which comes from Newton's Second Law of Motion. You can then make the substitution and substitute that in to get an equation of simple harmonic motion. Confusing Manifestation(Say hi!) 01:28, 27 August 2008 (UTC)
- Can you give more details. I'm trying it now, but the d^2 never disappears, and the cosines never apprears. —Preceding unsigned comment added by 65.92.231.82 (talk) 02:27, 27 August 2008 (UTC)
- The key equation of SHM is (which you'll have after the substitution suggested by ConMan - the a will be replaced by an appropriate constant of proportionality involving ks and ms), that is acceleration is proportional to displacement and in the opposite direction. You then simply observe that the sine (or cosine - which one you use depends on boundary conditions) function fits that equation, so you can use it as a solution. Just assume that (this is called an "ansatz"), substitute it in and solve for the constants, then you have your answer. --Tango (talk) 02:40, 27 August 2008 (UTC)
- Is this (using an ansatz) the only way of getting the equation? —Preceding unsigned comment added by 65.92.231.82 (talk) 02:55, 27 August 2008 (UTC)
- It's the only way I know of. It's certainly the easiest. --Tango (talk) 03:40, 27 August 2008 (UTC)
- SEPARATION....OF....VARIABLES. GAHHH. This is the VERY FIRST TECHNIQUE THEY TEACH YOU FOR SOLVING DIFFERENTIAL EQUATIONS. YOU LEARN IT DURING YOUR FIRST SEMESTER OF INTRODUCTORY CALCULUS. AND THEN YOU GO OFF AND GET ADVANCED DEGREES IN ENGINEERING OR APPLIED MATHEMATICS AND LEARN FOURIER TRANSFORMS AND LAPLACE TRANSFORMS AND NUMERIC SOLUTIONS AND FIFTY NINE VARIATIONS ON HOW TO SOLVE DIFFERENTIAL EQUATIONS ... AND YOU FORGET SEPARATION OF VARIABLES. Nimur (talk) 21:36, 27 August 2008 (UTC)
- Firstly, calm down! There is no need to shout. Secondly, it's been a while since I learned how to separate variables and I'm probably just being blind, but how do you separate a 2nd derivative? What am I missing (unless you mean to use ConMan's substitution first, in which there really wasn't a need to shout since he'd already said it)? --Tango (talk) 21:56, 27 August 2008 (UTC)
- SEPARATION....OF....VARIABLES. GAHHH. This is the VERY FIRST TECHNIQUE THEY TEACH YOU FOR SOLVING DIFFERENTIAL EQUATIONS. YOU LEARN IT DURING YOUR FIRST SEMESTER OF INTRODUCTORY CALCULUS. AND THEN YOU GO OFF AND GET ADVANCED DEGREES IN ENGINEERING OR APPLIED MATHEMATICS AND LEARN FOURIER TRANSFORMS AND LAPLACE TRANSFORMS AND NUMERIC SOLUTIONS AND FIFTY NINE VARIATIONS ON HOW TO SOLVE DIFFERENTIAL EQUATIONS ... AND YOU FORGET SEPARATION OF VARIABLES. Nimur (talk) 21:36, 27 August 2008 (UTC)
- It's possible to do using the substitution and a bunch of rearranging (and fiddling around with a plus-or-minus sign), but I agree the ansatz is much neater. More generally, you could use an exponential ansatz, as long as you're prepared to work with complex numbers (and know the relationship between the exponential and trigonometric functions). Confusing Manifestation(Say hi!) 05:10, 27 August 2008 (UTC)
- It's the only way I know of. It's certainly the easiest. --Tango (talk) 03:40, 27 August 2008 (UTC)
- Can you give more details. I'm trying it now, but the d^2 never disappears, and the cosines never apprears. —Preceding unsigned comment added by 65.92.231.82 (talk) 02:27, 27 August 2008 (UTC)
- There is a very good underlying question here. We know that or, more generally, gives a family of solutions to the equation of motion - but how do we know that this covers the whole solution space ? How do we know that there isn't some set of initial conditions that will result in an entirely different solution with very different behaviour ? Well, in this particular case we know that the differential equation is linear, which allows us to use the superposition principle to create a two dimensional family of solutions which we are sure covers the whole solution space. But if the equation of motion is non-linear (if, for example, we exceeded the spring's proportional limit) then it becomes much more difficult to know that we have a complete family of solutions (or even to find any exact solutions in the first place). This is why physicists and engineers will always attempt to linearise a problem if they possibly can.Gandalf61 (talk) 10:19, 27 August 2008 (UTC)
- Is it obvious (I know it's true) that linearity immediately implies a 2d solution space? I might be being unusually stupid, but I don't see it. Algebraist 22:04, 27 August 2008 (UTC)
- It's not obvious to me, but that's not saying a lot - I avoid differential equations when I can help it! --Tango (talk) 22:17, 27 August 2008 (UTC)
- So do I, and (since I also like a big hefty result) I would prove this with the Picard-Lindelöf theorem, but that isn't obvious and doesn't require linearity. Algebraist 22:20, 27 August 2008 (UTC)
- Obviously we only have a 2 dimensional solution space if we have a 2nd order linear d.e., not for all linear d.e.s. For the general case, if f(x) is a solution of a nth order linear d.e. then the nth derivative and above are expressible as a linear combination of f and its first n-1 derivatives. So if f is well-behaved enough to have a Taylor series expansion, doesn't it follow that the values of the first n coefficients in the Taylor series uniquely determine the rest of the coefficients, and hence uniquely determine f, and so we have an n dimensional solution space ? Gandalf61 (talk) 16:11, 28 August 2008 (UTC)
- So do I, and (since I also like a big hefty result) I would prove this with the Picard-Lindelöf theorem, but that isn't obvious and doesn't require linearity. Algebraist 22:20, 27 August 2008 (UTC)
- It's not obvious to me, but that's not saying a lot - I avoid differential equations when I can help it! --Tango (talk) 22:17, 27 August 2008 (UTC)
- Is it obvious (I know it's true) that linearity immediately implies a 2d solution space? I might be being unusually stupid, but I don't see it. Algebraist 22:04, 27 August 2008 (UTC)
- There is a very good underlying question here. We know that or, more generally, gives a family of solutions to the equation of motion - but how do we know that this covers the whole solution space ? How do we know that there isn't some set of initial conditions that will result in an entirely different solution with very different behaviour ? Well, in this particular case we know that the differential equation is linear, which allows us to use the superposition principle to create a two dimensional family of solutions which we are sure covers the whole solution space. But if the equation of motion is non-linear (if, for example, we exceeded the spring's proportional limit) then it becomes much more difficult to know that we have a complete family of solutions (or even to find any exact solutions in the first place). This is why physicists and engineers will always attempt to linearise a problem if they possibly can.Gandalf61 (talk) 10:19, 27 August 2008 (UTC)
- I think the sinusoidal representation is the simplest solution to understand. All motions can be broken down into sine and cosine waves by Fourier analysis any way. Its just that in a linear spring mass system, there are no harmonics above the fundamental. —Preceding unsigned comment added by LargoRhythm (talk • contribs) 21:44, 27 August 2008 (UTC)
Yawning
I was yawning earlier today, twice, while other people were yawning. It made me wonder, why is yawning contagious? —Preceding unsigned comment added by Earthan Philosopher (talk • contribs) 03:18, 27 August 2008 (UTC)
- See Yawn#Contagiousness, in short - no-one knows. Nanonic (talk) 03:24, 27 August 2008 (UTC)
- The cited article actually agrees with original research and common experience: yawning is highly contagious. Edison2 (talk) 03:57, 27 August 2008 (UTC)
- Yep - I'm pretty sure we know it's contageous. I believe there was a Science Friday piece on NPR that talked about this. What that show said was that yawning is nothing to directly do with tiredness - it's body-language that means something like "We all need to stop what we're doing and do something else now"...which could include going to bed. The reason it's contagious is because other people are subconsciously agreeing that there needs to be a change of group activity. SteveBaker (talk) 04:31, 27 August 2008 (UTC)
- Okay I wrote out a response but lost it since it was blocked. Anyway I'm rather surprised about the article, it mentions yawning in non-human animals but mostly seems to treat these as seperate issues. To me that doesn't make much sense, I see no reason to presume humans are special here, whatever reasons for yawning hold for us likely hold for at least some other animals particularly those close related to us [1]. There are quite a lot of theories of why animals yawn [2], one of them is to show off their teeth. This would also explain why yawning is contagious, if another animal is showing off it's teeth you'd want to as well. Incidentally I came across this [3] paper of a Biology 103 student "We cannot be certain that humans have evolved from monkeys" do people really right that sort of thing in Biology papers? Nil Einne (talk) 08:03, 27 August 2008 (UTC)
- I was under the impression that we can be certain that humans aren't descended from monkeys, but humans and monkeys are both descended from some proto-simian. Algebraist 10:45, 27 August 2008 (UTC)
- I don't know much about the details of yawning, but the picture in that article could make a great lolcat. the wub "?!" 12:09, 27 August 2008 (UTC)
- (Humans are a member of the Ape family - apes are not monkeys) Well, we aren't descended from any living species of ape. All apes are descended from a common antecedent - but that antecedent must in turn have been an animal that you would describe as an ape. That in turn must have descended from some kind of a simian (a "monkey") - but, again, probably not any living species. So the correct statement is: "Humans are descended from an animal that would be classified as an ape. Humans are not descended from any living species of ape." - I don't quite know why people fixate on "monkey" - the closest classification is "ape" and there are plenty of earlier species we descended via - so we're also descended from small furry mammals, fish-like things...bacteria. SteveBaker (talk) 13:38, 27 August 2008 (UTC)
- I think the problem is most people don't understand that apes are usually not considered monkeys, particularly historically when the monkey thing first arose nor do they understand that monkeys (and apes) nowadays are not in fact the same as the ancesteral 'monkeys' (and apes) we all evolved from we so from their POV we are descended from historic modern day monkeys and we get silly questions like why did monkeys stop evolving (what?). (Of course it gets worse when they think humans are the end all of evolution and so ask why monkeys don't evolve into humans but that's another issue.) I'm still rather surprised the student thought it wise to demonstrate that ignorance in a biology paper but anyway... Nil Einne (talk) 20:04, 27 August 2008 (UTC)
- (Humans are a member of the Ape family - apes are not monkeys) Well, we aren't descended from any living species of ape. All apes are descended from a common antecedent - but that antecedent must in turn have been an animal that you would describe as an ape. That in turn must have descended from some kind of a simian (a "monkey") - but, again, probably not any living species. So the correct statement is: "Humans are descended from an animal that would be classified as an ape. Humans are not descended from any living species of ape." - I don't quite know why people fixate on "monkey" - the closest classification is "ape" and there are plenty of earlier species we descended via - so we're also descended from small furry mammals, fish-like things...bacteria. SteveBaker (talk) 13:38, 27 August 2008 (UTC)
- Okay I wrote out a response but lost it since it was blocked. Anyway I'm rather surprised about the article, it mentions yawning in non-human animals but mostly seems to treat these as seperate issues. To me that doesn't make much sense, I see no reason to presume humans are special here, whatever reasons for yawning hold for us likely hold for at least some other animals particularly those close related to us [1]. There are quite a lot of theories of why animals yawn [2], one of them is to show off their teeth. This would also explain why yawning is contagious, if another animal is showing off it's teeth you'd want to as well. Incidentally I came across this [3] paper of a Biology 103 student "We cannot be certain that humans have evolved from monkeys" do people really right that sort of thing in Biology papers? Nil Einne (talk) 08:03, 27 August 2008 (UTC)
- Yep - I'm pretty sure we know it's contageous. I believe there was a Science Friday piece on NPR that talked about this. What that show said was that yawning is nothing to directly do with tiredness - it's body-language that means something like "We all need to stop what we're doing and do something else now"...which could include going to bed. The reason it's contagious is because other people are subconsciously agreeing that there needs to be a change of group activity. SteveBaker (talk) 04:31, 27 August 2008 (UTC)
- The cited article actually agrees with original research and common experience: yawning is highly contagious. Edison2 (talk) 03:57, 27 August 2008 (UTC)
I was told that we yawn because our brain lacksz oxygen. Philosophia X Known(Philosophia X Known) 15:06, 27 August 2008 (UTC)--Earthan Philosopher
- No - that would simply cause a slightly increased rate of respiration. Lack of oxygen in the brain is, however the cause of ridiculously large fonts in signatures. SteveBaker (talk) 03:54, 28 August 2008 (UTC)
- Its psychological. Just take a look at this word.
- Y-A-W-N and again Y-A-W-N and again Y-A-W-N
- Now dont you feel like yawning? (I do --- and did it) —Preceding unsigned comment added by 79.76.200.98 (talk) 01:54, 29 August 2008 (UTC)
man without a face
hi, on channel 7 last night there was a story about a man (or kid?) with a strange growth on his face. Does anyone know if we have an article on this, and what is the condition called? 203.35.135.133 (talk) 07:38, 27 August 2008 (UTC)
- I didn't see that report - but some possibilities are Proteus syndrome, von Recklinghausen's disease, Elephantiasis, Neurofibromatosis. SteveBaker (talk) 13:29, 27 August 2008 (UTC)
- The program might have been aboutJoseph Merrick.
- Wanderer57 (talk) 13:33, 27 August 2008 (UTC)
- (That's what I was thinking - and that's where I got the list of diseases from!) SteveBaker (talk) 14:58, 27 August 2008 (UTC)
- Wanderer57 (talk) 13:33, 27 August 2008 (UTC)
- It might also be McCune-Albright syndrome or polyostotic fibrous dysplasia as shown on television recently in some countries with the case of Marlie Casseus, a young Haitian girl who had an 18 pound "growth" removed from her face. Nanonic (talk) 15:06, 27 August 2008 (UTC)
- And strangely enough, being Wikipedia, we do have a fair few articles on people known for little more than having an odd tumour. Jose Mestre was featured on a Discovery Channel program called The Man with No Face in which he underwent surgery to have an enormous abnormal growth removed from his face.[4] Nanonic (talk) 15:12, 27 August 2008 (UTC)
- Which was on the TV series My Shocking Story which is being shown on the Seven Network in Australia, so that's probably the person the original poster was talking about. Also see Mark Tatum who had to have his face effectively removed because of mucormycosis. Graham87 16:00, 27 August 2008 (UTC)
Cat Eyes
Do cats have a second set of eyelids under their regular ones? A little while ago I was petting my cat, and his eyes opened a bit, and there was skin covering them, which opened when I woke him up. Black Carrot (talk) 08:05, 27 August 2008 (UTC)
- Sounds like the nictitating membrane. Algebraist 08:20, 27 August 2008 (UTC)
- It's worth noting that if you can still see the membrane on your cat, you should take it to see a veterinarian. It's usually a sign of illness in cats. —Cyclonenim (talk · contribs) 11:29, 27 August 2008 (UTC)
- Agree, this sounds like an unusual appearance and you should consult a veterinarian. Otolemur crassicaudatus (talk) 15:58, 27 August 2008 (UTC)
- It's worth noting that if you can still see the membrane on your cat, you should take it to see a veterinarian. It's usually a sign of illness in cats. —Cyclonenim (talk · contribs) 11:29, 27 August 2008 (UTC)
- I think that's a bit hasty. It only happened once, and it fits the conditions mentioned in the article - he was asleep, and his eyelids slid open a bit. Thanks for the reference. Black Carrot (talk) 03:27, 28 August 2008 (UTC)
Can tapirs pick things up with their nose?
I asked this on the miscellaneous reference desk a while ago but no-one really knew - basically, can they use their nose anything like how an elephant uses his trunk? Can they pick up food and pop it into their mouths? Can they hold objects with their noses? Bradley10 (talk) 09:24, 27 August 2008 (UTC)
- This National Geographic video shows them using their noses at least to assist in getting food into their mouths. Fribbler (talk) 11:35, 27 August 2008 (UTC)
Jurassic Park question
Is what they did in Jurassic Park possible if we did have any dinosaur blood? I know that the mosquito/amber technique wouldn't actually work, but could you, say, clone a dodo from the taxidermy dodos they have in Tring? Or could they make some new Yahzee River Dolphins? Bradley10 (talk) 09:27, 27 August 2008 (UTC)
- DNA doesn't last forever - I don't think the mosquito/amber trick does work. While the shape and tissues of the mosquito are still there - the actual chemical composition of the DNA in the blood in it's stomach is completely wrecked. This is acknowledged in Jurassic Park too - they claim that they had to carefully reassemble the DNA - and fill in the missing bits with Frog DNA (this actually turns out to be the reason for the downfall of the park - most people miss that point in all of the action!)
- A Dodo is much more do-able - the last viable DNA is only 350 or so years old, so perhaps it could be made to work. The Yangtze River dolphin is even easier because the DNA may still actually exist in the last few animals.
- But using this technique to bring a species back is problematic. To get a viable breeding colony requires a sufficiently diverse gene pool. It's not enough to have one male and one female because all of their offspring would share genes from the same set of two archetypes - and the resulting in-breeding would soon produce all sorts of major health problems. Opinions vary on the minumum number of individuals needed - I heard the number 50 at one time - but a lot depends on how genetically diverse those 50 individuals are - and on how closely scientists could control their subsequent breeding to maximize diversity over the succeeding generations. We probably don't have DNA from 50 Dodo's - and we might not have it from 50 River Dolphins either.
- With Jurassic Park, there are even bigger problems - the plants that the herbivores ate are probably all extinct - or perhaps have evolved chemical defenses that the dinosaurs would not be able to counteract (this too comes up in the movie - but very briefly). The idea that the T-Rex can survive on a diet of goat and lawyer is ridiculous - they'd need contemporary animals, which in a real Jurassic Park would be worth vastly too much money to feed them to carnivores. There might also be issues with them surviving modern diseases - but it's also possible that their great antiquity would mean that modern diseases were not able to infect them. It's hard to say. Worst of all - the amount of oxygen in our atmosphere is much less than it was in Jurassic times - the odds are good that these gigantic creatures wouldn't have sufficient lung capacity to survive at all in the modern world.
- Another issue (which is carefully skirted in Jurassic Park - but which would affect the Dodo and River Dolphin too) is that having the DNA isn't enough. You also need to turn that DNA into a fertilised egg cell and implant it into the womb of a suitable donor species. In the case of a Yangtze River dolphin - you might be able to implant the egg into a Ganges River dolphin (or one of the other handful of river dolphin species) - but those are also under pressure. You probably can't use one of the more common oceanic dolphins as a surrogate because they live in salt water and river dolphins don't. Same problem with the Dodo - can you find a sufficiently close surrogate mother bird who can lay an egg containing the right nutrients, of sufficient size to contain the chick of a 50 pound bird? The nearest living relatives of the Dodo are pigeons...so no hope there. Perhaps some other large, flightless bird would be able to form a large enough egg - but there is no guarantee that it would have the right shell thickness with the right amount of oxygen transport and the right nutritional stuff.
- With dinosaurs, you're in deep problems there. There are no relatives of the dinosaurs left to act as surrogates. This is quietly swept under the carpet in Jurassic Park.
- There are some strong candidates for "bringing back" - I think the Mammoth is the most likely. Modern elephants are probably "close enough" to birth a mammoth calf - and we have freeze-dried meat with (perhaps) viable DNA from a significant number of individuals to make a breeding herd. Mammoths lived in sufficiently recent history that diet and such like wouldn't be a major issue.
- It's an interesting matter - I think we'll see some of this kind of thing happening...but almost certainly not with Dinoasaurs.
- This is intriguing. Thank you.
- I'm curious about your point that a T. rex could not survive on a delicious diet of modern goats and lawyers. Based on no knowledge of the matter at all, my impression was that an ancient carnivore such as a dinosaur could live on modern reptiles or mammals. What dietary requirements might be missing? Presumably the fat and protein in modern animals would provide the necessary energy supply and amino acids for growth (or am I wrong on that point as well?) Wanderer57 (talk) 13:59, 27 August 2008 (UTC)
- No doubt the proteins and amino acids are there - but who knows what else too? Perhaps there are missing 'trace elements'? Look at modern animals: A lion can't live if fed only dog food (believe it or not - I know someone who tried that!)...and lions and dogs those are both modern carnivores. Chocolate is poisonous to dogs. Who knows what wierd stuff a dinosaur needs in it's diet? Who knows what stuff in modern foods would be poisonous to it?
- Let's do a thought experiment. Consider even a human - cloned into an alien society a few million years in the future when humans and all of the apes and monkeys are long-extinct and giant super-intelligent cockroaches are now running the place. They find a mosquito trapped in amber with human blood in it's gut...they do the Jurassic Park thing and make a baby...so what happens?
- The baby is brought into the world from an artificial placenta in an utterly sterile environment to avoid infection. They roach scientists have carefully researched all of the foods humans ate and they've even cloned tomatoes, corn, cows, etc. They somehow reproduce all of the nutrients present in human milk and make sure the baby gets all of that good stuff. But the scientists rapidly discover that the kid's blood won't clot. They have no clue why. They've double-checked the diet - cloned all the right foods - but eventually it dies. Well, maybe there was a cloning problem - maybe the DNA they got was from a haemophiliac. But no. They try this a dozen times with a dozen different clones from different DNA sources - always the exact same problem. They are totally at a loss.
- What happened? Well, they cloned the human - but they didn't clone any "gut flora" - the 300 different species of bacteria that are specialised to live in the intestines of humans. A baby naturally gets those from it's mother - through all sorts of mechanisms. But a clone grown in a sterile lab a million years from now wouldn't pick them up. Our gut flora provide us with vitamin K2 - which is needed for blood clotting. No gut flora (or the wrong kind of gut flora or something that's poisoning the gut flora) - no K2 - the clone dies.
- How would those future alien scientists figure that out with no living humans or human-descendants to study? Even if they do figure it out - perhaps no suitable bacteria exist in their future world that could substitute for the ones we get naturally. If there are 300 species of them living in our gut - then they would need an ungodly amount of research to figure out which ones they need to clone (and where the heck they'd get the DNA from is anyone's guess) in order to "fix" the poor clone's vitamin K2 deficiency.
- We have no clue what subtle things like that we'd have to provide to a baby dinosaur...it's very likely we'd miss a whole bunch of things - even if we DID clone all of the right food plants and such. The problems of raising a creature from the Jurassic would be overwhelming...not just that some species of berry in the park happens to be poisonous - as chocolate is to a dog.
- Also, what would happen if the aliens used DNA from one of the uneaten lawyers? They'd get their butts sued off for negligent cloning over the blood clotting issue. Litigious Park! Franamax (talk) 18:21, 27 August 2008 (UTC)
- The only (partly) serious attempt I know of to clone an extinct animal is that of the Thylacine which survived until the 1930s. You may want to read the article for some of the challenges faced, and this is with an animal relatively recently extinct Nil Einne (talk) 19:45, 27 August 2008 (UTC)
- P.S. Just remembered after reading SB's post there has ben some talking regarding the Woolly mammoth too and this is mentioned in the article although I don't think there is a specific effort yet Nil Einne (talk) 19:51, 27 August 2008 (UTC)
Is there any animal which lacks self-preservation instinct?
So that's the question: does any animal which lacks survival instinct, and survives just "by chance"? Maybe sea sponges? Leptictidium (mt) 11:31, 27 August 2008 (UTC)
- Sea sponges don't have a nervous system - they certainly don't have a brain - so they lack pretty much ALL instinct. So you've certainly answered your own question there. In general, there are lots of animals that don't have brains - or whose brains are far to primitive - so we could probably form a long list that would meet your criterion. But you're probably thinking of higher animals - so let's look in that direction a bit. I'm not sure what you mean by "survival instinct". Survival isn't a single ability - it's a HUGE range of abilities - and "instinct" implies something we're born with - not learned behavior. Humans lack the instinctive ability to eat the right things and exercise properly - that certainly affects our ability to survive in SOME circumstances - yet even newborn babies have the instinct to hold on tightly to your fingers if you try to lift them up by their hands. So to find an animal that lacks some survival instincts is easy - probably all animals have some gaps - but to find one that has no instincts for surviving any set of circumstances probably does require finding one with almost no brain at all. SteveBaker (talk) 12:49, 27 August 2008 (UTC)
- Thank you. Leptictidium (mt) 12:52, 27 August 2008 (UTC)
- Your body doesn't crave certain food when it is missing essential nutrients? I most certainly do have instincts for nutrition and exercise. It is only through years of practice that we manage to suppress those instincts. Plasticup T/C 13:52, 27 August 2008 (UTC)
- Thank you. Leptictidium (mt) 12:52, 27 August 2008 (UTC)
- The problem is that we evolved in a time when certain high-energy foods (animal fats for example) were in exceedingly short supply. We have the "instincts" to eat large quantities of those foods whenever they are available - but they were so rare and hard to obtain without taking a lot of exercise - that it was impossible to overeat on a routine basis. Therefore we never evolved a mechanism to stop us from eating them when we start to get fat. In a world where you needed to put on a few pounds in the summer in order to survive a long, hard winter - having an instinct to NOT stuff our faces with egg, bacon and cheese McMuffins would have been pretty fatal - so it would be evolved away in no time flat. Worse still - it's pretty clear that we've evolved to crave foods that have high nutritional content - so we not only lack an instinct NOT to over eat - but we also HAVE an instinct to shovel down as much fatty food as we can find! Now we know (intellectually) how bad this instinct is - we can use our big-brain intellects to fight against it...but (for some people at least) it's tough.
- It's a similar deal with exercise - we didn't need to evolve a mechanism for craving "pointless" exercise because we got plenty of exercise doing our daily food hunting and the issue of "Death by under-exercise" simply never needed a "survival instinct". Now we no longer do that by necessity - we pretty much lack any instinctive desire to leap on a treadmill a couple of times a week. SteveBaker (talk) 14:13, 27 August 2008 (UTC)
Glaucoma / abd pain
While reading Cope's "Early Diagnosis of the Acute Abdomen", a trusted reference on the surgical abdomen, I came across the following statement: ...the observer must remember that certain extra-abdominal conditions may cause deceptive pain within the abdomen; thus...even acute glaucoma may temporarily mislead the practitioner. Can anyone explain how glaucoma could possible be misinterpreted as an acute abdomen? Tuckerekcut (talk) 16:38, 27 August 2008 (UTC)
- The short answer is referred pain—conditions which cause pain in one of the body to present as pain elsewhere. The mechanism of pain referral is not well-characterized, but our article addresses some possibilities. This article notes that referred pain in the abdomen can be caused by acute angle-closre glaucoma, and is symptomatic of a loss of blood supply to the eyeball. TenOfAllTrades(talk) 17:48, 27 August 2008 (UTC)
- For anyone else interested in a more complete answer (though your short answer is appreciated Ten), this [5] has a well thought out mechanism. In short, Noxious stimulation of trigeminal nerve afferents activates the paratrigeminal nuclei in the medulla with secondary stimulation of the vagus nerve. Tuckerekcut (talk) 18:23, 27 August 2008 (UTC)
- Wow, and I thought computer-speak was nonsensical =P --mboverload@ 04:36, 28 August 2008 (UTC)
What happens when blood goes into tissue?
![]() Done
Done
I know, I know, I know - no medical advice!!! So use some discretion here - but I can't seem to find anything on google..
A moment after I had some blood taken for tests, I noticed a large bulge near the site. Apparently the blood had (at some point) flown into tissue.
Any information I can have on this? Does blood typically get absorbed? How does it get absorbed?
Thanks Rfwoolf (talk) 18:18, 27 August 2008 (UTC)
- Why ask if you know we can't answer? It could be a bruise, or it could be something entirely different. If you are seriously concerned about it, please seek the advice of a physician. --Russoc4 (talk) 18:25, 27 August 2008 (UTC)
- On the contrary you can answer general medical questions so long as it isn't tantamount to medical advice. My question is: What happens when blood is injected (leaked) into tissue? Does it get absorbed? In what way would it get absorbed? Rfwoolf (talk) 18:29, 27 August 2008 (UTC)
- The lesion you describe is known as a hematoma. The entrapped blood will slowly degrade by several mechanisms. Some blood cells will be broken down by natural and mechanically accelerated hemolysis. Macrophages and similar cells may consume and degrade some constituents. Some white blood cells are capable of migrating back to the intravascular space. The plasma will likely diffuse through the tissue and migrate in and out of spaces based on osmotic forces. Some cellular debris may stay in the hematoma site for a long time. Tuckerekcut (talk) 18:35, 27 August 2008 (UTC)
- Thank-you! Hopefully this question will be indexed by google and your answer will be helpful to others will similar questions. Rfwoolf (talk) 18:48, 27 August 2008 (UTC)
Evolution and the "no new information" argument
I really just don't understand this one. What do creationists even mean when they say "no new information is ever created through mutations/evolution"? Do they mean more genetic code isn't being added? (gene duplication) Or are they ignoring the fact that ACC and CAC, while the same base amino acids, code for completely different proteins? -- MacAddct 1984 (talk • contribs) 19:09, 27 August 2008 (UTC)
- I've never heard this one before (I don't spend much time listening to creationists), so can you give us a link to somewhere the argument is presented? Algebraist 19:13, 27 August 2008 (UTC)
- I'm currently at work and sites such as Answers in Genesis [6] and Dr. Dino [7] are blocked because of its content "religion" (har har). Talk.origins has a brief description, but it's not very informative. I'll provide more links later, if no one expands upon it. -- MacAddct 1984 (talk • contribs) 19:26, 27 August 2008 (UTC)
- My understanding is most creationists claim mutations are always deleterious and/or there is no evidence/it is impossible to form new, more complicated, structures from mutation. Nil Einne (talk) 19:32, 27 August 2008 (UTC)
- See [8] for example (does [9]) work? A mutation, being a random change in highly specified information contained in the nucleic acid base sequence, could almost never do anything but scramble the information; that is, reduce the information. Now sometimes such a loss of information results in a new trait—for example, purple or red flowers where there were only blue ones before (yes I know how dumb that is and how they're missing duplications etc but that's not the point) Nil Einne (talk) 19:36, 27 August 2008 (UTC)
- Thanks Nil and Macaddct. Algebraist 19:39, 27 August 2008 (UTC)
- Yeah, that's exactly the kind of argument that I often seen getting used. (Thanks for the cached link). -- MacAddct 1984 (talk • contribs) 19:59, 27 August 2008 (UTC)
- (edit conflict x5) Sounds like a misunderstanding/misapplication of the Second law of thermodynamics. Although entropy increases overall in a universe sense, biological/living systems can organize/reorganize. --mboverload@ 19:38, 27 August 2008 (UTC)
- Indeed you may also want to see Specified complexity for further understanding of their flawed thinking Nil Einne (talk) 19:40, 27 August 2008 (UTC)
- [EC]The attempted argument is a bastardization of information theory. Objections to evolution#Evolution cannot create information discusses it, but here's the gist: in order to justify a necessisary "intelligent designer", some folks have decided to make the nonscientific (and clearly false claim) that only a intelligent agent can add information into a system because natural systems can, allegedly, only lose information. This natural-systems-can-only-lose-information paradigm stems from a mischaracterization of thermodynamics and incorrect assumptions about the nature of genetic mutation. The Specified complexity arguments of William Dembski are commonly cited (outlined in his "Law of conservation of information"). — Scientizzle 19:47, 27 August 2008 (UTC)
- Oh okay, that's pretty much what I suspected. Thanks for the links. (I was trying to find it listed in criticisms of evolution article maybe I'll redirect it) As always, just blatant and willful ignorance of facts. -- MacAddct 1984 (talk • contribs) 19:59, 27 August 2008 (UTC)
- Various experiments have shown to everyone's satisfaction that species do change over time, so the Creationists have been backed into saying those changes are going "downhill" by losing genetic information:
The rare ‘beneficial’ mutations to which evolutionists cling all appear to be like wingless animals, blind cave animals, and many examples of antibiotic resistance. They are downhill changes, losses of information which, though they may give a survival advantage, are headed in precisely the wrong direction for evolution. (source)
- This rebuttal to the recent successes of the critters in the E. coli long-term evolution experiment concludes with "it is only possible to obtain truth about the past if we start with the only source of absolute truth in the present—the inerrant Word of God". So there you are. --Sean 19:55, 27 August 2008 (UTC)
- So they aren't trying to convince him to hand over his "data" then unlike some other creationists? Nil Einne (talk) 20:07, 27 August 2008 (UTC)
A side note (and hopefully not to stray too far off-topic), Arguments creationists should not use is a beautiful example of back-peddling and apologetics. -- MacAddct 1984 (talk • contribs) 19:59, 27 August 2008 (UTC)
- Oh, I don't know. It's actually much more honest than most Creationist groups in the respect that they recognize that some arguments which are incredibly common that Creationists have been using forever are wrong to the point of making the Creationists look stupid or dishonest (e.g. the Darwin eye misquote, which is always a useful way to spot totally ignorant Creationists). --98.217.8.46 (talk) 21:26, 27 August 2008 (UTC)
Creationists either don't understand - or refuse to acknowledge - the most basic science on this. The things they say are (without exception) ridiculously easy to demolish - but the people they are aiming these bogus arguments at are desperate to believe whatever crap is hurled in order that they can continue to cling to horribly outmoded beliefs. So there is guaranteed to be a breakdown in communication.
Here is the truth:
Evolutionary change comes about through at least THREE mechanisms:
- Sexual reproduction - where two sets of similar (but not identical) genes are shuffled together.
- Viral gene insertion where some virus comes along and inserts its DNA into that of the host species.
- Mutation - where either a transcription error in the DNA or a zap from radiation or some nasty chemical agent in the environment (a carcinogen, for example) causes the insertion, deletion or corruption of a part of the genetic code - resulting in a brand new gene popping up.
Mutation is a genuine source of completely new genes - they come from absolutely nowhere in the environment - it's a truly random change.
The other two mechanisms are indeed nothing more than a reshuffling of existing genes. But even that can result in totally new "features" in the resulting organism because the consequences of two old, well-established proteins meeting in the same animal for the first time in history can easily result in some bizarre - but useful - side-effect.
So - sure, new 'features' can arise that have never been seen before - there is no problem with some limited pool of information being the only source of genetic material...that's obviously not true. Creationists who claim this are simply sticking their fingers in their ears and singing "La, la-la, la-laaaa. I can't hear you!" because they've been told this plenty of times and clearly enough.
The reason the entropy argument is bogus is because entropy only increases in closed systems - and it's a statistical increase - not an absolute cast-iron increase. So within a group of animals stuck in a "closed system" (on an island or something), the arrival of new mutations results in a very few, rare improvements that survive into the future and a much larger number of disasterously 'damaged' creatures that don't survive long enough to pass on their genes to the next generation. The total entropy of the island does exactly what the laws of physics predict - it increases.
Life on earth is evolving and improving - but at the cost of increasing the chaos on the planet at a much higher rate than would occur if there was no life (or life without evolution).
We can simulate this in a computer relatively easily. When we do this, we can actually measure the entropy of the system and watch it increase as predicted by physics while the synthetic "animals" befome more and more well-suited to their artificial environment.
SteveBaker (talk) 03:40, 28 August 2008 (UTC)
- I have seen the computer models before but it was awhile ago. Is this mostly university stuff or are there public resources on it? It would be most excellent if I could get a simplified version to just watch =P --mboverload@ 04:33, 28 August 2008 (UTC)
- Artificial life and Evolutionary algorithm are the articles you need. I'd be surprised if there were no decent public-domain evolutionary demonstrations. However, it would be rash to assume there was anything to watch - firstly, even computer-based evolution can take a LONG time to develop anything interesting - and secondly, the systems involved are not necessarily graphical things you can watch. However, there are a few programs out there that run over a few days and produce brief graphical output - I have no idea whether any are in the public domain.
- Some of these are pretty amusing - and they are rarely what you either want or expect. One guy built a system where each creature was made up of a set of cuboids hinged together with simulated "muscles" operated by an elementary neural network "brain". The size and shape of the cuboids, the positioning of the muscles and the connections within the brain were all determined by a set of "genes" that could be passed on from one critter to another with cross-breeding and mutation for variation. His first attempt was to try to evolve something that could walk - so he'd set up a bunch of randomly generated synthetic creatures and he'd have his software watch them move. Initially, some of them just 'twitched' - that nudged them SLOWLY in some direction. The one that moved furthest over some period of simulated time would go on to provide the genes that would be mutated to form the next generation. He ran the program for days and found from the readout that the winning creature could move amazingly fast - much faster than he expected. Viewing the "design" of the winning creature revealed that it had just one cuboid - no muscles and no brain whatever. It was very tall and very narrow and beat all the other creatures merely by falling over! Its center of gravity moved by half its height in just a few simulated seconds - so it met the criteria of moving the furthest in the allowed time - and it simply evolved to get taller and taller and narrower and narrower. Later, he fixed that problem by changing the fitness criteria - and found that his creatures would evolve to exploit bugs in his code - he fixed bugs and then found that many of his creatures merely consisted of two blocks, one muscle and a very simple brain. They simply vibrated at high frequencies and mysteriously slid across the ground - it turned out that they had evolved to exploit matematical roundoff error as a source of energy.
- You might consider these as 'failures' - but in truth they are elegant demonstrations that creatures (even synthetic ones) will evolve to fit perfectly into their environment. If their environment inadvertently provides 'free energy' from roundoff errors - then for them, that's as important as sunlight is to plants out here in the real world. Making synthetic creatures evolve to look and behave like real creatures turns out to be virtually impossible. SteveBaker (talk) 06:08, 28 August 2008 (UTC)
- Thanks Steve! I guess the "models" I saw were just an "envisioning" of possible evolution =( --mboverload@ 06:11, 28 August 2008 (UTC)
- See List of digital organism simulators
- Also : "it turned out that they had evolved to exploit mathematical roundoff error as a source of energy." It would be a great line in a science fiction short story. APL (talk) 16:20, 28 August 2008 (UTC)
- It would have made a much better (albeit much geekier) ending to The Matrix. SteveBaker (talk) 19:50, 28 August 2008 (UTC)
- YouTube user cdk007 has some excellent videos of computer programs he wrote that show how mutations and natural selection take place. -- MacAddct 1984 (talk • contribs) 14:02, 28 August 2008 (UTC)
The evolution of intelligence
It seems odd to me that at least three, probably four, groups of animals have developed highly complex and intelligent brains independently (corvids, parrots, cetaceans, and primates), during the Cenozoic era, while no known fossil from the Mesozoic has an encephalization quotient (EQ) much above that of an ostrich (the highest are troodontids). (I say "possibly four" because there were parrots in the Cretaceous, but I'm not sure about their intelligence.)
Is there any speculation as to why this is? (Is the Mesozoic fossil record poor enough that we could have missed an entire taxon as significant as, say, parrots or corvids?) —Preceding unsigned comment added by Vultur (talk • contribs) 23:33, 27 August 2008 (UTC)
- One of the principles of evolution, articulated by Stephen Jay Gould among others, is that in any sufficiently large evolving population even a purely random exploration of all possible niches will lead to increasing diversity as one moves away from ancestral forms and tries new paths of life. Applied to the EQ in particular, this implies that since animals started with no brains (0 EQ), then even randomly directly evolution will lead to an expanding diversity of EQs. As a result, both the average and top performing EQ across any taxonomic group is likely to improve over time. If you look at the work of Jerison, for example, he generally concludes that the average EQ of birds and mammals has been increasing essentially since the inception of these groups, but that this random exploration of the "intelligence" space proceeds very slowly (perhaps max EQ doubles every 25 million years or so). If the average intelligence of birds and mammals has been improving gradually for a very long time, it is not too surprising that the intelligence of the brightest birds and mammals is also at or near a historical maximum today.
- There is also a view that reptiles and fish, which still have low EQ even today, may be unable to evolve a high EQ due to the metabolic requirements of a large brain. In other words, high intelligence may be only possible in warm blooded organisms. However, if you believe that at least some dinosaurs were warm-bloodied then that still doesn't explain why 180 million years wasn't long enough for dinosaurs to develop interstellar travel. So ultimately I dont have a good answer for you. Dragons flight (talk) 07:03, 28 August 2008 (UTC)
- I don't mean to contend with Gould here but that sounds awfully teleological. --98.217.8.46 (talk) 14:33, 28 August 2008 (UTC)
- Teleology would assume there is a plan or purpose beyond the evolution. Gould's point, somewhat oversimplified, is that given enough time random evolution will try nearly all possible stratagies for survival. Hence starting at any fixed taxa, the diversity of approachs is likely to increase across the range of species that are its descendants. That some descendants happen to become brainy requires only a sufficiently large pool of randomly evolving species and an assumption that increasing brain power is both physically possible and not so deleterious as to cause a species extinction. Dragons flight (talk) 18:49, 28 August 2008 (UTC)
- A useful analogy is a simple random walk on the Euclidean plane. The expected position of the random walker (i.e. (expected x, expected y)) remains at the starting point no matter how long the walk has been going on, but the expected distance from the starting point increases with time (I think it goes as the square root of the number of steps). The reason is that x and y coordinates are equally likely to go up or down, but distance starts out at zero and can only go up. Evolution is also a kind of random walk, and any metric which starts at zero and can be positive and can't be negative will increase with time for the same reason. It won't increase forever—since there are also downward pressures which have no analogue in the simple random walk—but it will increase for a while from zero to some stable nonzero average. -- BenRG (talk) 19:33, 28 August 2008 (UTC)
- Thank you. Perhaps the question was a bit poorly worded; I get that intelligence tends to increase over time [though I'm not sure it's a pure random walk; has any clade ever *reduced* its brain/body ratio over time? I'm sure one has, but I can't think of any.] I was wondering because we've only had 65 million years since the last big "reset" mass extinction, and the dinosaurs/pterosaurs/freaky mesozoic weirdnesses had much more. —Preceding unsigned comment added by Vultur (talk • contribs) 00:14, 29 August 2008 (UTC)
Objects rotating in space
I contend that it is impossible for an object to be rotating in more than one axis simultaneously without a continual force acting upon it, but there are others that disagree with me. Picture a pencil spinning along its axis, nudged so that it also spins end over end. Doesn't the gyroscope effect prevent an object that is rotating on one axis from also rotating on another axis such that after it has rotated 180° it is then rotating in the opposite direction on the original axis of rotation? Surely this periodic reversal of rotation (in an absolute, external observer sense) requires continuous application of force? How can I more clearly elucidate my point, or am I mistaken? — PhilHibbs | talk 23:38, 27 August 2008 (UTC)
- Any two rotations in 3D space around any two axes (that intersect - presumably at the object's centre of mass) combine to make a single rotation around a single axis, see Euler's rotation theorem. This means that if you try to make something rotate simultaneously around two axes you'll actually find that it's rotating about one, different, axis. --Tango (talk) 23:56, 27 August 2008 (UTC)
- And that rotation can't change without external force, by the conservation of angular momentum. Algebraist 23:57, 27 August 2008 (UTC)
- This is actually not true, see below. -- BenRG (talk) 23:25, 28 August 2008 (UTC)
- I don't see how what you've said below contradicts what I've said. I've just quoted a mathematical theorem, I'm pretty sure it's right, Euler knew his stuff! --Tango (talk) 20:15, 29 August 2008 (UTC)
- This is actually not true, see below. -- BenRG (talk) 23:25, 28 August 2008 (UTC)
- Certainly you can combine any number of rotations in to a single rotation about some other axis. Whether you can change the rotation without an external force is a little tricky. The classic example of the spinning ice-skater who - with arms outstretched - can change the SPEED of her rotation by pulling her arms inwards. That's a change in speed - but not of the axis of rotation. However if she pulls in just one arm, she'll start spinning around an axis that DOESN'T run through her head and her feet. But she's still spinning about the same axis from a point of view of conservation of rotational momentum because her head/body moved one way as the arm moved the other way - the axis of rotation for the entire system is still vertical with respect to the ice - but from the perspective of the skater, it changed.
- This principle is important for spacecraft. Using thrusters to change your axis of rotation consumes reaction mass - rocket fuel - which is valuable stuff...but instead you can have a big flywheel inside and use solar power to spin it up and to keep it spinning against friction. When the spacecraft needs to rotate, it applies forces to the flywheel - the flywheel spins one way - the remainder of the spacecraft rotates the other way in order to conserve rotational momentum. The spacecraft APPEARS to rotate without any external force being applied - where in physical terms, it's total rotational momentum didn't change. This mechanism is how the Hubble Space telescope turns to point where it needs to point without ever running out of fuel.
- Gyroscopes are not magical - they use the same principles. When you see a kid's toy gyro spinning on a table - you do indeed see it "precessing" (it's axis of rotation changes) - but that's because there IS an external force - friction with the table (or whatever mounting points it uses) and gravity.
- I don't know, I still find gyroscopes kind of freaky. Confusing Manifestation(Say hi!) 03:59, 28 August 2008 (UTC)
- An ice skater in outer space can change her axis of rotation by redistributing her weight, see below. -- BenRG (talk) 23:25, 28 August 2008 (UTC)
- An ice skater in outer space? How can you skate in zero-g? — PhilHibbs | talk 07:59, 29 August 2008 (UTC)
- I'll bet you could skate around the inside of a cylinder. Starting and stopping would be problematic though. APL (talk) 20:28, 29 August 2008 (UTC)
OK here's a follow-up. What does torque-free precession mean in this context? It looks to me like it's referring purely to the precession of an axis of symmetry, not the precession of an axis of rotation. — PhilHibbs | talk 13:28, 28 August 2008 (UTC)
- Angular velocity (ω) can change without a torque. Angular momentum (L) is constant in the absence of torque, but L is not a scalar times ω, it's Iω where I (the moment of inertia) is a symmetric 3×3 tensor. Any real symmetric matrix is orthogonally diagonalizable, so you can think of the tensor as three orthogonal axes (the principal axes) with a scalar moment of inertia associated with each one. You can convert between L and ω by individually scaling their components along the three principal axes (thus L and ω do not generally point in the same direction). The trick is that as the object rotates, its principal axes rotate with it. If L is aligned with a principal axis (say x) then it doesn't matter; L = Ixω and that doesn't change as the y and z axes rotate into each other. If L is not aligned with a principal axis (or any combination of axes with the same scalar moment of inertia) then the angular velocity, hence the physical axis and speed of rotation, changes with time even though there's no torque. If the object is not solid then the moment of inertia may change in more complicated ways; objects tend to deform such that one of their principal axes aligns with L. -- BenRG (talk) 14:46, 28 August 2008 (UTC)
- Thanks, I almost understood that! (need to re-read it and mull over it a little more). 4179 Toutatis appears to be behaving oddly, though. What's going on there? — PhilHibbs | talk 16:50, 28 August 2008 (UTC)
- That's what free rotation looks like when L isn't aligned with a principal axis. See Poinsot's construction, which is summarized by the delightful phrase "the polhode rolls without slipping on the herpolhode lying in the invariable plane". The angular velocity is tracing out a herpolhode. The angular momentum, if it were plotted on the frame, would point toward the lower left. To directly answer your question, an isolated pencil in outer space can't spin around its long axis while also spinning end over end (tracing out a disc), but it can spin around its long axis while also tracing out a double cone, sort of like that asteroid is doing. -- BenRG (talk) 23:25, 28 August 2008 (UTC)
- If Spaceman Biff sets his #2 yellow pencil spinning around it longitudinal axis, then he flips it to make it rotate around an axis perpendicular to the initial axis of rotation, it will precess. Edison2 (talk) 05:14, 29 August 2008 (UTC)
- I've heard that claimed, but I'm not convinced that it is true. — PhilHibbs | talk 07:59, 29 August 2008 (UTC)
- You can try the effects of rotations yourself using Orbiter_(sim). The physics model is very elaborate. It takes some time to get a grasp of the controls, but it is really worth the effort. —Preceding unsigned comment added by 84.187.95.86 (talk) 20:34, 29 August 2008 (UTC)
August 28
Spider silk
After watching a spider lower itself from the ceiling, I got to wondering. What happens to such a strand after the critter is done with it? Does it hang there until it disintegrates or somebody comes by? Or does the spider have some way to reel it in and recycle it? Spider silk answereth not. Clarityfiend (talk) 02:10, 28 August 2008 (UTC)
- See spider web —Preceding unsigned comment added by 79.76.196.178 (talk) 02:38, 28 August 2008 (UTC)
- It says "It is not uncommon for spiders to eat their own web daily...", but how would a spider do that with a single strand when there's no other strands to move about on, unlike a web? Clarityfiend (talk) 03:41, 28 August 2008 (UTC)
- This is original research but I find that any time I do something dusty (like sand wallboard or saw wood in the garage), a lot of "left-over" spider silk gets revealed by the dust that it traps. So my assumption is that spider silk hangs around until disturbed by external forces (like the wind or your vacuum cleaner). Once on the ground, doubtless some biological process breaks it down.
What eats starfish?
What kinds of animals are predators of starfish? The starfish article mentions predators in at least two places, but does not elaborate. -- Dominus (talk) 03:58, 28 August 2008 (UTC)
- Human. They taste really weird... --antilivedT | C | G 04:54, 28 August 2008 (UTC)
- Thanks. Anything else? -- Dominus (talk) 05:15, 28 August 2008 (UTC)
- This says "sharks, manta rays, and large bony fish", as well as other starfish. This turgid bit of academic writing supports the fishy notion. Clarityfiend (talk) 05:46, 28 August 2008 (UTC)
- Thanks. Anything else? -- Dominus (talk) 05:15, 28 August 2008 (UTC)

- Gull. They're difficult to swallow whole (Google images has some wonderful pics if you plug in 'gull starfish') but they are definitely considered food if they happen to be there at the same time as the hunger. --Kurt Shaped Box (talk) 05:57, 28 August 2008 (UTC)
- Thank you both! -- Dominus (talk) 14:24, 28 August 2008 (UTC)
- Regarding manta rays, the Wikipedia article about them says they are filter feeders, which rules out eating starfish. Maybe that source meant some other kind of ray instead. -- Dominus (talk) 02:42, 29 August 2008 (UTC)
- Having dissected a starfish in biology lab, I'sd about as soon eat a Dobie pad: "A white nylon/polyester mesh wrapped around a yellow urethane ..."[10]. Edison2 (talk) 05:10, 29 August 2008 (UTC)
- Which is why you should never assume that something is good eatin' just because the gulls seem to be enjoying it... ;) --Kurt Shaped Box (talk) 19:23, 29 August 2008 (UTC)
Do today's dyes weaken fabrics still?
My mom just told me that back in the days, yellow (I think!) clothing ripped most easily because yellow dye weakened the fabric. Is that true? Is it still true? What about other colors? Thanks. 67.243.6.204 (talk) 04:31, 28 August 2008 (UTC)
- Most of the damage that I am aware of is not from the dyes themselves but from the mordants and other chemicals used to make the dye strike and color evenly. Yellow can be made from a number of different dyes (partly depending on if you are dyeing plants like cotton or animal fibres like wool) so it is hard to imagine a blanket statement could be correct. Rmhermen (talk) 00:58, 29 August 2008 (UTC)
- Thanks. FWIW, I'm concerned with cotton here. 67.243.6.204 (talk) 14:08, 29 August 2008 (UTC)
ELISA kit aging
Hi guys. Do you know any sources that tell us how to conduct an accelerated aging test on ELISA kits? --Lenticel (talk) 06:58, 28 August 2008 (UTC)
- It's unlikely that such information exists - at least publically - which means you probably won't get a decent answer here. Presumably these kits come with a "use by" date - and the manufacturers must have used some technique to estimate that date. If the lifespan of the kits is short then it's possible they didn't need to do accellerated aging - they may have simply been able to use the kits to test samples with known properties and see how good the test is after aging naturally. If they know that the lifespan is at least long enough to be acceptable to their customers then the manufacturer may just put an arbitary cutoff date on them. It's true for many drugs that the manufacturer knows they last at least (say) three years - and puts three years on the label just to be safe. There are cases when the US Military has tested drugs that are much older than the "use by" date and found them to be perfectly usable. It's therefore far from certain that anyone has ever even thought of using accelerated aging techniques to estimate the lifetime of these test kits. (For others who might know about accelerated aging, an "ELISA kit" is an "Enzyme-Linked Immunosorbent Assay" (ELISA). They measure antibody counts.) SteveBaker (talk) 15:34, 28 August 2008 (UTC)
- Don't forget the link, Steve: → ELISA. TenOfAllTrades(talk) 15:38, 28 August 2008 (UTC)
- I suspect the poster meant accelerated aging disease test, which sounds like something that might be accomplished with ELISA, though I don't know where to get procedural info. Dragons flight (talk) 15:44, 28 August 2008 (UTC)
- Ah - that would maybe make more sense. I made the fatal mistake of following the link that the OP provided. SteveBaker (talk) 19:44, 28 August 2008 (UTC)
- No, you're right Steve, I was talking about the kit's lifespan. An industry expert said that they require at least have a verifiable 1-year shelf life. This gave us a "no go" signal for a university- invented ELISA kit as we don't have data regarding its shelf life. I was thinking of doing an accelerated aging test to this kit so they can be convinced to manufacture it. But judging from Steve's post, I think it's better to do a 1-year waiting game (my pity goes to the undergrad who unluckily gets this thesis).--Lenticel (talk) 09:55, 29 August 2008 (UTC)
- Ah - that would maybe make more sense. I made the fatal mistake of following the link that the OP provided. SteveBaker (talk) 19:44, 28 August 2008 (UTC)
Sliding filament theory
- How many ATP molecules are consumed per cycle of "sliding"? I believe it to be one, but would like a confirmation.
- When I'd learnt about it (around 6 years back), it was believed that the ATP molecule was required to detach the "flexed" myosin head from actin. But, the the 20th (2001) edition of Ganong's Review of Medical Physiology said that there was a controversy surrounding the exact stage at which the ATP bound to the myosin head, and the 22nd edition says that ATP bindng is required for the actual flexion of myosin head as well as for detachment. The advantage of the previous theory was that it so beautifully accounted for rigor mortis (flexed myosin heads required ATP to detach, which gets depleted, so the muscle remains contracted). Now, rigor mortis is believed to be due to accumulation of calcium ions in the cytosol as its sequestration back into the sarcoplasmic reticulum also requires ATP.
So, it would be nice if some one could comment on this confusion, and I'd appreciate it further if some updated source is cited, too.
Thanks in advance!
—KetanPanchaltaLK 07:52, 28 August 2008 (UTC)
Metaphase of mitosis
At the end of metaphase, are there 46 or 23 pairs of sister chromatids at the equator (in humans)? I feel it should be 46 pairs (homologous chromosomes will not be attached to each other), but am not sure of this assumption. My idea is that in the new daughter cells, there have to be 46 chrmosomes in all, so at metaphase, there should be 46 pairs of ("to-be-chromosomes") chromatids. The doubt is if the homologous chromosomes (of respecitve paternal and maternal origin) are also attached to each other by the kinetochore/centromere, or if they (pairs of homologous chromatids) remain widely separated?
If I'm sounding confusing (as am myself confused), let me give an example. Let's say, chromosome 7. A diploid cell is suppose to have a maternal and a paternal copy each of chromosome 7. Now, no doubt during metaphase two copies each of a maternal and paternal chromosomes would be present, which can be for convenience called maternal chromatids 7 (2 copies attached to each other) and paternal chromatids 7 (2 copies attached to each other), 4 copies of chromosome 7 in all. Now there are two possibilities—situation 1: the two pairs of chromatids remain attached to each other, in which case we'll get 23 copies of "chromatid-complexes" at the equator, and the other possibility—situation 2: the two pairs remain detached, meaning the two copies of maternal chromatids (which are attached to each other), remain separate from two copies of paternal chromatids (which are also attached to each other), in which case we'll get 46 "chromatid-complexes" at the equator. So, my doubt is which situation, 1 or 2, describes the event correctly?
Thanks in advance!
—KetanPanchaltaLK 08:34, 28 August 2008 (UTC)
- Human cells have 46 chromosomes that can be divided into 23 homologous chromosome pairs. During mitosis, each chromosome is duplicated, resulting in 92 chromatids distributed in 46 chromatid pairs (i.e. sister chromatids).
- I think that is what you were saying, but frankly, I stopped trying to read what you wrote. Dragons flight (talk) 08:53, 28 August 2008 (UTC)
- Thanks. Well, I understood your answer that the homologous chromosomes are not attached to each other. It'd be nice if you could cite some source to support this. If you're finding the doubt too bothersome to understand, could you please go through specifically the second paragraph in which I've ginen an example to explain my doubt? —KetanPanchaltaLK 09:57, 28 August 2008 (UTC)
- In reference desk answers, it is considered sufficient to refer you to Wikipedia articles rather than to supply hard references. If you click through to the articles that User:Dragons flight linked to - you'll find references to the facts therein. SteveBaker (talk) 15:20, 28 August 2008 (UTC)
Name that plant
[[11]]
Would like to load it on Wikipedia, but don't know what it's called! Joshua.c.j (talk) 11:03, 28 August 2008 (UTC)
- If you identify it, put it in commons, not wikipedia. -- kainaw™ 12:22, 28 August 2008 (UTC)
- That's a very attractive plant. If you get no joy here, I suggest that you show the picture to experts at your local museum or botanical garden. Fleebo (talk) 06:02, 4 September 2008 (UTC)
Flying cats
Can anyone explain what may be the cause of this? Otolemur crassicaudatus (talk) 15:04, 28 August 2008 (UTC)
- Very probably a genetic mutation.. Leptictidium (mt) 16:16, 28 August 2008 (UTC)
- That's assuming the condition is real and not a hoax, of course. Perhaps I'm too suspicious, but considering that the only person named in the article is some cat owner "only identified as Feng" and she wasn't talking to the Telegraph, but to a local paper (meaning that the reporter who wrote this up is very unlikely to have personally confirmed the existence of these cats), and the research into this has allegedly been conducted by unnamed scientists and equally unnamed "veterinary experts", and that even the place this happened in is only identified as "Sichuan province", an area roughly the size of Spain, my first thought certainly isn't "gee, that that winged cat story sounds really, really believable". The picture's lack of detail doesn't help things any. Not that I'd be shocked to hear that it's true, but you don't exactly get a plethora of reliable sources and convincing details from the article. -- Captain Disdain (talk) 18:13, 28 August 2008 (UTC)
- There was something very similar in Fortean Times a couple of years back, Here. Oh, and we have an article on winged cats! — PhilHibbs | talk 19:36, 28 August 2008 (UTC)
- IF we believe the report (and maybe we don't) - the claim is that this only happens to male cats. That pretty much ensures it's a genetic thing. But these "wings" develop later in life - they aren't born with them - and we're told that a whole bunch of male cats started growing them at more or less the same time. That somewhat suggests it's not genetic. SteveBaker (talk) 19:42, 28 August 2008 (UTC)
- Another problem is that the picture clearly shows a calico, which is nearly always female (a male calico would have to be XXY). --Joelmills (talk) 22:25, 28 August 2008 (UTC)
- Oh! Beautiful! Thank you Joel! A wonderful piece of detective work. BUSTED! SteveBaker (talk) 23:00, 28 August 2008 (UTC)
- Yeah, I think we're done here. =) -- Captain Disdain (talk) 05:27, 29 August 2008 (UTC)
- That winged cat article of ours is pretty interesting in that it claims that this whole thing with this Feng and her cat actually happened in 2007, in another province. That doesn't improve the original poster's news item's credibility (but of course, that may merely be a case of bad journalism by the Telegraph). The citations in the article are pretty vague, so they don't help us a whole lot on this particular case -- or, actually, at all, because the latest reference listed appears to be from 2003... But wait! The External links section includes a link to this unfortunately undated article, which also includes a little better picture, the same one as in the Fortean Times (always the most credible of sources!) article -- though unfortunately that one's pretty much worthless as proof; the bits she's holding up could be glued on, or even completely unattached; there's just no way to tell from the picture. That link was added on May 26, 2007, well over a year ago. All in all, this strikes me as a case where it's kind of hard to know whether there's any truth to the story at all, let alone what the cause of these "wings" in this instance is. -- Captain Disdain (talk) 21:28, 28 August 2008 (UTC)
Who would win?
OK, I know this may seem like a soapbox question or a joke, but I am serious and would like to keep the debate with scientific arguments: Who would win a fight to death, a fit adult man or a fit adult Velociraptor?.
Velociraptor is perceived by the general public as one of the most lethal dinosaurs ever. Yet, the fact that it is only turkey-sized, and the fact that The Truth About Killer Dinosaurs says that the dino's infamous claw was more a grappling device than a killer weapon, make me doubt. A human could break a Velociraptor's neck, or could sent it reeling with powerful kicks to vital organs... But the dino would still have its sharp teeth.
So... More or less, what are the scientific arguments for and against a human beating a Velociraptor in a fight to death? Leptictidium (mt) 16:15, 28 August 2008 (UTC)
- Some OR needed. Get a turkey, fit it with very sharp claws, teeth etc and then start to annoy it. Report findings back here.--79.76.200.98 (talk) 17:09, 28 August 2008 (UTC)
- I don't know much about dinosaurs, but I think it's reasonable to assume that they couldn't be more deadly than anything currently alive of about the same size, after allowing for the hunting-out we've done of large predators. That wouldn't apply to something the size of a turkey. So, it'd probably be comparable to a wildcat or small wolf, or maybe one of the more dangerous reptiles like the komodo dragon. A fit human could, with some damage, take out any of those, so probably could beat a small dinosaur. Black Carrot (talk) 19:53, 28 August 2008 (UTC)

- With the aid of a firearm, I'd assume that human's could essentially kill any dinosaur that previously existed. Without firearms, I don't know. Velociraptors were fast and had incredibly sharp claws. It's essentially like a dog running up, jumping at you, knocking you down and then slashing you to pieces. Who would win that? —Cyclonenim (talk · contribs · email) 20:05, 28 August 2008 (UTC)
- I know it's not scientific but a picture comes to mind of a full speed approaching velociraptor and a human sticking out his spear, uttering "kebab" 93.132.167.145 (talk) 21:21, 28 August 2008 (UTC)
- If it helps modern humans sleep better to belittle dinosaurs equipped with formidable claws and fangs, so be it. I'd bet on the raptor. He also might not come to the fight alone. He might choose his battles so as to have numeric advantage. Edison2 (talk) 05:06, 29 August 2008 (UTC)
- Human against more than 2 Velociraptor = humans is dead.--mboverload@ 19:03, 31 August 2008 (UTC)
- The thing only just barely reached up to you knees...it's like a small dog. Please! Jurrassic Park has a lot to answer for! SteveBaker (talk) 20:39, 31 August 2008 (UTC)
- Human against more than 2 Velociraptor = humans is dead.--mboverload@ 19:03, 31 August 2008 (UTC)
- If it helps modern humans sleep better to belittle dinosaurs equipped with formidable claws and fangs, so be it. I'd bet on the raptor. He also might not come to the fight alone. He might choose his battles so as to have numeric advantage. Edison2 (talk) 05:06, 29 August 2008 (UTC)
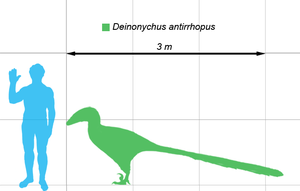
- Deinonychus would probably be a better match, all things considered. --Kurt Shaped Box (talk) 21:24, 31 August 2008 (UTC)
- Are you, by any chance, an xkcdian? Avnas Ishtaroth drop me a line 05:39, 1 September 2008 (UTC)
- How small is small? According to the Velociraptor it had a height of 0.5 metres and estimated to weight around 15kg. This compares to the lower end of the range of the American Pit Bull Terrier. This is a dog which has killed people, of course not all of these are fit and some of them may not have wanted to kill the dog and probably all were not prepared for a fight and indeed a number would have been surprise attacks. But definitely I would say it's going to be a rather difficult fight for an unarmed fit humans against a (smallish) pitbull intending to kill with the fight starting from close range. I would say it's probably even worse with a velociraptor. While there's still a lot we don't know (and may never know) about them, they are likely to go for either the throat or the stomach and neither of these are particularly well protected in humans (it's primary a matter of if it can find them). I'm not saying the velociraptor is guaranteed victory but I definitely don't think the human comes close to being guaranteed victory either. Even more when there are multiple of them. Of course if you are talking about an armed human with the fight starting from long range, with a spear or something and perhaps some rocks then things become much better for the human. And with a gun even more so. Remember in wildlive terms, humans aren't exactly good predators unarmed. Our intelligence allows us to make weapons and come up with clever strategies to win (with a velociraptor the smartest thing may be to just ignore it unless it's making problems for you, why risk injury when you don't need to? And if you did want to kill it, since I don't think these could climb, you may want to get a bunch together and attack it from above or just set some sort of trap for it)
- We don't know anything about what sort of temperament Velociraptors had. They may have been the sort of creature that was brave against small prey but immediately turned tail and ran away as fast as their legs would carry them as soon as something bigger than them (or approximately the same size) took one step towards them whilst looking annoyed. Much like a housecat, for instance... --Kurt Shaped Box (talk) 00:03, 3 September 2008 (UTC)
- "More or less, what are the scientific arguments for and against a human beating a Velociraptor in a fight to death?"
- There isn't one. This isn't a scientific question, its fanwankery based on Godzilla and Pokemon. ;) Clearly, individual circumstances will always come into play, so there can never be one answer. It's like asking "who would win in a fight: Mike Tyson or Evander Hollyfield?" Clearly... it depends on the fight. Dinoguy2 (talk) 01:07, 2 September 2008 (UTC)
cryptozoology and biology
I've always had an interest in cryptozoology and the interesting creatures that come up. Recently, I was watching a program about some "researchers" who were looking for a new species of giant octopus (octopus gigantius with a tentacle span of 200 feet) in the Pugent Sound, Washington area. Their rationale was that since the Sound is so deep, it could easily hide one of these creatures. No surprise: thery found absolutely no evidence!
My question is this: from a biological point of view, is it POSSIBLE for such a creature to exist and what would the parameters be (i.e food supply, effects of the water pressure at depths of 700 feet or so, longevity, etc.)? —Preceding unsigned comment added by 216.154.16.106 (talk) 16:30, 28 August 2008 (UTC)
- I don't know anything about theoretical octopi, but the sperm whale can dive as deep as 2,200 meters (7,200 feet). Giant squid have been captured at a depth of 1,000 meters (3,280 feet). —Preceding unsigned comment added by OtherDave (talk • contribs) 16:47, 28 August 2008 (UTC)
- The problem is that it's not enough for the Puget Sound to have room to hide one of these creatures. There doesn't appear to be any means for the creatures to sneak into and out of the sound without being noticed - a 200' creature in water that's 175 feet deep in such a busy shipping area is going to get noticed. If they aren't sneaking in and out then there would have to be room for an entire breeding colony with sufficient genetic diversity to suvive...maybe a hundred octupi that size...plus a food supply large enough to support such a vast colony. So, I'd say no. SteveBaker (talk) 23:59, 28 August 2008 (UTC)
- Okay, let's get hypothetical here. ;-) Octopuses generally die a short time after mating; it would seem a small change (genetically) to turn off that endocrine trigger that kills them. How large could they get if they didn't die after mating? We don't know yet because there are complications regarding feeding behaviour, but lobsters for example (who are not closely related at all), exhibit "negligible senescence" and can simply keep growing and growing. Maybe you're not looking for a whole breeding colony of monsters, you're looking for a population of normal octopuses that sometimes throw the genetic dice the right way for a monster to show up. It could happen maybe, but it's still a long way from could to is. Matt Deres (talk) 16:34, 2 September 2008 (UTC)
humidification operation
air at atmospheric pressure has a wet bulb temperature of 80 degree Fahrenheit and a dry bulb temperature of 150 degree Fahrenheit. 1.estimate the humidity,molal humidity, relative humidity and dew point of the air. 2.calculate the weight of water in 100 cubic feet of entering air. 3.the air is heated to 150 degree Fahrenheit and cooled adiabatically to 115 degree Fahrenheit.estimate the humidity,percentage saturation and dew point of the air41.205.166.241 (talk) 16:53, 28 August 2008 (UTC)
- Welcome to the Wikipedia Reference Desk. Your question appears to be a homework question. I apologize if this is a misevaluation, but it is our policy here to not do people's homework for them, but to merely aid them in doing it themselves. Letting someone else do your homework does not help you learn how to solve such problems. Please attempt to solve the problem yourself first. If you need help with a specific part of your homework, feel free to tell us where you are stuck and ask for help. If you need help grasping the concept of a problem, by all means let us know. Thank you. -- kainaw™ 16:55, 28 August 2008 (UTC)
- Please refer the questioner to the relevant articles, rather than merely scolding him for asking you to answer his homework, That is what a Real Life Reference Librarian would do. That is what Ref Desk volunteers should do. So to the questioner, this is an encyclopedia. Please read the Wikipdeia articles Relative humidity , Psychrometrics , Dry-bulb temperature , Wet-bulb temperature and Dew point. Feel free to ask a further question if needed. Thanks. Edison2 (talk) 04:57, 29 August 2008 (UTC)
Yeti and Bigfoot
Is it possible they are similar species?--79.76.200.98 (talk) 17:06, 28 August 2008 (UTC)
- Sure, although it should be noted that virtually anything is possible within the realm of imagination. Should both prove to be real, however, it's entirely reasonable to expect them to be related. — Lomn 17:13, 28 August 2008 (UTC)
- Hi. Well, that depends on what you mean by "similar". A book on primate cryptids notes categorises bigfoot into "neo-giants" (human-like footprints) and yeti into "unknown pongids" (big toe out at an angle). This suggests that if they exist then yeti might be more closely related to the skunk ape. Thanks. ~AH1(TCU) 17:38, 28 August 2008 (UTC)
- I would say they are both of the genus-species Homo sapien, but one in a snowy environment in a white fur suit and another in the forest in a brown fur suit. -- MacAddct 1984 (talk • contribs) 18:25, 28 August 2008 (UTC)
- So how many 9 ft tall humans do you know who go prancing about in gorilla suits in the forest of North America or the frozen heights of Tibet? —Preceding unsigned comment added by 79.76.200.98 (talk) 01:26, 29 August 2008 (UTC)
- So how many 9 ft tall Yetis and Bigfeet (?) do you know? Fribbler (talk) 15:08, 29 August 2008 (UTC)
- So how many 9 ft tall humans do you know who go prancing about in gorilla suits in the forest of North America or the frozen heights of Tibet? —Preceding unsigned comment added by 79.76.200.98 (talk) 01:26, 29 August 2008 (UTC)
- They both appear to be Primates, so if they're both real they're going to be pretty similar. How similar are you looking for (Do you consider a Chimp similar to a Gorilla?). There are arguments either way. They have some pretty different territories though, the most recently they could have possibly interbred would be the last Ice Age. Still, that's not that long ago for a primate, and they both seem to share the startling evolutionary adaptation of being completely impossible to prove the existence of, so perhaps they're even the same species separated from each other for thousands of years. The only real way to know for sure is in-detail genetic tests or seeing if they're capable of interbreeding. When you catch one of each let us know. APL (talk) 02:49, 29 August 2008 (UTC)
- You could also be interested in the Gigantopithecus article. --jjron (talk) 16:10, 29 August 2008 (UTC)
- The Yeti article states that “The Yeti can be considered a Himalayan parallel to Bigfoot (Sasquatch).” The [[Bigfoot] article is even more specific: it says “Believers in its existence contend that such an animal, or close relatives of it, may be found around the world under different regional names, such as the Yeti of Tibet and Nepal, …” So yes, they are similar species. – b_jonas 20:02, 30 August 2008 (UTC)
- I am agree with MacAddct. Both Yeti and Bigfoot are Homo sapiens, one in white suit other in brown suit. Otolemur crassicaudatus (talk) 03:20, 2 September 2008 (UTC)
spider web sacs in trees?
Hi. Recently I came upon some spider-web like sacs in trees. The trees were all decidous, and I live in Southern Ontario. The material was likely spider webbing, but it also resembled thisn plastic bags or fine mesh nets. There were roughly 2 - 30 cubic ft in volume, and roughly 2 - 10 m off the ground. There was also some dark plant like material in the "webs", which were roughly elliptoidical, which might have been leaves, bark, dirt, etc. I didn't see any spiders present, but they reminded me of birds' nests, except larger and nearly transparent. I also came upon spider silk on another low-lying tree close to another one which had one of these web sacs. Any idea what it might be? Is there such thing as a "tree spider" (no article), and if there is, do they exist in Ontario and how large are they? Or, could this have been made by something else? Thanks. ~AH1(TCU) 17:33, 28 August 2008 (UTC)
- If you are able to check those trees again, you may find some or all of the leaves eaten, or perhaps find caterpillars busy eating the leaves. Wanderer57 (talk) 18:44, 28 August 2008 (UTC)
what is animal.
[['Link title'[Bold text]]] —Preceding unsigned comment added by 202.125.143.73 (talk) 18:12, 28 August 2008 (UTC)
- Animal is a character on The Muppet Show. -- kainaw™ 18:27, 28 August 2008 (UTC)
- I added a wikilink for you. Dragons flight (talk) 18:57, 28 August 2008 (UTC)
- "Animal" is also a clue given in the quiz game Twenty Questions. In that game, "animal" generally means an animal or something created from some part of an animal. Eg, a leather saddle is "animal", a shark's tooth is "animal", a ivory carving is "animal", a wooden carving is "vegetable", and a meershaum carving is "mineral". Wanderer57 (talk) 19:00, 28 August 2008 (UTC)
Might as well quote the dab page:
- An animal is a taxonomic member of the Kingdom Animalia. Animal may also refer to:
- A.N.I.M.A.L., an Argentinian heavy metal band
- The Animals, a British rock band
- Animals (album), a 1977 concept album by Pink Floyd
- "Animal" (song), the name of several songs
- Animal (video game), a video game by Microtime
- Animal (book), full title Animal: The Definitive Visual Guide to The World's WildLife, a 2003 non-fiction book by David Burnie and several co-authors
- ANIMAL (image processing), an interactive software environment for image processing
- The Animal, a 2001 film starring Rob Schneider
- Animal, a 2005 film starring Ving Rhames and Terrance Howard.
- Animals is an episode of The Vicar of Dibley.
- Animal (Muppet), a drummer in the Muppet Show's Dr. Teeth and The Electric Mayhem
- George Steele, professional wrestler nicknamed "The Animal"
- Joseph Laurinaitis, professional wrestler nicknamed "Road Warrior Animal"
- David Batista, professional wrestler nicknamed "The Animal"
- Animal, nickname of Takeshi's Castle regular character, played by Japanese/American baseball player Brad Lesley
- Animal, nickname of Brazilian association footballer Edmundo.
There - now we have all the bases covered. SteveBaker (talk) 22:56, 28 August 2008 (UTC)
- Wiktionary has a few more... JessicaThunderbolt 20:17, 29 August 2008 (UTC)
BIOLOGY
PLEASE I WILL LIKE TO GET MORE INFORMATION ABOUT (THE ROLE OF MICROORGANISMS IN THE PRODUCTION OF ANTIBIOTICS). FOR MY PROJECT. THANKS —Preceding unsigned comment added by 80.250.32.5 (talk) 19:35, 28 August 2008 (UTC)
- First, STOP SHOUTING (using capital letters), it's considered rude on the internet.
- Secondly, we can't help directly with your homework, but we can give you hints. Check out Antibiotic and Production of antibiotics. If you have any more questions that are more specific (remembering that we can't give you direct answers), then do feel free to come back. —Cyclonenim (talk · contribs · email) 19:57, 28 August 2008 (UTC)
- You have an project now? You Nigerians have a strange schoolyear. --99.237.101.48 (talk) 20:16, 28 August 2008 (UTC)
- It is the northern hemisphere schools that have strange school years that don't align with the calendar year. Graeme Bartlett (talk) 21:18, 28 August 2008 (UTC)
- Actually a number of northern hemisphere schools do have school years that partially or completely align with the calendar year. It's only those in temperate regions that have to follow the silly weather Nil Einne (talk) 23:49, 28 August 2008 (UTC)
- It is the northern hemisphere schools that have strange school years that don't align with the calendar year. Graeme Bartlett (talk) 21:18, 28 August 2008 (UTC)
- You have an project now? You Nigerians have a strange schoolyear. --99.237.101.48 (talk) 20:16, 28 August 2008 (UTC)
- You might also want to read Biochemical engineering, Biopharmaceuticals, andPenicillin#Production --Shaggorama (talk) 00:55, 30 August 2008 (UTC)
Spider eggs?
Ages ago, there were these little cotton wool like things (about the size of the cotton on a cotton bud, only more spherical) in about groups of four and just about smaller than a pea, outside in a little crack by the front communal door. I assumed them to be spiders eggs, only they were a little big. Any idea what they are? I'm in the UK. Thanks, 86.148.47.145 (talk) 21:29, 28 August 2008 (UTC)

- You're right - spiders wrap their eggs in web silk and roll them up into large balls. SteveBaker (talk) 22:49, 28 August 2008 (UTC)
- At a certain point of its development, if you carefully slit it open, you might see tiny spiders running around. At a later stage of development, spiders of some species can extrude strands of web which act like kites to carry them to remote locations, where by virtue of their small size they can enter a dwelling through the most minute cracks. Edison2 (talk) 04:53, 29 August 2008 (UTC)
dog
has a dog really ever eaten maths homwork? —Preceding unsigned comment added by 86.128.102.247 (talk) 21:42, 28 August 2008 (UTC)
- Yes. Although, even more amazingly, the dog ate a USB memory stick, not paper. —Cyclonenim (talk · contribs · email) 22:08, 28 August 2008 (UTC)
- Hey - no homework questions! SteveBaker (talk) 22:40, 28 August 2008 (UTC)
- If a human infant can eat large portions of a newspaper (original research)and a dog can eat a paper bag containing a ham sandwich (original research) then it is plausible (WP:SYNTHESIS) than a dog could eat math homework. Especially if food had ever been near it. Edison2 (talk) 04:50, 29 August 2008 (UTC)
- Hey - no homework questions! SteveBaker (talk) 22:40, 28 August 2008 (UTC)
- Edison2: If you are doing this type of research with an infant, may I suggest you do not publicize it. Wanderer57 (talk) 05:38, 29 August 2008 (UTC)
- There was no problem, because the paper was still readable once it was extracted from the infant's mouth, dried out a bit and sorted back to the pages it had been torn from. Edison2 (talk) 20:57, 29 August 2008 (UTC)
- Edison2: If you are doing this type of research with an infant, may I suggest you do not publicize it. Wanderer57 (talk) 05:38, 29 August 2008 (UTC)
- When my wife was in vet school, her cat ate the budget for the Animal Behavior Club. That was a tough one to explain. -- Coneslayer (talk) 11:43, 29 August 2008 (UTC)
- My Peach-faced Lovebird once shredded my homework. She worked from the top of the paper down, chewing it into little strips. Luckily, there was enough of it left to show the teacher the next day, so I was given the benefit of the doubt... --Kurt Shaped Box (talk) 23:09, 29 August 2008 (UTC)
- I can't remember if it was maths or not, but our dog genuinely ate my homework on more than one occasion. — PhilHibbs | talk 13:52, 2 September 2008 (UTC)
has our buddy broke his leg?????
Conflicting information on effects of neutering dogs
There is a conflict of info on the effects of neutering of dogs with respect to the likelihood of contracting prostrate cancer. The article on castration directly contradicts the article on neutering. —Preceding unsigned comment added by 202.59.164.121 (talk) 23:30, 28 August 2008 (UTC)
- I guarantee that neutering has no effect whatsoever on prostrate cancer. Prostate cancer is another matter, and I have no information about its incidence in dogs. — OtherDave (talk) 23:41, 28 August 2008 (UTC)
- On the contrary, every source I can find on Google says that neutering a dog reduces it's risk of prostate cancer. —Cyclonenim (talk · contribs · email) 00:32, 29 August 2008 (UTC)
- Common misconception. Neutering a dog does about double the risk of prostate cancer. I didn't know the castration article was covering this, but I'll fix it. --Joelmills (talk) 02:11, 30 August 2008 (UTC)
- Just checked the castration article - it actually said it reduces the incidence of prostate disease. This is true, since benign prostatic hypertrophy is much more common in dogs than prostate cancer, and neutering does eliminate the risk of it. I did clarify the point about cancer in the article. --Joelmills (talk) 02:19, 30 August 2008 (UTC)
- I think you might have missed the joke in OtherDave's post. --Bowlhover (talk) 06:35, 29 August 2008 (UTC)
- Haha, probably :( It was late and I'd hate a few beers... (any excuse!) —Cyclonenim (talk · contribs · email) 09:36, 29 August 2008 (UTC)
- Common misconception. Neutering a dog does about double the risk of prostate cancer. I didn't know the castration article was covering this, but I'll fix it. --Joelmills (talk) 02:11, 30 August 2008 (UTC)
- On the contrary, every source I can find on Google says that neutering a dog reduces it's risk of prostate cancer. —Cyclonenim (talk · contribs · email) 00:32, 29 August 2008 (UTC)
August 29
Motorcycle
I have seen motorcycles turn, not by turning their steering wheel, but by leaning their bikes towards the ground. Why does this work. I've draw a free-body diagram, but I can't find the centripetal force. —Preceding unsigned comment added by 65.92.231.82 (talk) 00:27, 29 August 2008 (UTC)
- Bicycle and motorcycle dynamics has everything you could possibly want to know. Algebraist 00:31, 29 August 2008 (UTC)
- I think it's because motorcycles are fast enough that you need to lean significantly even with a wide turning radius. In short, the amount you lean the bike is noticeable, but the amount you turn the front wheel isn't. In addition, do the the way bikes are built, leaning causes the front wheel to turn. — DanielLC 00:52, 29 August 2008 (UTC)
- In response to DanielLC, I'm sure it's a centripetal force causing the acceleration, and I'm also sure it's firction. I checked the article Bicycle and motorcycle dynamics, specifically turning, but they didn't why the friction force appears, or the derivation of the formula. —Preceding unsigned comment added by 65.92.231.82 (talk) 01:24, 29 August 2008 (UTC)
- Friction provides centripetal force on a flat corner. On a banked corner, centripetal force is a combination of horizontal components of friction and normal force. Gandalf61 (talk) 10:22, 29 August 2008 (UTC)
- Also note... They are turning the wheel, slightly in the opposite direction that they are turning. Let me see if have an article on it... counter steering. Wow! -- kainaw™ 14:12, 29 August 2008 (UTC)
- Well, that article on counter steering was great. I never understood the physics. Now I know. OrangeMarlin Talk• Contributions 00:51, 30 August 2008 (UTC)
Clorox as hair lightener/bleach
Regardless of safety, can clorox (or similar) bleach effectively lighten hair? As an oxidizer, I would think that it would be mightily effective. Please also describe potential side effects of this procedure. Thank you. Kenjibeast (talk) 01:05, 29 August 2008 (UTC)
- (from accidental experience) Yes, it will alter the color of hair. No, it will not make it blond. Yes, it will turn it a weird copper-green color. No, it will not look healthy. Yes, it will become thin and stiff and break when you try to brush it. No, it will not feel good. Yes, it will burn and leave a permanent thinning-hair spot on the back of your head that is still visible after 20 years. -- kainaw™ 01:35, 29 August 2008 (UTC)
- Concur from the memory of the appearance of a friend's hair and the state of his scalp after he tried to bleach his hair using toilet bleach. It started falling out in clumps the day after - and the sores, they did weep. Not the best idea. --Kurt Shaped Box (talk) 19:27, 29 August 2008 (UTC)
Perception of Musical Pitch
Is there any evidence that peoples perception of musical pitch deteriorates with age?--79.76.200.98 (talk) 02:07, 29 August 2008 (UTC)
- It's mentioned in Cecil Adam's column on Perfect Pitch but it's not referenced. APL (talk) 02:59, 29 August 2008 (UTC)
- Under Limits of Perception, http://en.wikipedia.org/wiki/Psychoacoustics it says, "The human ear can nominally hear sounds in the range 20 Hz to 20,000 Hz (20 kHz). This upper limit tends to decrease with age, most adults being unable to hear above 16 kHz." In fact, hearing ability in the entire upper audio range typically tends to decrease as a person ages. I cannot find an authoritative source for that statement, but I am sure there are authoritative sources. Andme2 (talk) 04:32, 29 August 2008 (UTC)
- I don't think that was exactly the question. Yes, the upper audible limit deteriorates markedly with age but the questioner is instead asking is the human ability to reproduce an exact frequency age related. For instance, when an orchestra is tuning up before the start of a concert, the lead violin will play a note A 440Hz for the rest of the violins to reference to. There are no frets on a violin - the note is placed at the correct frequency entirely by ear. It is essential that the lead violin has perfect pitch to accurately reproduce the required note. 440Hz is a relatively low frequency, well below the normal age related deterioration. If the player has lost the ability to hear this frequency then he/she has substantially gone deaf. I don't know the answer to the original question but my guess is that perfect pitch is not related to the physiology of the ear and that deterioration in the function of the ear (provided it has not gone too far) does not affect this ability. I think that this rather wonderful skill is more likely an ability of the brain. SpinningSpark 06:16, 29 August 2008 (UTC)
- It's almost always the oboe that sets the orchestra's pitch, and not a violin, precisely because the oboe's pitch is more or less unalterable by the player once it's established, while the same is not true, as you point out, for the strings. - Nunh-huh 06:59, 29 August 2008 (UTC)
- Also, because of tuning forks and electronic tuners, no one in an orchestra needs perfect pitch. It's a cool ability, but many (I expect most) professional musicians don't have it. --Allen (talk) 19:48, 29 August 2008 (UTC)
- It's almost always the oboe that sets the orchestra's pitch, and not a violin, precisely because the oboe's pitch is more or less unalterable by the player once it's established, while the same is not true, as you point out, for the strings. - Nunh-huh 06:59, 29 August 2008 (UTC)
- The Mosquito, as well as the ringtone derived from it, were based on this assumption. As for references, I found this webpage from Hypertextbook.com, which claims:
- "The range of hearing for a healthy young person is 20 to 20,000 hertz. The hearing range of humans gets worse with age. People lose the ability to hear sounds of high frequency as they get older. The highest frequency that a normal middle-aged adult can hear is only 12-14 kilohertz."
- and
- "The normal range of hearing for a healthy young person is 20 to 20,000 Hz; hearing deteriorates with age [...]"
- I once used Frequency Generator to test whether the maximum frequency decreases with age, and found the correlation was startlingly strong; a sound that one person considered unbearably loud was inaudible to a person only several years older. --Bowlhover (talk) 06:29, 29 August 2008 (UTC)
- I don't have a source at hand at the moment, but I think that statistically speaking, on average the upper limit of hearing decreasing with age. As for whether the ability to perceive pitch changes at any frequency, including the mid-range, decreasing with age, I haven't seen anything to suggest that. It could be, but there are plenty of elderly musicians who play instruments such as the violin that require sensitive pitch perception. In any case it would have something to do with the inner ear's hair cells. It wouldn't surprise me if over time there is some kind of loss there. Pfly (talk) 07:22, 30 August 2008 (UTC)
- I don't think that was exactly the question. Yes, the upper audible limit deteriorates markedly with age but the questioner is instead asking is the human ability to reproduce an exact frequency age related. For instance, when an orchestra is tuning up before the start of a concert, the lead violin will play a note A 440Hz for the rest of the violins to reference to. There are no frets on a violin - the note is placed at the correct frequency entirely by ear. It is essential that the lead violin has perfect pitch to accurately reproduce the required note. 440Hz is a relatively low frequency, well below the normal age related deterioration. If the player has lost the ability to hear this frequency then he/she has substantially gone deaf. I don't know the answer to the original question but my guess is that perfect pitch is not related to the physiology of the ear and that deterioration in the function of the ear (provided it has not gone too far) does not affect this ability. I think that this rather wonderful skill is more likely an ability of the brain. SpinningSpark 06:16, 29 August 2008 (UTC)
- I remember reading somewhere that Benjamin Britten had complained of standard pitch seeming to grow sharper as he aged. Google throws up an article about an NPR program which bears that out: "Our results clearly show that as people get older, they are perceiving things sharper than when they are younger," William Avery (talk) 15:07, 30 August 2008 (UTC)
- I don't know how this relates to perceived musical sharpness, but the perceived sound of musical instruments becomes somewhat different as a person ages. All musical instruments produce complex (non-sine wave) tones. The sensitivity to higher pitches typically decreases as a person ages. Therefore an older person hears a less complex tone, somewhat closer to a sine wave. This would occur even at low fundamental frequencies. Also, of course, the perceived loudness of pitches with a high fundamental frequency decreases - that is a second effect. Andme2 (talk) 06:34, 31 August 2008 (UTC)
Transverse waves
Why can't transverse waves pass in liquids? I can see why they can't pass in gasses, but why not in liquids? —Preceding unsigned comment added by 65.92.231.82 (talk) 02:11, 29 August 2008 (UTC)
- Clearly they propagate nicely along the interface between water and the air above it. It seems that to propagate within a body of liquid, there would have to be an interface within the liquid, such as a layer of fresh water above a layer of salt water where a river flows into the ocean, or the interface between oil and water. Longitudinal waves propagate nicely within a homogeneous liquid. Edison2 (talk) 04:46, 29 August 2008 (UTC)
- The liquid has no strength or stiffness. It does not resist movement like a solid, so there is no restoring force to make a wave. However the liquid does resist compression, so you can get a compression wave. Graeme Bartlett (talk) 05:55, 29 August 2008 (UTC)
- Why would it work in gasses but not liquids? The only difference is that gasses compress when under pressure. — DanielLC 17:22, 30 August 2008 (UTC)
Why is mescaline a controlled substance?
What are the detrimental effects of it, if any? False Tournament (talk) 02:41, 29 August 2008 (UTC)
- Why is it controlled? See Convention on Psychotropic Substances. -- kainaw™ 02:47, 29 August 2008 (UTC)
- I heard of on- and off-pills used by the military so the pilots are guaranteed awake for combat and have a sound sleep afterwards. Can't find any reference on the net right now, though. Does anybody know about that and why it's allowed? If it is allowed, can I have access even if I'm not at war with anybody? 93.132.129.229 (talk) 19:53, 29 August 2008 (UTC)
- The USAF (and probably other air forces) prescribe amphetamines to some of their pilots (e.g. B2 and B52 operations to Iraq based in the US and the UK respectively) where the pilots had to stay awake for long periods of time (reference). They do this under the control of a flight surgeon (a doctor), who is supposed to make sure their use is appropriate and the side effects managed. You may wish to consider whether you're comfortable with someone taking a drug which has side effects including nervousness, irritability, over stimulation, restlessness, euphoria, and feelings of suspicion and paranoia (reference) is a fit person to be in charge of weapons and aircraft. Lots of things are legal for the military to have (machine guns, tanks, nuclear armed submarines) which are nevertheless illegal for ordinary people. -- Finlay McWalter | Talk 20:03, 29 August 2008 (UTC)
- I see, so this is because they have machine guns, tanks, nuclear armed submarines and so on, so no one can effectively deny those rights to them. 93.132.129.229 (talk) 20:32, 29 August 2008 (UTC)
- No! That's completely untrue - and I'm very sure you know that. They have the right to use these drugs AND they have the right to use tanks, etc because the law/constitution of the country (rightly or wrongly) permits it. Your take on it would suggest that they demand the drugs and get them because they're heavily armed...that's a totally ridiculous point of view. Correlation does not imply causation. SteveBaker (talk) 22:06, 29 August 2008 (UTC)
- "That's a totally ridiculous point of view".... is it really? Nimur (talk) 22:27, 29 August 2008 (UTC)
- SteveBaker: yes, I know that it is totally ridiculous, but I really wonder why. Why does a government deny psychoactive substances to their subjects (probably because they could do dangerous things while druged, like drunken driving) and at the same time allow it to those in charge of have machine guns, tanks, nuclear armed submarines? 93.132.129.229 (talk) 23:02, 29 August 2008 (UTC)
- No! That's completely untrue - and I'm very sure you know that. They have the right to use these drugs AND they have the right to use tanks, etc because the law/constitution of the country (rightly or wrongly) permits it. Your take on it would suggest that they demand the drugs and get them because they're heavily armed...that's a totally ridiculous point of view. Correlation does not imply causation. SteveBaker (talk) 22:06, 29 August 2008 (UTC)
- The stimulants given to military members are available to the US public by prescription. You just have to convince a doctor that you have a need for them. Combat pilots are given then on the theory that they need to stay alert or else they might die. Presumably if you had an equally compelling need (like combating certain sleep disorders), then you could use them too. In neither case are they simply given out for experimentation though. Dragons flight (talk) 23:10, 29 August 2008 (UTC)
- These things are given to people rarely and under medical supervision. The people involved are generally pretty fit (mentally and physically) - and it's done for reasons of "national security". That's nothing like a typically unfit drug addict taking them unsupervised - getting them in probably impure form from who-knows-what source - and taking them far too often - for reasons that are deleterious to society in general - not beneficial. SteveBaker (talk) 00:47, 30 August 2008 (UTC)
- Your argument is quite good. Actually, I would be more interested in off-pills as I have a longer than circadian rhythm. Do you think I would get a prescription in your country? 93.132.129.229 (talk) 23:19, 29 August 2008 (UTC)
- We aren't allowed to answer medical questions on the WP:RD - please seek the advice of a qualified doctor. SteveBaker (talk) 00:47, 30 August 2008 (UTC)
- The stimulants given to military members are available to the US public by prescription. You just have to convince a doctor that you have a need for them. Combat pilots are given then on the theory that they need to stay alert or else they might die. Presumably if you had an equally compelling need (like combating certain sleep disorders), then you could use them too. In neither case are they simply given out for experimentation though. Dragons flight (talk) 23:10, 29 August 2008 (UTC)
Note also that amphetamines and mescaline are quite, quite different. Pfly (talk) 07:29, 30 August 2008 (UTC)
- Then there's nodoze tablets, which are available at most drug stores. They are taken voluntarily and without prescription. Andme2 (talk) —Preceding undated comment was added at 22:46, 30 August 2008 (UTC)
- ...yes - but active ingradient in NoDoz is caffeine which is not exactly a controlled drug! As I recall, they have about the same amount of the stuff as two cups of good, strong coffee! SteveBaker (talk) 15:32, 31 August 2008 (UTC)
- Then there's nodoze tablets, which are available at most drug stores. They are taken voluntarily and without prescription. Andme2 (talk) —Preceding undated comment was added at 22:46, 30 August 2008 (UTC)
Emit Visible Light by Radio Transmitter
According to article Electromagnetic spectrum, radio waves and visible lights are both electromagnetic waves. The only difference I can see between them is they have different frequencies. So is it possible for a radio transmitter to emit visible light if the radio transmitter can emit radio wave of extremely high frequency? - Justin545 (talk) 09:35, 29 August 2008 (UTC)
- In principle, yes. In practice, the required frequencies are unobtainable in any system that would resemble a normal radio transmitter. Dragons flight (talk) 10:10, 29 August 2008 (UTC)
- A radio transmitter that emitted visible light it would look very similar to a Incandescent light bulb --Shniken (talk) 14:45, 29 August 2008 (UTC)
- Not really. A light bulb's output is a byproduct of heat, not a modulated frequency. — Lomn 17:28, 29 August 2008 (UTC)
- Yes, if you use the right equipment. Electromagnetic radiation in radio transmitters is an oscillating field caused by an oscillating current of electrons. That is, we accelerate the electrons back and forth. It's the acceleration in itself that is the trick and another word for electromagnetic radiation caused by the acceleration of a charged particle is Bremsstrahlung. With a current in a wire we only manage radio waves, but when sending the particles round in circles in a vacuum it's a different matter. This is called Synchrotron radiation and you can indeed achieve frequencies from radio waves, into and past the visible spectrum and into ultraviolet and x-rays. EverGreg (talk) 18:31, 29 August 2008 (UTC)
- It's a matter of frequency, certainly - but it's easier to think in terms of wavelengths. (Frequency and wavelengths are opposite sides of the same coin here). So let's look at how long the actual waves are:
- TV and FM radio waves are around a meter long...AM radio is out at 300 meters maybe.
- Radar systems and microwave ovens operate at - between 10cm and 1cm wavelengths (there is an elegant experiment involving chocolate chips that lets you measure that in your microwave oven!)
- Waves smaller than a centimeter are called "millimeter band" and are used for short, precise, distance measurements - short range radar - such as the 'reversing sensors' that some cars have.
- Millimeter waves at 1mm are right next to the "far" infrared part of the spectrum - which extends down to "near" infrared at a millionth of a meter. For some reason, we call infrared "light" - not "radio" or "radar" - but it's all the same stuff - it's just a matter of wavelength.
- Infrared light is right next to visible red light in the spectrum. Visible light waves are around half of a millionth of a meter long.
- So "radio" waves are about a million times bigger than "light" waves - but they are EXACTLY the same phenomenon - it's just a matter of frequency and wavelength.
- To answer the question then: To operate efficiently, Radio antennae need to be about a wavelength long. The antenna on your car is about a meter long - so it can pick up radio waves. The little stubby antenna on your cellphone reflects the fact that it's operating at wavelengths of a few centimeters. So the "antenna" for visible light would be less than a millionth of a meter long! But the systems that make radio transmitters work efficiently simply aren't designed to put out waves that small. The other side of the coin - the "frequency" is to do with how fast the electronics have to oscillate to make waves of the appropriate length. To make radio waves, you only have to oscillate a few million times a second. This is easy to engineer - there are crystals that oscillate at those rates - also you can build all sorts of circuits that'll do that. But as the frequencies get higher, it gets harder and harder to make systems that'll vibrate fast enough. We have computers that run at frequencies as high as 3 to 4 GHz - that's barely as fast as microwaves. Making electronics oscillate faster than that starts to get tough because the size of the electronics has to be small enough to let the electrons get across the circuit in a small enough amount of time. By the time you get into the infrared region, you need to have atoms oscillating - not big things like crystals. So we can't make a 'crystal radio' oscillate anywhere near fast enough to make light. We make infrared by stimulating atoms to vibrate - similarly with visible light. So while light and radio are "the same thing", in practice, it's not just a matter of retuning the transmitter and reducing the size of the antenna to convert a radio into a lightbulb. SteveBaker (talk) 19:10, 29 August 2008 (UTC)
- Tiny question for clarity: I thought antennae needed to be half a wavelength long for optimal efficiency? Franamax (talk) 21:24, 29 August 2008 (UTC)
- That would be the distinction between a Marconi antenna[12] (archaic nomenclature) and a half-wave dipole antenna; but in modern antenna theory, there are ten million variations on the theme, so "optimal efficiency" may be traded for directionality, bandwidth, narrow-band frequency-specific coupling effects, active control, etc. Nimur (talk) 21:32, 29 August 2008 (UTC)
- Yes - and in any case, it's a pretty rough requirement. For the purposes of answering this question we don't need to get into horrible details of the 'black art' of antenna design! You can pick up a perfectly good signal over a wide range of radio or TV channels with a fixed size antenna of roughly a wavelength...but ten times the wavelength or a tenth the wavelength doesn't work nearly as well. The point is that a radio transmitter that's set up with an antenna suitable for AM radio won't make light (which would require an antenna a MILLIONTH of that length). SteveBaker (talk) 21:54, 29 August 2008 (UTC)
- That would be the distinction between a Marconi antenna[12] (archaic nomenclature) and a half-wave dipole antenna; but in modern antenna theory, there are ten million variations on the theme, so "optimal efficiency" may be traded for directionality, bandwidth, narrow-band frequency-specific coupling effects, active control, etc. Nimur (talk) 21:32, 29 August 2008 (UTC)
- Tiny question for clarity: I thought antennae needed to be half a wavelength long for optimal efficiency? Franamax (talk) 21:24, 29 August 2008 (UTC)

- There have been some experiments in radio-induced airglow or (artificial) aurora, such as this IEEE publication on work performed at the HAARP facility. This is an indirect effect and requires certain ionospheric conditions. The beam is a "High Frequency" radiowave, meaning ~5 MHz, and it is through ionspheric interactions that this energy can be converted in to optically observable light. Nimur (talk) 21:30, 29 August 2008 (UTC)
- Here's a link to the Navy's description of optical emissions: [13]. "The exciting result was that by pointing the HF beam directly along a geomagnetic field line, artificial emissions of greater than 200 Rayleighs (R) at 630.0 nm and greater than 50 R at 557.7 nm could be produced. This intensity was nearly an order of magnitude larger than that produced by heating directly overhead." Nimur (talk) 21:40, 29 August 2008 (UTC)
- Let's not confuse the OP though. HAARP and the Navy work are NOT a matter of retuning a radio transmitter to broadcast up in the PetaHz (1015Hz) range! We have no idea how to make a "radio transmitter" that works at such spectacularly high frequencies. The things you are discussing are systems causing secondary effects in the atmosphere. That's not at all what the OP is asking - so let's not muddy the waters! SteveBaker (talk) 22:01, 29 August 2008 (UTC)
- I only half-agree. The generation of optical frequencies is a special class of frequency mixing, where the frequency mixer just happens to be a particular natural phenomena / atomic property. Though this effect occurs at high altitude, it's not so very different from using a diode mixer on a circuit board, where a different other atomic effect is responsible for signal conditioning suitable for changing frequency. Nimur (talk) 22:10, 29 August 2008 (UTC)
- There have been some experiments in radio-induced airglow or (artificial) aurora, such as this IEEE publication on work performed at the HAARP facility. This is an indirect effect and requires certain ionospheric conditions. The beam is a "High Frequency" radiowave, meaning ~5 MHz, and it is through ionspheric interactions that this energy can be converted in to optically observable light. Nimur (talk) 21:30, 29 August 2008 (UTC)
- As you increase in frequency the techniques of producing the oscillations produces less and less power, and amplifiers produce less and less gain until you reach the technology limit around one terahertz. Your antennas will have to be on the same scale as light waves, much bigger than atoms and molecules, and potentially on the same scale as silicon chip technology. But really the problem is generating the arbitrary waveform at the required frequency. As you get to light frequencies the quantization in to photons also takes effect, raising your noise floor. Graeme Bartlett (talk) 08:54, 30 August 2008 (UTC)
Bat vs bird populations
White nose syndrome has had a crippling effect on bats here in the Northeast US. Add to that the heavy rainfall in June and July. It looks like these factors have caused a huge increase in the number of mosquitos in my yard. I normally don't use bug dope but while mowing the lawn just now, I was getting eaten alive! So, how long will it take for the bug-eating bird population to kind of fill the gap that the bats have left? Can I expect a change this year or do I have to wait till next year? Dismas|(talk) 17:45, 29 August 2008 (UTC)
- Wow... It's so quiet, you can hear them buzzing in your ears... Seriously, there aren't any ornithologists in the house? Dismas|(talk) 00:23, 31 August 2008 (UTC)
- Ornithologists study birds - not bats. SteveBaker (talk) 03:14, 31 August 2008 (UTC)
- I think you missed the last two sentences of my original posting. I wasn't asking about the bats. Dismas|(talk) 00:19, 1 September 2008 (UTC)
- Ornithologists study birds - not bats. SteveBaker (talk) 03:14, 31 August 2008 (UTC)
- Is it possible that the current bug-eating bird population has been held in check by a voracious predator? Imagine Reason (talk) 22:35, 6 September 2008 (UTC)
What's with this leaf?


The leaf you see here is covered with round green nodules that look rather like tiny apples. Does it normally look like that? Or is it evidence of some sort of parasite or leaf disease or something else?
Sorry, I don't remember what sort of plant it came from. -- Dominus (talk) 18:45, 29 August 2008 (UTC)
- Could be lots of things. See gall. 93.132.129.229 (talk) 19:17, 29 August 2008 (UTC)
It looks quite a lot like hackberry leaf gall. See here [14]Richard Avery (talk) 06:43, 30 August 2008 (UTC)
- I'm satisfied with "gall". Thanks, folks. -- Dominus (talk) 02:39, 31 August 2008 (UTC)
- It was in fact a hackberry leaf. Thanks again. —Mark Dominus (talk) 13:29, 11 September 2011 (UTC)
Not be exactly with this leaf but quite similar with some mango leaves in my garden. There are some kind of insects implant their eggs in the leaves, making leaf tissue to swell up and the larvae live inside....Ninjaw —Preceding unsigned comment added by 124.120.215.21 (talk) 14:57, 31 August 2008 (UTC)
Freaks
I knew a girl with an additional vertebra in her neck. I knew someone whose uncle had three kidneys. My own mother grew a third tooth in her early 50th. I heard of people who have the heart not only in the right place but also on the right side, instead of the left. All those people are healthy and without examination undistinguishable from those people the anatomy books write about. How common are those uncommon features the like described above? What else can there be? 93.132.129.229 (talk) 19:15, 29 August 2008 (UTC)
- It's possible to have an extra one (or an extra set) of many body parts, but often the extra parts are not fully formed. The supernumerary nipple article says the incidence for nipples is 1 in 18 people, but an extra one is often mistaken for a mole. The article on polydactyly (extra fingers and toes) gives an incidence of 1 in 500, but again, most of these are not fully developed. --Anonymous, 19:50, August 29, 2008.
- Having the heart on the wrong side is call dextrocardia, that article says it occurs in approximately 1 in 12,019 people. --Tango (talk) 20:18, 29 August 2008 (UTC)
- I have that but with all the organs. JessicaThunderbolt 20:24, 29 August 2008 (UTC)
- And I wouldn't say it's the wrong side, just the less popular. Sure you have seen alot of dumb faces from new doctors? 93.132.129.229 (talk) 20:36, 29 August 2008 (UTC)
- Nah, they all know what it is, but sometimes they get a bit excited as it's the first time they've seen it IRL. JessicaThunderbolt 21:01, 29 August 2008 (UTC)
- I'm intrigued...are you left or right handed? And do you think you conform to the stereotype for whichever handed you are? (Wondering whether your brain is also left/right flipped). SteveBaker (talk) 21:40, 29 August 2008 (UTC)
- Nah, they all know what it is, but sometimes they get a bit excited as it's the first time they've seen it IRL. JessicaThunderbolt 21:01, 29 August 2008 (UTC)
- And I wouldn't say it's the wrong side, just the less popular. Sure you have seen alot of dumb faces from new doctors? 93.132.129.229 (talk) 20:36, 29 August 2008 (UTC)
- There's a vast range of asymptomatic variation from the 'normal' human body. Perhaps the biggest culprit is the circulatory system, where just about any variation on a theme is possible. Check out the entry in Gray's Anatomy on the aortic arch. Normally three blood vessels (the innominate, the left common carotid, and the left subclavian) branch off the aortic arch. However, variations have been observed with but a single branch and with as many as six branches. (If you go through the 1918 Gray's, you'll find that a large number of sections contain information about 'Peculiarities'.)
- This much more recent article notes that external landmarks are unreliable in locating the internal jugular vein in about a quarter of all patients. Moving away from the circulatory system, here's an article about a patient who was missing the upper lobe of his right lung (the rest of the lung just expands to fill the space; he showed no symptoms). In somewhere from 0.1 to 0.7% of people, the gallbladder is beneath the left liver: [15]. TenOfAllTrades(talk) 01:41, 30 August 2008 (UTC)
Standard Units for Quantities of Lumber
Can someone please tell me what is the "standard unit" used for quantities of lumber? My question was triggered by a note taken from Wikipedia:Manual of Style.
"if the text contains an obscure use of units (e.g., five million board feet of lumber), annotate it with a footnote that provides standard modern units, rather than changing the text of the quotation."
Thanks, Wanderer57 (talk) 20:09, 29 August 2008 (UTC)
- Board foot says it's a measure of volume, so the standard unit would be metres cubed. --Tango (talk) 20:19, 29 August 2008 (UTC)
- 1,000 board feet is 2.36 cubic meters. SteveBaker (talk) 21:07, 29 August 2008 (UTC)
- "Board feet" estimates the recoverable sawn lumber based on the size of the smaller end of the log, and "log rules" based on old growth timber with 1/4 inch saw kerfs do not accurately estimate the board feet of dimensional lumber recovered with modern thin kerf saws and computerized sawing. The delivered dimensions of nominal 2 x 4 , 2 x 10 inch etc lumber was made smaller in the 1960s, further changing the board feet contained in a tree. A modern 2x4 is 1.5 inches by 3.5 inches, or .656 actual board foot per nominal board foot. The "board feet" in a log changes with definition of a 2x4 and with sawing technology. The cubic meters includes the "total volume of sound wood" including dimensional lumber, chips and sawdust [16]. It is not at all as direct as converting feet to meters. The 1950 conversion was 4.53 m3 per thousand board feet, but conversion factors up to 6.7 m3 have been found to apply. Edison2 (talk) 21:12, 29 August 2008 (UTC)
August 30
about electrons...
why are electrons easier to add or remove from an atom than a proton or neutron ? —Preceding unsigned comment added by 122.163.232.226 (talk) 04:20, 30 August 2008 (UTC)
- Because they're held by the electromagnetic force, whereas protons and neutrons are subject to the much stronger nuclear force. Clarityfiend (talk) 04:53, 30 August 2008 (UTC)
- Or, equivalently, because they are farther distant from the nucleus. --Ayacop (talk) 07:20, 30 August 2008 (UTC)
- Protons will be taken up by bases. In fact a loose proton will react with almost any other substance, (even including helium to make helium hydride or hydrogen to make H3+) so a proton is not hard to add to something, it is extremely reactive! Neutrons are easily absorbed in many nuclei. Electrons are very light, but yet not many negative ions are stable. Graeme Bartlett (talk) 09:00, 30 August 2008 (UTC)
- Ayacop, that isn't equivalent. They're held by different forces. The protons are actually repulsed by the force that holds the electrons in.
- Graeme, You're talking about adding and removing protons from a molecule. 122 is talking about adding it to an atom. If you added a proton atomically to a hydrogen atom, you'd get Helium-2, which would immediately decay into Deuterium, and emit a beta particle. This is nuclear fusion, and is very different, and far more difficult, than if you just added a proton molecularly and made H2+. — DanielLC 15:58, 30 August 2008 (UTC)
What's stopping electrons from actually hitting and joining the nucleus along with the protons and neutrons? ScienceApe (talk) 03:24, 31 August 2008 (UTC)
- Among other reasons, the Pauli_Exclusion_Principle. However note that electrons on s orbitals have the peak of their wavefunction right in the center of the core. —Preceding unsigned comment added by 84.187.60.4 (talk) 12:08, 31 August 2008 (UTC)
- In the case of the s orbitals, it's because the nucleus is so tiny. The waveform of the electron can collapse anywhere in the s orbital, and is more likely to do so closer to the nucleus, but it's still not likely enough to make the electrons hit such a small target. The Pauli Exclusion Principle just explains why not all of the electrons are in the smallest orbital, and doesn't have much to do with the question. — DanielLC 16:12, 31 August 2008 (UTC)
Purple skin
Why do some people's skin turn purple when they are cold? Does it have something to do with the blood? Minor Contributer (talk) 05:25, 30 August 2008 (UTC)
- yes, the blood has its circulation rate reduced in the cold skin so that it does not lose heat. When the blood has oxygen consumed it changes colour from bright red to a dull red or purple. Combined with the bluish colour of empty skin it can look purple. Graeme Bartlett (talk) 09:04, 30 August 2008 (UTC)
- Why do some people turn purple more quickly or more oftenly than others? Minor Contributer (talk) 15:24, 30 August 2008 (UTC)
- Having poor circulation can do it. You may find Raynaud's syndrome interesting. --Tango (talk) 18:50, 30 August 2008 (UTC)
- Another obvious thing, the lighter your skin the easier it would be to see any change in blood colour Nil Einne (talk) 01:16, 31 August 2008 (UTC)
- Having poor circulation can do it. You may find Raynaud's syndrome interesting. --Tango (talk) 18:50, 30 August 2008 (UTC)
- Why do some people turn purple more quickly or more oftenly than others? Minor Contributer (talk) 15:24, 30 August 2008 (UTC)
Optical Activity
Hi all,
I was just wondering how do you determine if a moleucle is optically active and why (or explaining why it is/isn't optically active). I know if a molecule has symmetry (i.e. it is achiral) it is not optically active, also, if a molecule does not have a chiral carbon it too is not optically active. But how do i determine if something like 2-bromopentane or 2-bromo-3-methylpentane (both formed from an addition reaction) is optically active?
Thanks heaps —Preceding unsigned comment added by 122.108.169.75 (talk) 05:26, 30 August 2008 (UTC)
- If a molecule is not symmetric, it is optically active. So 2-bromopropane is optically active (and you can look up its specific rotation in the usual literature sources, maybe even on wikipedia). Now whether a particular sample of the compound has optical activity, that takes a physical measurement in a polarimeter. If you have a 50/50 mixture of both enantiomers of a compound, the effect of each will cancel the other and the net rotation will be zero. DMacks (talk) 06:35, 30 August 2008 (UTC)
So does optical activity only depend on the symmetry of a molecule? what if I know that there was a satureated molecule (alkene) and it under went an addition reaction to give me 2-bromopentane ? does the addition reaction tell me anything? Also, does a chiral carbon tell me anything about the optical activity of a molecule? —Preceding unsigned comment added by 122.108.169.75 (talk) 07:04, 30 August 2008 (UTC)
- Optical activity does not depend on symmetry, nor on chirality, i.e. asymmetric molecules need not have a specific activity. That's why you see compounds with (+) and (-) labeled in addition to D- and L- specification. See also cryptochirality. Optical activity has to be measured. --Ayacop (talk) 07:17, 30 August 2008 (UTC)
- Knowing the optical activity of a sample of a compound that is not symmetric can tell you whether the sample is just a single isomer or is a mixture of stereochemistries. But you would need some (potentially difficult) experiment or (very easy) literature search to figure out which isomer you have based on its optical activity. If you know whether your sample is "optically pure" (i.e., a single enantiomer) vs a racemic mixture, you can know something about the reaction mechanism. DMacks (talk) 07:36, 30 August 2008 (UTC)
My homework question asks to explain why 2-chloropentane is not optically active after it is formed via the addition reaction of pent-1-ene and HCl. the molecule is not chiral, so i thought would be optically active, however, how do i explain it is not? —Preceding unsigned comment added by 122.108.169.75 (talk) 08:22, 30 August 2008 (UTC)
- one molecule of 2-chloropentane is chiral, with the number 2 carbon being substituted with hydrogen chlorine methyl and propyl. However which side would the chlorine add on? There is nothing to determine a left or right hand chirality, so both are made. Graeme Bartlett (talk) 09:11, 30 August 2008 (UTC)
Sorry i had meant to say that i know the molecule (2-chloropentane) is not achiral, so being that it is not symmetrical, shouldn't it be optically active? so why isn't it? —Preceding unsigned comment added by 122.108.169.75 (talk) 10:19, 30 August 2008 (UTC)
- I think DMacks hinted at the answer above and Graeme Bartlett gave you an even stronger hint. Take a look at racemic mixture. Gandalf61 (talk) 15:46, 30 August 2008 (UTC)
Distortion in half-wave rectifier.
Why distortion happened in half-wave rectifier with high frequencysRonilove (talk) 09:29, 30 August 2008 (UTC)
- Because it chops off half the wave, so the output does not resemble the input. This is if you are not using it as an amplitude modulation demodulator. Distortion could arise in demodulcation, because after rectification you filter with a capacitor to remove RF, and this could reduce high frequencies. Graeme Bartlett (talk) 10:13, 30 August 2008 (UTC)
- I wonder if there might be more distortion at high frequency because of the time of the rectifier to turn on, and the bias level for turn on, and if this might cause part of the waveform to be clipped off. What is "high frequency" depends on the response of the rectifier, its capacitance and other factors. Edison2 (talk) 19:30, 30 August 2008 (UTC)
- Some context from the OP would help here. Fourier analysis of an unfiltered half-wave rectified sine wave shows that it has a very high harmonic distortion content anyway. SpinningSpark 21:35, 30 August 2008 (UTC)
Cause of Quantum Decoherence?
According to the section Problems of the article Quantum computer, there are number of practical difficulties in building a quantum computer. One of the major difficulties is keeping the components of the computer in a coherent state. But I still get no sense of what interaction will cause the system to decohere... Is it environmental temperature? some form of noise? CMB? movement of planets, stars? cosmic inflation? Any ideas? Thanks - Justin545 (talk) 11:45, 30 August 2008 (UTC)
- What I've usually heard is thermal noise. With say a silicon chip with a quantum dot on it, the chip emits a photon which hits the dot and ruins its superposition. Or the dot could emit a photon that hits the chip. Because of this, proper cooling is a preequisite of many quantum computer blueprints. EverGreg (talk) 15:43, 30 August 2008 (UTC)
Inventing VS Engineering
What exactly is the difference between an inventor and an engineer?
I was under the impression that an inventor is just an amateur engineer, is this true? —Preceding unsigned comment added by Kenjibeast (talk • contribs) 15:41, 30 August 2008 (UTC)
- Inventor and engineer should help you differenciate the two. "Engineers are concerned with developing economical and safe solutions to practical problems, by applying mathematics and scientific knowledge while considering technical constraints." and "An inventor is a person who creates or discovers a new method, form, device or other useful means." are the key sentences. —Cyclonenim (talk · contribs · email) 15:46, 30 August 2008 (UTC)
- An inventor is someone who had the idea for a device and usually also built it. This someone dosn't have to be an engineer by education. An engineer plan, develop, improve and build devices among other things, but can do their job fine without coming up with a new, original device. There's of course a grey area between an improved and a new device, but you can generally say that the person is an inventor of a new device if he/she can get a patent on it. Also see the term inventor in patent law EverGreg (talk) 15:53, 30 August 2008 (UTC)
- There's no reason an inventor has to be an amateur. And there are plenty of inventors that are not engineers. Consider how many patents are filed each year by physicists, biologists, etc. --98.217.8.46 (talk) 17:22, 30 August 2008 (UTC)
- "engineer" is more of a job title. "Inventor" is just a description of something you've already done. APL (talk) 19:09, 30 August 2008 (UTC)
- In modern times to call yourself an "engineeer" you need to be a graduate of an accredited college in an engineering curriculum. Experience and the passing of tests are required to be a "Professional engineer" entitled to do consulting for others. Some famous inventors of 19th century electrical gadgets, like Samuel Morse and Alexander Graham Bell knew virtually nothing about electricity and had assistants who understood electricity actually develop and build the invention, based on their idea. Others like Thomas Edison , Nikola Tesla and the Wright Brothers were knowledgable of the intricacies of most of their "inventions" and many qualified as engineers by the standards of the time. An invention is often an improvement on someone's earlier idea that did not quite work in a usable sense, perhaps incorporating other improved technologies. (edited)Edison2 (talk) 19:27, 30 August 2008 (UTC)
Spider in My Front Yard

Can somebody identify this spider I found in my front yard? Thanks.--Xp54321 (Hello! • Contribs) 19:21, 30 August 2008 (UTC)
- When asking this kind of question it's important to tell us what part of the world you live in. Australia, Scotland and Mexico have very different bugs. --Sean 19:35, 30 August 2008 (UTC)
- Actually it looks a lot like a Orb-weaver spider which is found in almost every corner of the globe. (If someone finds one on Mars I would be surprised but not shocked.) —Preceding unsigned comment added by 71.100.4.91 (talk) 21:00, 30 August 2008 (UTC)
- I have re-arranged your photo so that it doesn't go into the next section. Right-floating is better for left-to-right languages like English.--antilivedT | C | G 00:18, 31 August 2008 (UTC)
- Thanks!--Xp54321 (Hello! • Contribs) 00:22, 31 August 2008 (UTC)
- I have re-arranged your photo so that it doesn't go into the next section. Right-floating is better for left-to-right languages like English.--antilivedT | C | G 00:18, 31 August 2008 (UTC)
Helicopters unable to fly above a pit
Here is an interesting question found on this talk page of this article Udachnaya pipe
"should there be something noted about helicopters unable to fly above the pit? from what has been said or what ive heard, flight above the area causes damage to the helicopter, for example being completely destroyed. i am unsure of the research required, and would appreciate help in this area as with being unable to relocate the source of information, however use of the search query in google may provide sufficient sources of information. thanks."
16@r (talk) 19:48, 30 August 2008 (UTC)
- It seems really impossible that flight over such a thing would result in some mysterious damage to the helicopter. THe person who posted that doesn't seem to have any references or proof of any kind. I'd ignore it until/unless proof is produced. If that person attempts to add that information into the article without some pretty decent references - just revert it. Extraordinary claims require extraordinary evidence. SteveBaker (talk) 21:19, 30 August 2008 (UTC)
- I'm thinking something along the lines of very strong, unpredictable updraughts coming up from the pit walls - or perhaps some odd wind vortex effect due to the particular shape of the excavation... --Kurt Shaped Box (talk) 22:05, 30 August 2008 (UTC)
- Given the north latitude and the depth, it seems plausible that heat release from the relatively deeper earth might cause turbulent updrafting over the pit. Wind effects are also an interesting possibility. Franamax (talk) 22:25, 30 August 2008 (UTC)
- I'm thinking something along the lines of very strong, unpredictable updraughts coming up from the pit walls - or perhaps some odd wind vortex effect due to the particular shape of the excavation... --Kurt Shaped Box (talk) 22:05, 30 August 2008 (UTC)
- I've heard many, many times before that you can't fly over it with a helicopter, precisely because air currents -- but downdrafts rather than updrafts. I don't know whether that's true or just a story, but in any case, the idea of something that big affecting air currents certainly doesn't sound implausible to me. That said, though, they certainly wouldn't damage the helicopter by themselves, but they would make flying over the pit dangerous and could cause a chopper to crash. An example of these stories can be found at here. -- Captain Disdain (talk) 01:24, 31 August 2008 (UTC)
- A halfway decent pilot can cope with updrafts and downdrafts if he/she knows they are likely. Unexpected vertical air motion is kinda dangerous - but if you were flying over a big hole in the ground, you'd be expecting it. Look at the number of helicopter flights they take into an out of the Grand Canyon for example. Any steep slope or cliff-face can be the source of an updraft (if the wind is blowing up the slope) or a downdraft (if it's blowing the other way). Helicopters can operate reasonably close to cliff faces and large buildings without problems. So this can't be true. I wouldn't find it hard to believe that helicopters have crashed there - perhaps even due to some careless piloting and that might be the source of some kind of an urban legend - but I used to make flight simulators for helicopters and we'd simulate all kinds of vertical air motion (both expected and unexpected) that pilots would train for. This pit might make it harder to fly a helicopter over/into - but there is no way it's some kind of magical helicopter crusher that mangles anything that comes nearby - that's just ridiculous. SteveBaker (talk) 15:26, 31 August 2008 (UTC)
- I think (well, I've always assumed, anyway) that this has involved situations where the chopper is coming in low and actually going into the pit rather than flying a safe distance above it. That's got no basis on anything other than my imagination, though. -- Captain Disdain (talk) 17:59, 31 August 2008 (UTC)
- But even that should be no worse than landing on a helipad on top of a tall building. The up/down-drafts around those things are phenomenal but pilots seem to have no problem sticking landings on them all the time. SteveBaker (talk) 20:15, 31 August 2008 (UTC)
- I think (well, I've always assumed, anyway) that this has involved situations where the chopper is coming in low and actually going into the pit rather than flying a safe distance above it. That's got no basis on anything other than my imagination, though. -- Captain Disdain (talk) 17:59, 31 August 2008 (UTC)
- Sure. I mean, maybe it was some cowboy pilot with a couple of shots of vodka under his belt. Or just someone who wasn't really qualified to pilot a helicopter; after all this is Russia; I wouldn't be surprised to hear of someone who would never pass the FAA's standards screwing up. And I'm not putting the country down, mind you, but, uh, a lot of the time, they do things a little differently over there. Or maybe it never happened at all. Who knows?
- (Speaking of the way things are done in Russia, an unrelated, but amusing anecdote: friend of mine was shooting a science fiction action movie over there, and the production included cooperation from the local army guys, who were hired as extras. Someone in the prop department screwed up and they didn't have the scifi guns for them, so they simply arranged to dress up the troops' rifles on the spot. That was a couple of hundred AK-74s that were actually army property, altered so they could no longer be fired or even properly aimed, because of the scifi crap they welded on them. I mean, I'm sure they compensated them, but from what I understand, the decision was pretty much made on the spot, because they were in a hurry, but I kind of doubt the guy making the sale actually had authorization to sell working weapons to a movie crew. Nobody thought it was a big deal, but my friend, not being Russian, was kinda nervous about the whole thing. Later on they also blew up a whole bunch of aircraft... allegedly because it was cheaper, faster, and easier than using special effects. So, you know, the idea of some unqualified guy just jumping in a helicopter and crashing it when the air currents prove surprisingly treacherous strikes me as entirely credible. But really, who knows?) -- Captain Disdain (talk) 00:48, 1 September 2008 (UTC)
- I personally blame the Russian army's experimental EMP howitzer firing range just down the road. The one they haven't told us about yet. --Kurt Shaped Box (talk) 19:27, 31 August 2008 (UTC)
bipolar transistor
Is there a replacement or substitute for the old (1980) bipolar 100mhz, 115 volt, 10 amp, 100 watt complementary PNP/NPN transistors? —Preceding unsigned comment added by 71.100.4.91 (talk) 20:57, 30 August 2008 (UTC)
- Do you have a part designation for that? SpinningSpark 21:24, 30 August 2008 (UTC)
- You might find something here that will do the job. SpinningSpark 22:01, 30 August 2008 (UTC)
- Are you sure about the 100MHz? Finding a 100W Pdiss, 115v Vcb, 10A Ic transistor with that fT is going to be difficult if not impossible.--79.76.176.172 (talk) 02:15, 31 August 2008 (UTC)
Butterfly Bee Insect (Unknown)
Hello,
I have a question about an insect that keeps returning to my Butterfly Bush here in lower Delaware. The insect aforementioned is about the size of a large Bumble-Bee, it consists of a black and yellow striped pattern, wings like a bee, mouth and feelers of a butterfly, and an odd fan shaped tail. It does not seem aggresive as I have been studying it for the last two months, and mingles with the other insects feeding on the bush without incident. I could not find it or any reference in any insect books I have, or google. Any help you could give me would be outstanding.
- Thank You,
- Brent —Preceding unsigned comment added by 209.244.188.217 (talk) 23:13, 30 August 2008 (UTC)
- A bee hawk moth perhaps? Nanonic (talk) 23:23, 30 August 2008 (UTC)
- Or a related bug in the family Sphingidae. Deor (talk) 00:12, 31 August 2008 (UTC)
- Hemaris thysbe [17] is found in Delaware and would fit the bill.--Eriastrum (talk) 00:28, 31 August 2008 (UTC)
- Side note: I planted a few Butterfly Bush plants near the end of my sojourn in my ex-Ontario home with the hard-fought backyard naturalized (wilderness) area. This was for the express purpose of atrracting more butterflies, which they did. Much to my chagrin, given my "naturist" mindset, when I looked up the plant species, they both turned out to be of Asian origin and listed as potential invasive species in temperate North America. Luckily, I didn't water them enough at the start, so I didn't have to actually kill a living thing, my problem was solved after two months. But beware! - Butterfly Bush is not a native-adapted plant. Franamax (talk) 00:37, 31 August 2008 (UTC)
August 31
Magnetic "field lines"
Reflecting my utter confusion on some parts of physics, what are magnetic field lines? The classic grade-school experiment with iron filings on a piece of paper with a magnet below shows field lines; the aurorae are produced by "recombination of field lines in the magnetopause"; and every diagram concerning the magnetic force has those dashed lines from N to S. So is the magnetic force expressed along physically real lines through space? Why isn't the magnetic force expressed on a uniform gradient, why does it have to go through certain "lines"? Which of Maxwell's equations result in a field line where the magnetic field is especially concentrated? And how do we predict that "here be a magnetic field line"? Is this a conceptual tool or a real phenomenon? Confusedly yours, Franamax (talk) 01:04, 31 August 2008 (UTC)
- Magnetic field#Visualizing the magnetic field seems to deal with the 'field line' usage. We also have an article field line. I haven't read either, so I don't know if there're any good. Algebraist 01:08, 31 August 2008 (UTC)
- Conceptual tool. Magnetic field lines are contour lines that give the direction of the magnetic field at any point in space. There are an infinite number of (hypothetical) field lines.--79.76.176.172 (talk) 02:11, 31 August 2008 (UTC)
- The field lines indicate the direction a tiny compass needle would point if placed at that spot. It not the case that magnetic fields are present along certain lines, with points between the lines where the magnet would have no effect on a compass needle. As for iron filings, they tend to clump together and establish a pattern of lines or curves. If the magnet were left and the filings were removed and new filings shaken over the piece of cardboard, the clumpy curves woould be similar but in slightly different places. The closeness of the spacing of the "lines" is a helpful approximation the the flux density variation. Edison2 (talk) 06:23, 31 August 2008 (UTC)
- Hmmm. Why do the filings conveniently clump together into lines? Clarityfiend (talk) 06:26, 31 August 2008 (UTC)
- The field lines indicate the direction a tiny compass needle would point if placed at that spot. It not the case that magnetic fields are present along certain lines, with points between the lines where the magnet would have no effect on a compass needle. As for iron filings, they tend to clump together and establish a pattern of lines or curves. If the magnet were left and the filings were removed and new filings shaken over the piece of cardboard, the clumpy curves woould be similar but in slightly different places. The closeness of the spacing of the "lines" is a helpful approximation the the flux density variation. Edison2 (talk) 06:23, 31 August 2008 (UTC)
- I guess that each small splinter attains a N / S-pole. As they are linear in shape, the N-pole of one then "sticks" to the S-pole of the next one, thus forming curves / field lines. --Cookatoo.ergo.ZooM (talk) 10:03, 31 August 2008 (UTC)
- The ontological status of field lines can get a little complicated. I have read discussions on this very point by physicist/historian/philosophers. Sometimes they are treated as a mathematical construction, sometimes as a real physical entity. As far as I can tell, at least by conversations with plasma physicists, it's not totally straightforward. --98.217.8.46 (talk) 17:15, 31 August 2008 (UTC)
- This is the SCIENCE desk - and science is all about experiments. Here is an easy experiment: Take your fridge magnet, a sheet of paper and some iron filings. Mark the location of the magnet under the sheet of paper, sprinkle your iron filings and trace along the lines that appear with a pencil. Now remove the magnet, shake all the iron filings off of the paper and do the exact same experiment again (making sure you replace the magnet in the exact same spot. If the field lines from the second experiment consistently line up with those from the first experiment no matter how many times you try - then you have good evidence that field "lines" exist because they are a reproducible phenomenon. If they don't line up - if the lines appear in different places each time you do it - then they are a side-effect of the way iron filing rotate to align with the direction of the field and clump together to make "lines". It's my belief that the lines are not real - magnetic fields are continuous functions just like any other field. But hey...go ahead and prove me wrong! SteveBaker (talk) 19:47, 31 August 2008 (UTC)
- I get confused when I read in Nature a summary of a paper in Science about "a realignment of Earth's magnetic field lines...lead to luminous polar auroras". It does go on to say the "field snaps back into place". On reflection, I can however buy into the idea that the iron filings clump due to N-S attraction between the individually ferromagnetized particles, presumably the clumping mechanism is stochastic. I'm interested though in 98.217's comment - the geo(/space?)-physicists do tend to talk explicitly about "a" field line reconnecting. Franamax (talk) 22:58, 31 August 2008 (UTC)
- Right, that's the issue, I believe. Usually they are regarded as SteveBaker describes but often they are described as being able to break, reconnect, etc., which implies something a little more concrete. I seem to recall the person in question mentioning magnetic reconnection as one of the issues, but honestly, it has been awhile and this sort of intimacy with physics has never been to my taste. --98.217.8.46 (talk) 05:17, 1 September 2008 (UTC)
Where could I buy lead ball bearings?
Preferably 20mm in diameter or so. ScienceApe (talk) 02:49, 31 August 2008 (UTC)
- Musket balls?--79.76.176.172 (talk) 02:57, 31 August 2008 (UTC)
- Nobody is going to make 'bearings' out of lead - the point of a bearing is to remain perfectly spherical under load - and lead would deform and be an utterly useless material - it's far too soft. However, you can buy spherical BB-gun pellets in lead. As '79 says, you could look for musket balls. Quite a few antique gun enthusiasts must need large diameter lead projectiles. However, I think a lot of them make their own by pouring liquid lead into suitable molds. You could do that too. SteveBaker (talk) 03:02, 31 August 2008 (UTC)
- Are you looking for ball mill grinding media? United Nuclear sells grinding media made of lead and antimony which are 12.7 mm in diameter. I'm sure other retailers sell grinding media closer to 20 mm if you search on the internet. Coolotter88 (talk) 20:01, 31 August 2008 (UTC)
Asteroids and comets
What plans are in place to deal with the asteroids and comets currently on a direct collision course with Earth?--79.76.176.172 (talk) 02:55, 31 August 2008 (UTC)
- Many asteroid deflection strategies have been proposed, but there are no concrete plans in place. In most cases, the choice of approach would have a lot to with the amount of preperation time available and the size of the threatening rock. Dragons flight (talk) 03:03, 31 August 2008 (UTC)
- And composition. A solid lump of rock requires a different approach to a loose pile of rubble. --Tango (talk) 03:06, 31 August 2008 (UTC)
- NASA have looked at a range of possibilities - but so far, none of them have been brought to the point where we could actually deploy them. Further testing is needed because there is a severe risk of simply breaking up an asteroid into smaller chunks - which would be just as dangerous and have a larger probability of splattering something important on impact. There are also concerns that errors in calculating the precise orbit of the body might result in us inadvertently turning a one in one ten chance of a problem into a certainty. The best chance we have to do this cleanly is to detect the problematic body many years before impact. The smallest nudge at that time will avoid a problem - but if you leave it until the body is merely months away, it's vastly harder. Right now, we can't detect mountain-sized bodies until they are weeks away. Before we do anything about deflection or destruction - we first need to put serious funding into detection - and that's really not happening. The short answer is that we currently have absolutely zero protection - and at present rate of progress, we probably won't have such protection for decades into the future. SteveBaker (talk) 03:09, 31 August 2008 (UTC)
- Oh shit! Well wouldn't it be a good idea if both Russia and America and anyone else were to get together to form a plan to detect and divert these objects which would probably terminate most of the life on earth rather than have petty squabbles about whose got more missiles in the others back yard?--79.76.176.172 (talk) 03:18, 31 August 2008 (UTC)
- Why? That is nothing more than opinion. This is a reference desk, not a place to try and get a debate going about how important it is to drop everything and try to fix a problem that will likely never happen while humans are still alive on our tiny little speck of a planet. -- kainaw™ 03:21, 31 August 2008 (UTC)
- Likely never? 99942 Apophis —Preceding unsigned comment added by 79.76.176.172 (talk) 04:38, 31 August 2008 (UTC)
- Actually it was a question (as indicated by the ? at the end) not opinion, but thats a remarkably cool attitude you have there! Just how sure are you that its probably not going to happen sometime soon?--79.76.176.172 (talk) 03:36, 31 August 2008 (UTC)
- See opinion and fact. Any question beginning with "Well wouldn't it be a good idea if..." is asking for opinion, not fact. This is not a discussion forum. There are thousands of discussion forums on the Internet. Use one of them if you feel such a desire to discuss opinions. -- kainaw™ 03:40, 31 August 2008 (UTC)
- He did follow up with a legitimate question regarding the probability of an impact event during which humans inhabit the Earth. ScienceApe (talk) 04:38, 31 August 2008 (UTC)
- What is your source for that statement that we can't detect mountain-sized objects until they are weeks away? My understanding was that our detection programs were pretty good. --Tango (talk) 03:46, 31 August 2008 (UTC)
- We detect and follow a great many objects that are only tens or hundreds of meters in size [18]. Most known objects are followed many years in advance. Yes there are unknown asteroids, and yes some could be very large, but the idea that we only see mountains right before they show up is simply not true. Dragons flight (talk) 05:16, 31 August 2008 (UTC)
- I think there is a big difference between detecting a NEO for the first time and tracking it once it has been detected - tracking is much easier than detection. If you look at this NASA list of recent and upcoming close approaches, you can see that some of the objects have been tracked over several years. But if you drill down to the (rather cool) Java interactive orbital simulation for each object, you can see that most of them (especially in the sub-1km diameter range) were very close to the Earth when then were first detected. 1998 UP1, for example, has been tracked for 10 years, but it makes a close approach once a year (because its orbital period is almost exactly 1 year) and when it was discovered in October 1998 it was a few days past that year's close approach. NEO tracking programs seem to concentrate on detecting objects that repeatedly cross the Earth's orbit and make close approaches every few years, so that we can track them and get an accurate enough fix on their orbits to determine whether they will be a danger in, say, 50 or 100 year's time. Gandalf61 (talk) 13:32, 31 August 2008 (UTC)
- That was, as you say, detected 10 years ago. Our detection programs have come on a long way since then - I would hope we would have spotted it sooner had it made the same close approach now. Also, I wouldn't consider sub-1km to be mountain sized. --Tango (talk) 14:22, 31 August 2008 (UTC)
- I don't know - a cubic kilometer of rock would look a lot like a mountain if you put it in your back yard! But let's consult (say) an encyclopedia: Mountain#Definitions says that in the US, a mountain has to be more than 610m tall and in the UK, over 914m. Something that's 1000m tall qualifies as a mountain. SteveBaker (talk) 16:09, 31 August 2008 (UTC)
- Yeah, I looked at those definitions and decided 1km was a good cutoff, so sub-1km wouldn't be mountain sized. Remember, these aren't going to be nice regular shapes, so the 1km presumably refers to the longest axis, the other will probably be significantly smaller. --Tango (talk) 19:30, 31 August 2008 (UTC)
- I don't know - a cubic kilometer of rock would look a lot like a mountain if you put it in your back yard! But let's consult (say) an encyclopedia: Mountain#Definitions says that in the US, a mountain has to be more than 610m tall and in the UK, over 914m. Something that's 1000m tall qualifies as a mountain. SteveBaker (talk) 16:09, 31 August 2008 (UTC)
- That was, as you say, detected 10 years ago. Our detection programs have come on a long way since then - I would hope we would have spotted it sooner had it made the same close approach now. Also, I wouldn't consider sub-1km to be mountain sized. --Tango (talk) 14:22, 31 August 2008 (UTC)
- I think there is a big difference between detecting a NEO for the first time and tracking it once it has been detected - tracking is much easier than detection. If you look at this NASA list of recent and upcoming close approaches, you can see that some of the objects have been tracked over several years. But if you drill down to the (rather cool) Java interactive orbital simulation for each object, you can see that most of them (especially in the sub-1km diameter range) were very close to the Earth when then were first detected. 1998 UP1, for example, has been tracked for 10 years, but it makes a close approach once a year (because its orbital period is almost exactly 1 year) and when it was discovered in October 1998 it was a few days past that year's close approach. NEO tracking programs seem to concentrate on detecting objects that repeatedly cross the Earth's orbit and make close approaches every few years, so that we can track them and get an accurate enough fix on their orbits to determine whether they will be a danger in, say, 50 or 100 year's time. Gandalf61 (talk) 13:32, 31 August 2008 (UTC)
- The object that caused the Tunguska event and the object that created Meteor Crater in Arizona were both about 50 m in diameter - tiddlers, but large enough to cause explosions in the 1-10 megatons range. NASA's minimum size threshold for counting an object as a potentially hazardous asteroid is 150 m diameter - see "What Is A Potentially Hazardous Asteroid (PHA)?" at this FAQ. An impact event from a sub-1 km object can still cause massive damage. Gandalf61 (talk) 17:49, 31 August 2008 (UTC)
- Sure, they'll hurt, but they're not end-of-world type events. Deaths would probably be comparable to other fairly frequent natural disasters. --Tango (talk) 19:30, 31 August 2008 (UTC)
- Well, the 50 meter object that caused the Tanguska event flattened 800 square miles of forest - certainly not an end-of-the-world event...for that you need something like a 1km rock. But translate Tanguska into 800 square miles of a major city - and you have perhaps a million people dead. That would make it the second or maybe third biggest natural disaster ever (after floods in China in 1931 and again in 1887). Of course the probability of such a thing is low simply because cities don't cover much area compared to the surface of the entire planet - so the probability of a direct city impact is small. But as I said before, the size of the disaster is potentially so huge that even with the relative rareity of the event - we really should be more concerned than we are. SteveBaker (talk) 15:57, 1 September 2008 (UTC)
- Sure, they'll hurt, but they're not end-of-world type events. Deaths would probably be comparable to other fairly frequent natural disasters. --Tango (talk) 19:30, 31 August 2008 (UTC)
- The object that caused the Tunguska event and the object that created Meteor Crater in Arizona were both about 50 m in diameter - tiddlers, but large enough to cause explosions in the 1-10 megatons range. NASA's minimum size threshold for counting an object as a potentially hazardous asteroid is 150 m diameter - see "What Is A Potentially Hazardous Asteroid (PHA)?" at this FAQ. An impact event from a sub-1 km object can still cause massive damage. Gandalf61 (talk) 17:49, 31 August 2008 (UTC)
Here is some data for the not-so-worried:
- Tunguska_event#Similar_events shows that megaton-range events happened in 1908 and 1930 and many multi-kiloton (bigger-than-Hiroshima) sized events have happened in the last 50 years or so.
- 50m rocks hit us about every thousand years and can level 800 square miles of land (such as in the Tunguska event in 1908) - an explosion with minimum 5 megatons.
- 1km rocks hit us about every half million years.
- 5km rocks hit us about every ten million years and produce a 100km crater and perhaps 50 megatons of explosive power - the debris would blot out the sun and to terrible things to our crops...billions of people could die of starvation within a year.
- The dinosaurs were likely wiped out by a 10km rock - some of humanity might survive it - but probably 99% of humans would die.
So - there is a 1 in 1000 chance per year of a random 800 square mile chunk of the planet getting wiped out with the force of a very large nuclear weapon - from a rock that's WAY smaller than we're able to detect even at the very last minute. Every decade or so we get hit with rocks that could kill a million people in the very unlikely event they might hit a city. Previous replies suggest that a 1km rock may or may not be detectable soon enough to do something - but it could take out an enormous amount of people and/or cause massive tsunamis.
Once every half million years for a really big rock may sound like a pretty remote chance - but the consequences would be extreme.
The problem with this kind of thing is to balance the probability of it happening versus the scale of the consequences. We spend an immense amount of money to protect ourselves from very probably events (car crashes, house fires, medical mishaps) that are very likely - but which don't affect many people at a time. We have a blind-spot to very infrequent/unlikely events that would take out half the planet. That's an odd thing. It really doesn't matter (on the grand scheme of things) how many people get killed in car crashes - but if a mountain of rock were to wipe out almost all of humanity - then that's something we need to pay attention to.
If there is a one in 500,000 chance of a meteor killing (say) 5 billion people each year (that's only considering the really big rocks) - that's an average of 10 out of every 100,000 people per year. OK - now let's examine: List of causes of death by rate. 20 people per 100,000 get killed in car crashes worldwide (it's more likely in places like the USA - but less likely in (say) India) - so you are twice as likely to die in a car crash than by the consequences of a 10km meteor. The probability of dying in a killer meteor collision is comparable to the chances of dying from one of the more common cancers or a random act of violence (eg a crime, a mass murderer or a domestic dispute). So, from an individual person's perspective - we should be spending at least as much on looking for and deflecting 1km meteors as we do on trying to eliminate street violence. Death by meteor is quite a bit more probable than death by Altzheimer's disease. You are maybe twice as likely to die from a meteor than from your house burning down.
From a purely statistical perspective, we should spend more on meteor protection than on (say) airbags in cars, Altzheimer's research or smoke detectors because no matter how much you spend, you'll never eliminate those things - where there is every chance that would could provide 100% protection against 1km+ meteor strikes. As a species, we should place more 'weight' on a potential species-obliterating-event than on more probable events that (while killing the same number of people on average) are no risk whatever to the survival of our species. You can go one further by saying that we'd also be protecting most other species on earth from obliteration too.
That's only considering the very infrequent planet killers - I guarantee that the first time a once-a-decade sub-50m rock hits a major city, delivering a few hundred Hiroshima-sized bombs worth of damage and perhaps killing a million people, we'll suddenly become VERY focussed on those smaller rocks.
SteveBaker (talk) 19:13, 31 August 2008 (UTC)
- You need to take into account cost:benefit ratios. You can almost completely protect a family with one smoke detector for a couple of quid. The cost per family of protecting against small asteroids is probably far far greater (I don't have any figures). Remember, even if we detect them, the cost of actually doing anything about it is probably going to be prohibitive if it's "only" going to save a million people. Using Deep_Impact_(space_mission) as a benchmark (a deflection mission seems roughly similar in parameters), we're talking about $330 million, probably more due to the need to do it quickly. $330 per person sounds pretty reasonable at first glance, but when you consider what else that $330 could be spent on (smoke detectors for 100 families, say), it may not be the best use of limited funds (and funds are always limited). --Tango (talk) 19:30, 31 August 2008 (UTC)
- You have the math totally wrong. You can only spread the cost of $330 million over a million people once you know which million people will be affected. Before you know which million you're going to have to save, you get to spread the cost over the entire planet. If $330 million were all it would take to protect six billion people from a one in a thousand chance of death-by-meteor per year, it would be a total bargin! You'd have to put $3 smoke detectors into a lot more than 110 million homes to save a million lives. Remember - death from house fires is 5 per 100,000 people per year. To save a million people over maybe the ten year life of your smoke detectors in a year you'd have to put them 1,000,000x100,000/(5x10) homes...that's two BILLION homes...which would cost six billion dollars - not $330 million...and (furthermore) they'd have to be 100% effective in preventing death from fires - which they obviously are not.
- If you are thinking about an after-detection calculation - then look at it like this: We spent considerably more than $330 per person on fixing up New Orleans AFTER Katrina - forget the deaths - the cost of reconstruction alone would more than pay for the mission to deflect a small rock. If a big rock was to hit New York and you asked the million people within the strike zone to pay $330 each to save their homes - I'm pretty sure they (or their insurance companies) would be more than happy to pay up! $330 million is NOTHING compared to the cost of a million lives. The government could justify it in terms of lost taxation revenue alone!
Linked earlier in this discussion, The Sentry Risk Table is an interesting read. It's fascinating how many serious impact events have better odds than a lottery ticket.
For instance, you'd need to buy 3,398 powerball tickets before your odds of hitting the jackpot were higher than the odds of an impact by 99942 Apophis.APL (talk) 19:27, 31 August 2008 (UTC)
- So asking people to pay $3,398 each for meteor insurance should be a no-brainer since dying is a much worse event than winning a lottery is a good event. SteveBaker (talk) 20:09, 31 August 2008 (UTC)
- Yes, but not playing the lottery ought to be a no-brainer, yet people still do it. --Tango (talk) 22:36, 31 August 2008 (UTC)
- Steve, I've got you covered, where should I send my PayPal account information? Special this month only, $3,000 even if you act now! I'll insure you against winning Powerball too. However I won't cover you for the much more liekly chance of getting hit by lightning or contracting the flesh-eating virus (which amazingly I'm not able to find a wikilink for). Franamax (talk) 22:44, 31 August 2008 (UTC)
- The lottery is a tax on the statistically-challenged. Sadly, I can't afford meteor insurance because I'm spending all my money on other forms of insurance! SteveBaker (talk) 03:34, 1 September 2008 (UTC)
- Oh - and the chance of being struck by lighting in any given year is about one in 300,000 - so that's 0.3 deaths per 100,000 - about 30 times less likely than dying from a planet-killing meteor strike.Necrotizing fasciitis is about half as likely to hit you as lightning...so about 60 times less likely than dying from a meteor strike. Hence it would cost you barely 5% more to throw in insurance for those other two conditions along with your meteor insurance. You see what I mean about people's failure to understand statistics? SteveBaker (talk) 04:27, 1 September 2008 (UTC)
- The lottery is a tax on the statistically-challenged. Sadly, I can't afford meteor insurance because I'm spending all my money on other forms of insurance! SteveBaker (talk) 03:34, 1 September 2008 (UTC)
- Necrotizing fasciitis. --Tango (talk) 00:33, 1 September 2008 (UTC)
- Yeah, that's the one. Why did I use "virus" in my search? I tells ya, we should ask Google to take over our search box! Franamax (talk) 01:52, 1 September 2008 (UTC)
- Hey, at least it's not lupus. --mboverload@ 01:54, 1 September 2008 (UTC)
Poisonous things that taste good
I want to compile a list of notoriously carcinogenic/poisonous chemicals that also have a palatable flavor/scent. For example Lead(II) acetate, cyanide (I've heard it smells like bitter almonds), etc.. I would be eternally thankful if you could help me on my way, thank you. Kenjibeast (talk) 06:20, 31 August 2008 (UTC)
- How about fat, alcohol (I personally don't like the taste but a lot of people seem to like it), sodium chloride, sucrose ... Nil Einne (talk) 06:57, 31 August 2008 (UTC)
- What about water? 93.132.155.98 (talk) 10:14, 31 August 2008 (UTC)
- Some like the smokey note which is definitely carcinogenic. --Ayacop (talk) 07:47, 31 August 2008 (UTC)
- Fugu, anyone? --antilivedT | C | G 07:51, 31 August 2008 (UTC)
- I thought about that but in that case I think it's questionable whether they really like the taste or simple the effect of the nicotine they're inhaling. I don't think the same thing can be said for alcoholic beverages because altho some people just want to get drunk, many people claim to like the taste too Nil Einne (talk) 10:59, 1 September 2008 (UTC)
- Most kinds of ant poison taste good because it contains sugar to attract the ants. But most kinds also contain strychnine. (Refer to "strychnine" in Wikipedia.) Because of the sugar content, ant poison should be strictly kept away from children. Dogs like sugar too. The safest kind of ant poison comes in a little sealed can that has small holes for the ants to enter. Andme2 (talk) 15:32, 31 August 2008 (UTC)
- The ability to smell hydrogen cyanide is genetic, though I assume non-detection is a side-effect of some more useful trait:) Phosgene smells nice. Lots of molecules are made of various sugars (as a class of compounds, not the specific "table sugar" and other sweeteners usually seen in foods. Some saccharaides are wickedly toxic, but don't know how they smell. Even some simple acetals smell nice but have toxic effects (first-hand experience here). DMacks (talk) 19:58, 31 August 2008 (UTC)
- I'll nominate ethylene oxide which apparently has a "faintly sweet" odour which I've thankfully never detected - they have honkin' big signs around the EO facilities in refineries and gas plants. And of course, there's benzene, which gave the name to the whole class of aromatic compounds, because it smells sweet while it kills you. Franamax (talk) 22:29, 31 August 2008 (UTC)
- Anecdote: I remember driving in for my safety training to a really quite large Texas refinery (Texaco Port Neches) and smelling the kinda pleasant smells wafting in from the hot Texas breeze (I'm Canadian, we don't roll our windows up on hot days). I also remember, after my safety training, sitting in the smoking area smelling those pleasant odours wafting about, and sucking on my cigarette so that its nice familiar toxic compounds could keep me safe. :( Franamax (talk) 00:01, 1 September 2008 (UTC)
- Ethylene glycol - found in antifreeze - slightly sweet smell - especially if heated. Very sweet and pleasant-tasting too. Many children and pets have died from drinking small amounts of the stuff, being fooled by the pretty colors it comes in and the super-sweet taste. The merest taste of the stuff is enough to require hospitalisation for a child. Ethylene glycol poisoning has a good deal to say about this nasty stuff. SteveBaker (talk) 03:29, 1 September 2008 (UTC)
Death caps ScienceApe (talk) 21:33, 1 September 2008 (UTC)
Breakdown via anti-hydrogen
Could several anti-hydrogen atoms be used to take one proton, one by one, from a neculeus of a normal atom, stepping it through alot of different elements? Or would the normal atom usually decay into two seperate atoms if hit by a antiproton etc? —Preceding unsigned comment added by 58.108.249.161 (talk) 10:09, 31 August 2008 (UTC)
- The energy released by a proton-antiproton annihilation event is on the order of 2 billion electronvolts (2 GeV). The binding energy of an atomic nucleus is – for most nuclei – somewhere around 8 million electronvolts (8 MeV) per nucleon (proton and neutron in the nucleus). See our article on Binding energy#Nuclear binding energy curve for specific numbers there. The energy available from the annihilation is more than a hundred times the energy required to blow an extra proton or neutron out of the nucleus.
- To be fair, the actual efficiency of transfer of annihilation energy to the nucleus is actually nowhere near 100% efficient. The first publicly available study I found was this one (warning, 7.0 MB PDF). They found that 120 MeV (on average) is transferred to a carbon nucleus, and about 450 MeV is transferred to a uranium nucleus. (That pretty much covers the gamut of atomic weights.) In any event, those energies are quite sufficient to drive fission events, so 'stepping' through elements one by one isn't likely to occur. TenOfAllTrades(talk) 14:59, 31 August 2008 (UTC)
- Also, the probability of exactly one anti-hydrogen actually hitting your one atom is essentially zero. Even if this worked - you'd have to fire a lot of anti-hydrogen at a lot of your chosen material. Then you'll have no control of how many anti-hydrogens hit each atom in your material. So even if you could somehow evade the problems that TenOfAllTrades brings up - you'd still be doomed. SteveBaker (talk) 15:14, 31 August 2008 (UTC)
- Electron capture could be interesting, though I don't see how to force it on atoms not prone to it (using myons?). But this way you would have excess neutrons very soon. 93.132.155.98 (talk) 17:06, 31 August 2008 (UTC)
- (You meant muons, right?) --Anon, 17:36 UTC, August 31.
- Right! 93.132.155.98 (talk) 17:41, 31 August 2008 (UTC)
- (You meant muons, right?) --Anon, 17:36 UTC, August 31.
DNA inbreedinng
Do we have an article that explains why inbreeding/incest etc. causes DNA defects and why its eveolutionarily beneficial for genes too not 'mate' with genes that are similair to themselves?--58.108.249.161 (talk) 10:41, 31 August 2008 (UTC)
- Have you looked at inbreeding? SpinningSpark 11:05, 31 August 2008 (UTC)
when to plant nectarines from saved pits
I've been eating a bunch of nectarines and peaches lately and I've saved all the pits so I can plant them (mostly nectarines, but a few peaches too). I'd just like to know when would be a good time to plant them and any tips on growing the trees. In case you're wondering, I live in Northeast Kansas.
Also, I just noticed that some of the pits are getting tiny little spots of mold and I'd like to know how to safely store the pits until they can be planted. should I discard the pits that have mold?
One more thing. We have lots of little wild plums around here (I cannot identify the specific species since I'm not a botanist :P), but I know that plums are in the same genus as peaches and nectarines (prunus) and I'd like to know if they would accidentally cross-polinate. If so, would this negatively affect the quality of the fruit?
Thanks ahead of time for your help! 63.245.152.68 (talk) 11:48, 31 August 2008 (UTC)
- For growing you will have to crack the pit open as the actual seed is inside, but after that there are not really any special preparations needed, just put the seed straight into some ordinary soil and keep it damp. It will take a long time to germinate though. Also try planting several seeds to increase the chances as in nature it is very rare for all offspring to survive. Remember that if you are growing them to eat, fruit grown from a seed almost never tastes the same as the fruit the seed came from. This is because the fruit you buy in the shops is bred asexually (basically clones the plant) and may have been crossed thousands of times to get it just right, but when you grow a seed it has had its genes all mixed up in independent assortment so you could get just about anything, though most of the time they just taste really bitter. Then again you could get a better fruit than the original, it's a lottery. JessicaThunderbolt 14:47, 31 August 2008 (UTC)
- I've found that too. If you have a friend with a good tree - get the pit from that, then you'll have a vastly better chance - also, you'll know that that variety of tree will grow in your local climate. SteveBaker (talk) 15:11, 31 August 2008 (UTC)
- 'twould seem to me that when the fruit is ripe and falls to the ground would be about right? Saintrain (talk) 23:33, 31 August 2008 (UTC)
I did notice that if you crack the pit open there's a little seed in there that actually resembles an almond (which makes sense as almonds are also in the genus prunus), but it never occurred to me to plant that. I guess it makes a lot more sense that way, though. I'm aware that the fruit probably won't meet my expectations, but I have about 15 pits so I'm bound to get some good fruit someday. About how long does it take for the seeds to germinate? and after they do, how long does it take for them to grow big enough to make fruit?
I wonder if you can eat the almond-like seeds or if they have some sort of poison in them. I'm sure not gonna try, but I am curious.63.245.152.68 (talk) 11:50, 1 September 2008 (UTC)
- The peach trees I have seen grown from seed have fine tasting fruit, but the fruit is a little smaller.Polypipe Wrangler (talk) 03:36, 2 September 2008 (UTC)
- got about twenty fruit after 7 years, (tree about 2 m tall) but that was with a drought and slow growing conditions.Polypipe Wrangler (talk) 03:39, 2 September 2008 (UTC)
lipid movement in a liposome..
I would like to know a few methods using which one could track the movement of a lipid in a liposome. In the sense, suppose I want to keep observing a lipid molecule for say, 5 minutes, then how do I do that? —Preceding unsigned comment added by Psruthi16 (talk • contribs) 14:29, 31 August 2008 (UTC)
- An admitted wild guess here (which I'm not supposed to do), but our article on fluorescent proteins indicates that they can be palmitoylated to mGFP. Palmitoyl is a fatty acid so possibly the GFP-palmitoyl complex could be incorporated into a lipid molecule? However, getting that complex through the liposome membrane would be a different story. Other than that, I got nothin'. Franamax (talk) 22:21, 31 August 2008 (UTC)
Mozzy bites
What the best thing to rub on Mosquito bites? Does spit really help? --217.227.97.121 (talk) 14:56, 31 August 2008 (UTC)
- I'm surprised it doesn't mention witch hazel, which works for me every time. Use two applications five minutes apart.--Shantavira|feed me 15:25, 31 August 2008 (UTC)
- Wikipedia - the encyclopedia you can edit! SteveBaker (talk) 16:04, 31 August 2008 (UTC)
- I'm surprised it doesn't mention witch hazel, which works for me every time. Use two applications five minutes apart.--Shantavira|feed me 15:25, 31 August 2008 (UTC)
Unknown insect
Found a insect that was thought to be an armored snail, it has slimy feet of a snail, but the head of a catapiller, and the shell of a pill bug(rolly polly bug). Cannot find any information on this insect?216.236.163.183 (talk) 15:23, 31 August 2008 (UTC)
- It seems unlikely to be an insect. Adult insects all have six legs and a three-part segmented body (head, thorax, abdomen). SteveBaker (talk) 16:00, 31 August 2008 (UTC)
- This sounds like a joke question, but I'll assume good faith. You should provide the location and a photograph of the creature to help people here make an indentification. Jdrewitt (talk) 16:05, 31 August 2008 (UTC)
- It could be a cherry slug or pear slug. (Why is there no article on this topic?)Graeme Bartlett (talk) 21:35, 31 August 2008 (UTC)
- Cherry/Pear slugs (they are both Caliroa cerasi) aren't slugs at all - they are Sawfly larvae from the Tenthredinidae family - we don't have articles about any of that family. The photo at right comes from Wikicommons - but it doesn't seem to be used anywhere in Wikipedia. It looks like the sawflies are a taxonomic mess - a bunch of more or less unrelated species jammed together because they share the same lifestyle - not because they are genetically related. In these cases, the names of the animals may change - but the references I can find in agricultural web sites may well be using some older name. It kinda fits the OP's description - except for the feet. SteveBaker (talk) 03:18, 1 September 2008 (UTC)
- Maybe he thought that it has a snail's foot due to its slime secretion?--Lenticel (talk) 00:46, 2 September 2008 (UTC)
Magnetic cows
There is a story that's been all over the news media over the last few days about researchers looking at Google Maps and finding that cows (and other herd animals like deer) have a preference for aligning themselves North/South. I'm trying to find out whether they mean that the cows all face with their noses to the north - or whether 50% of them are facing north and 50% south. I've been thinking that a possible reason for doing this (None of these reports seem to be suggesting reasons) is that it might be a way for the cows to arrange for the herd to get good all-round vision for predators. They have eyes out on the sides of their heads to improve their field of view - but they must have a blind-spot behind their thick, juicy, tender rear ends. If the cows pointed in utterly random directions, they'd have pretty good 360 coverage - but if they all pointed North - that would mean that they deliberately evolved a blind spot! However, if 50% faced north and 50% south that would guarantee no blind-spots and they could be using the earth's magnetic field to dramatically reduce the probability of a blind-spot. Has anyone heard a theory for this? SteveBaker (talk) 15:55, 31 August 2008 (UTC)
- Am I the only one who has an image of a cow suspended on a piece of string slowly rotating to point north? Seriously, the first thing that crosses my mind is that they are putting the sun to their backs to avoid the glare. Is there any information on cows in Australia or South America? If they face South rather than North that would indicate that it is not magnetic field related. SpinningSpark 16:22, 31 August 2008 (UTC)
- Here is the study. It's not clear in the abstract, but I believe they were unable to determine from satellite images which way round cows were standing. The deer tend to be head-north though. To Spinningspark: they tested the magnetic hypothesis by looking at areas where magnetic north is significantly different from true north. It turned out magnetic north wins. Algebraist 16:34, 31 August 2008 (UTC)
- ...and, according to this summary in The Economist, the researchers averaged out the effects of sun and wind direction. Gandalf61 (talk) 17:13, 31 August 2008 (UTC)
- (ec) This report from the BBC says that the deer (all observed in Czech Rebublic) were 2:1 in favour of North over South and as Algebraist said, the satellite images of cattle could not resolve head from rear. It does say though, that cattle in Africa and S America are more NE/SW than N/S and speculate that this is because the field is weaker in these locations. SpinningSpark 17:16, 31 August 2008 (UTC)
- This sounds to me like what comes out of the back end of the cow. Cows point the way the boss cow is looking, because there might be something interesting there, and point the other way because they are a hierarchy and standing tranversely is inviting a fight. They orient to maximize warmth on cool days and into the breeze on hot days to keep the zillions of flies away from their eyes. However, if anyone has a copy of the full study, please email me, I'd love to read the whole thing. Franamax (talk) 22:05, 31 August 2008 (UTC)
- I drove past a herd of Texas longhorn on the way home an hour or so ago. They were definitely all facing the same way. Now I've checked on Google Maps - I'm 100% sure they were facing nore or less exactly South. So on one entirely not-statistically-valid sample, South wins. Of course that might be because all but one of them are facing the same way as the 'boss cow' - but that doesn't help answer the question: Why does the boss cow face predominantly along the North/South axis?
- Anyway - I'm bored with that one...I have a new theory. How do we feel about this one?
- At the map resolution at which these photos must have been taken in order to see cows at all, we must be talking about aerial photography done from a plane. Commercially-available satellite imagery is too fuzzy to pick out anything as small as a car - let alone a cow. To get pixels that resolve as a cow - but without telling you which is head and which is tail (as the researchers claim), you must be looking at image pixels that are perhaps a quarter of a meter across. Any higher resolution and the head end would be obvious. That would give you a "cow" that's perhaps 8 fuzzy off-white pixels. If the resolution were a half meter then a cow would be only be about two or three pixels. So what these guys are undoubtedly analysing is the direction based on perhaps just a handful of pixels.
- But suppose the aircraft doing the photography were flying east-west and taking a photograph on an oblique angle in order to cover a wide swath of land without having to fly backwards and forwards too many times. When Google stretch that photo to make it look like it was taken looking straight down, then a fuzzy ROUND splotch would turn into a fuzzy elliptical splotch that would be stretched along the north-south axis. So if the original resolution was not quite high enough to resolve the direction the cows were pointing then Google's image processing could easily make it look like they all face the same way...at right angles to the flight path of the aircraft. So...can anyone think why an aerial photography plane would always tend to fly East/West when doing it's photography run rather than North/South? Maybe some kind of air traffic control rules for the altitude they fly at? I know that aircraft flying in certain conditions are separated out at different altitudes depending on the direction they are flying...perhaps at the best altitude for photography, you have to be flying East or West?
- SteveBaker (talk) 02:40, 1 September 2008 (UTC)
- I only have one air chart (Helsinki), the Malmi and Vantaa approaches and holding zones are variously oriented. But wouldn't aerial surveys be flying in the uncontrolled zone anyway? On this chart, below 1000 feet near the airports and below 1500 feet everywhere else is fly-as-you-like. How high would a survey plane be flying? Franamax (talk) 09:43, 1 September 2008 (UTC)
- SteveBaker (talk) 02:40, 1 September 2008 (UTC)
- Thats the same thing i thought. I haven't seen the details of the study, at first you get the impression that its very professionally done, then later you get the impression that it is rather unprofessionally done. Also it should be pretty simple to test this in practice. Get some strong electromagents and put theem around a small field and see if you can get the cow to allign itself.
155.144.40.31 (talk) 05:36, 1 September 2008 (UTC)
- Or pick a cow up, move it a few thousand km east/west, put it down again and see if it turns slightly. What would happen if you put the magnet on the cow? Would it spin in circles? :) Franamax (talk) 09:32, 1 September 2008 (UTC)
- We just did it to freak you humans out. Joke's over, so moooove along. Sincerely, Elsie.
Anyone here who thinks to understand that article? It all looks like technobabble to me. The formula below the heading Time-slicing definition looks as if it's integrating over just one path, not over all possible paths. 93.132.155.98 (talk) 16:13, 31 August 2008 (UTC)
- Well, it's about quantum mechanics, what kind of babble did you expect? :) And it's about an idea developed by Richard Feynman, who had an extra several brains packed in his skull. Quantum theory is one of those areas where it becomes very difficult to meet the Wikipedia standard of "anyone should be able to read and understand the article". That can be done, but you'd need a box at the top saying "read these six hundred other articles first". Franamax (talk) 21:51, 31 August 2008 (UTC)
- You are right in general, this makes it so difficult to recognize technobabble. In this special case, line integral and path integral seem to be mixed up without warning. 93.132.132.172 (talk) 00:12, 1 September 2008 (UTC)
- The path integral is vastly easier to understand than that article makes it look; it's a very simple idea. There's a nice explanation on pages 7–9 of Chapter I of QFT in a Nutshell. We need an introduction like that in the article. If someone will draw the pictures, I'll write the text.
- The formula
- in the "time-slicing definition" section is integrating over all paths. A particular tuple of values represents a path in which the particle is at position at time , where are times between and . I don't know how they're choosing the n times or interpolating the position at intermediate times, but it doesn't matter because they're taking the limit (compare the definition of the Riemann integral).
- There isn't anything specifically quantum mechanical about the path integral. It works with any linear wave equation. Schrödinger's equation is linear, as are Maxwell's equations in vacuum. Maybe the article should introduce the path integral using water waves instead of quantum mechanics. -- BenRG (talk) 01:43, 1 September 2008 (UTC)
- Leaving the physics aside, I note that the integration variables are not part of the integrand. It is all but clear what the has to do with the . If the denoted one ot the it would be gone after integrating with it and of no use for the others. 93.132.132.172 (talk) 19:02, 1 September 2008 (UTC)
blood
what is the non-living part of blood? —Preceding unsigned comment added by 124.195.198.213 (talk) 16:39, 31 August 2008 (UTC)
- Blood plasma. And I'm not sure if red blood cells are considered 'living' as they don't contain DNA. 93.132.155.98 (talk) 16:47, 31 August 2008 (UTC)
- RBCs are certainly usually considered living. They have an active metabolism, and die in about 90 days. The definition of living doesn't usually include any criterion that DNA is present. Such a definition would classify some viruses as living and some as non-living on the basis of chemical constitutents rather than activity. - Nunh-huh 16:36, 1 September 2008 (UTC)
- I was to say that it didn't contain DNA (as usual in other human cells, and no RNA either, nor any other substance or information) to guide reproduction. (As a thought experiment, I consider candle flames alive and worker bees as dead. I don't really believe in that, but I can't pinpoint what would be wrong with that.) 93.132.132.172 (talk) 20:52, 1 September 2008 (UTC)
- Perhaps it would be best to keep such definitions of "living" very quiet...they're probably not helpful here, or if, say, you were to get a job as a coroner. Other than that, no harm no foul. - Nunh-huh 03:02, 2 September 2008 (UTC)
- The main objective of wikipedia is to give information to people, but what would that be to a people that doesn't also think? Making people think (for themselves, if ever possible) is a goal that I pursue and hope not to be in conflict with wikepedia goals. 93.132.180.102 (talk) 21:47, 2 September 2008 (UTC)
- I was to say that it didn't contain DNA (as usual in other human cells, and no RNA either, nor any other substance or information) to guide reproduction. (As a thought experiment, I consider candle flames alive and worker bees as dead. I don't really believe in that, but I can't pinpoint what would be wrong with that.) 93.132.132.172 (talk) 20:52, 1 September 2008 (UTC)
- RBCs are certainly usually considered living. They have an active metabolism, and die in about 90 days. The definition of living doesn't usually include any criterion that DNA is present. Such a definition would classify some viruses as living and some as non-living on the basis of chemical constitutents rather than activity. - Nunh-huh 16:36, 1 September 2008 (UTC)
Life in the most polluted environments?
Which land weeds or sea creatures are able to survive in the most polluted of environments?--Sonjaaa (talk) 19:13, 31 August 2008 (UTC)
- Pollution kills off lichens and bryophytes leaving algae. I can't help you with the toughest flowering plant. However there are different plants that can tolerate differing kinds of pollution. Some can tolerate high copper, others high phosphate, and yet others high acid. Is your pollution smoke, fluoride, acid rain, contaminated water or what? Graeme Bartlett (talk) 21:42, 31 August 2008 (UTC)
- Does radiation count as pollution by what you're asking about? Becuase, if so, I would say Chernobyl would be a good place to start, because some stuff does grow there, I think, and the article should have information on the aftereffects. I can't imagine that any other kind of pollution would be as toxic as what happened there, though I could just be basing on what I've heard about it.209.244.30.221 (talk) 14:24, 1 September 2008 (UTC)
- Forget the plants at Chernobyl. If you want real radiation resistance, you need Conan the Bacterium: Deinococcus radiodurans. In addition to tolerating huge amounts of ionizing radiation (surviving hundreds of times a dose lethal to humans), this bacterium tolerates dehydration, cold, acids, and vacuum. Genetically engineered versions of D. radiodurans have been developed for bioremediation of toluene and mercury contamination in radioactive waste.
- As Graeme notes, however, the answer comes down to what you define as 'pollution'. In the deep ocean, hydrothermal vents spew superheated water laden with hydrogen sulfide—a deadly toxin to most creatures. Whole ecosystems are based on bateria with a taste for this usually-toxic gas. TenOfAllTrades(talk) 17:45, 1 September 2008 (UTC)
Physics Degree
I just met this grad student studying Physics and was wondering what exactly people with advanced degrees in Physics do, job-wise, after they graduate. Do they mostly become scientists? Professors? Or what? 128.239.177.28 (talk) 20:23, 31 August 2008 (UTC)Curious
- Apart from your suggestions, I know planetarium operator, semiconductor factory developer, microwave nonlinear materials developer, geophysicist working for mining or oil companies, science teacher. Graeme Bartlett (talk) 21:47, 31 August 2008 (UTC)
- From what I've heard, lots of graduates go into computing. —Cyclonenim (talk · contribs · email) 22:29, 31 August 2008 (UTC)
- There's actually quite a lot of them on Wall Street as well. Mathematical abilities + major smarts = ideal candidate for a lot of big business. --98.217.8.46 (talk) 22:40, 31 August 2008 (UTC)
- The American Institute of Physics keeps track of this sort of thing. Just skimming over a report from a year ago (report here), 43% of people with MSc in Physics end up in the Private Sector, 21% end up at a university, 13% end up working for the government, 12% teach in high school, 8% go into the military, 3% do something else. The report omits this sort of discussion for PhDs but indicates that most of them aim for employment at universities, but only about 25% end up with jobs relating to directly to physics. Most end up doing engineering jobs of some sort. (p. 16 of the report)--98.217.8.46 (talk) 22:52, 31 August 2008 (UTC)
Red jelly growing in fridge
I have something unpleasant growing in my fridge. It is the colour and consistency of raspberry jam. It does not grow in the fridge compartment itself, but inside the small bore synthetic rubber tube that drains the condenstion from the fridge. Every few months the drain becomes blocked and the bottom shelf of the fridge gets wet which is how I know it is time to clean it out again. I have flushed the drain tube countless times, often with neat disinfectant but this alien substance always grows back after a few months. But strangely, it never seems to go anywhere outside the dark inside of the pipe. What is it? Is it dangerous? As it is only ever in the pipe and I don't seem able to kill it, I am wondering if it is a chemical reaction of the synthetic rubber rather than something biological. SpinningSpark 20:37, 31 August 2008 (UTC)
- Have you tried freezing it with a fire extinguisher and dropping it into the Arctic? --98.217.8.46 (talk) 23:00, 31 August 2008 (UTC)
- I am told Steve McQueen is no longer available for that kind of work. SpinningSpark 23:20, 31 August 2008 (UTC)
- Maybe, but I know for a fact that Kurt Russell died on just such a selfless mission. Franamax (talk) 01:41, 1 September 2008 (UTC)
- I am told Steve McQueen is no longer available for that kind of work. SpinningSpark 23:20, 31 August 2008 (UTC)
- This sounds to me like a slime mould (breaking the rules by guessing, again!). The rubber tube would provide a semi-porous substrate where the organisms could hide from your cleaning attempts. Also I think slime molds can form a biofilm which is resistant to disinfectants, although our articles seem to contradict me on this. The solution seems to be to buy yourself a new tube, or perhaps soak it in an acid or alkali solution for several days? I doubt it is a chemical reaction with the tube since presumably the tube would be gradually consumed in the reaction - unless the sulphur is acting as a catalyst, which I don't think would be the case. (On the subject of slime moulds, check out Dictyo for a really fascinating example of unicellular organisms turning into an animal when necessary - very Wikipedish!) Franamax (talk) 23:26, 31 August 2008 (UTC)
- My red jelly has never done anything like that. But by the way, I once saw a documentary about Dictyo or a similar creature which was attacked by an amoeba-like predator which could infiltrate and take over the Dictyo body while it was on the move. You don't know the name of that creature do you? SpinningSpark 00:13, 1 September 2008 (UTC)
- Well, Dictyo is an amoeba, so I'd like to see which bigger, tougher amoeba comes along to rough it up. You'd need to create a nutrient-deprived environment to see your mould rise up and fly away in any case, quit paying so much attention to keeping the stuff in your fridge cold. As far as takeovers go, I'm minded of the Wollbachia bacteria, which most unbelievably is a redlink. This is a virtuoso - it invades neurons and gonads, alters reproductive sex-ratios, forces bugs to climb up plants where they can be eaten, the whole nine yards. Please tell me we're not missing an article on Wollbachia!! Franamax (talk) 01:41, 1 September 2008 (UTC)
- Yeah - we're missing that one - but you could try Wolbachia instead! :-P SteveBaker (talk) 02:03, 1 September 2008 (UTC)
- As I understood it, the predator was another species of Dictyo which did not form moving "slugs" of its own. It hitched a ride on another moving Dictyo until it got somewhere good, then killed it from the inside and used the victims body to produce its own spores. There was a fascinating sequence of a Dictyo colony happily crawling along then suddenly stopping dead in its tracks and starting to grow spores from the invader. SpinningSpark 08:29, 1 September 2008 (UTC)
- Well, Dictyo is an amoeba, so I'd like to see which bigger, tougher amoeba comes along to rough it up. You'd need to create a nutrient-deprived environment to see your mould rise up and fly away in any case, quit paying so much attention to keeping the stuff in your fridge cold. As far as takeovers go, I'm minded of the Wollbachia bacteria, which most unbelievably is a redlink. This is a virtuoso - it invades neurons and gonads, alters reproductive sex-ratios, forces bugs to climb up plants where they can be eaten, the whole nine yards. Please tell me we're not missing an article on Wollbachia!! Franamax (talk) 01:41, 1 September 2008 (UTC)
- My red jelly has never done anything like that. But by the way, I once saw a documentary about Dictyo or a similar creature which was attacked by an amoeba-like predator which could infiltrate and take over the Dictyo body while it was on the move. You don't know the name of that creature do you? SpinningSpark 00:13, 1 September 2008 (UTC)
- What type of disinfectant are you using? Perhaps a bleach solution would work well. Flush the tube with a solution made from 1 part household bleach and 9 parts water. (Using neat bleach is much more likely to damage surfaces, and probably wouldn't be any more effective.) For full effectiveness, plug the bottom of the tube and let the solution stand for at least ten minutes. Afterwards, rinse the tube out with clean water (residual bleach may harm the material). For bonus points, flush the line with 70% ethanol (or 70% isopropyl alcohol/rubbing alcohol, if it's easier to obtain) and let air dry. Since it's possible – indeed, likely – that spores or bacterial colonies remain elsewhere on the lower shelves and trays of your refrigerator, you might also want to start the process by wiping down all the exposed surfaces with a 1 in 100 bleach dilution. Don't forget to wear gloves and work in a well-ventilated area, and remember that the bleach solutions may damage or discolour both your clothing and other stuff in the kitchen. TenOfAllTrades(talk) 17:58, 1 September 2008 (UTC)
Why do we find some animals attractive?
Why do humans(at least some humans) think that animals like big cats, wolves, birds, and even certain reptiles, as well as some invertebrates such as insects, are beautiful? Because some of these creatures might be dangerous, standing around gazing at, say, a tiger could be quite an evolutionary disadvantage. 68.123.238.140 (talk) 21:51, 31 August 2008 (UTC)
- Just to be sure, are you really asking: "Why do humans(at least some humans) think that animals like big cats, wolves, birds, and even certain reptiles, as well as some invertebrates such as insects, are beautiful?" --hydnjo talk 22:37, 31 August 2008 (UTC)
- Are you refering to sexual attraction or "awwww they're soooo cute" beautiful? --mboverload@ 22:39, 31 August 2008 (UTC)
- Not quite either of those, actually, but closer to the "cute" interpretation-- why people like to look at these animals, that's what I was getting at. 68.123.238.140 (talk) 23:02, 1 September 2008 (UTC)
- The last time a similar question came up here, someone suggested that our concept of 'cuteness' is an inbuilt safety mechanism to prevent us from eating our own offspring. --Kurt Shaped Box (talk) 00:11, 2 September 2008 (UTC)
- Not quite either of those, actually, but closer to the "cute" interpretation-- why people like to look at these animals, that's what I was getting at. 68.123.238.140 (talk) 23:02, 1 September 2008 (UTC)
- Bilateral symmetry, anthropomorphic features (like the big eyes of a seal pup or panda bear), flowing "elegant" features would be some of the factors. Also, many sexual adaptations such as the "eyes" on a peacock's tail feathers and the brilliant colouration of male birds would weigh in. Of course, the concept of beauty is like your gas mileage - it varies. I personally find a preying mantis quite beautiful, but others might freak out. Then there's spiders - both beautiful and repellent. Franamax (talk) 23:34, 31 August 2008 (UTC)
- The article on furries may be of interest to you. JessicaThunderbolt 12:28, 1 September 2008 (UTC)
- At the risk of sounding unscientific, anything that looks beautiful to you follows the Golden ratio at some level or the other. Look at its relation to beauty on goldennumber.net. Sandman30s (talk) 14:58, 1 September 2008 (UTC)
- Yeah also could it be said to be a sort of sexual feeling towards said animals esp when you pet them?? —Preceding unsigned comment added by LCMk2 (talk • contribs) 21:16, 1 September 2008 (UTC)
- Are you confusing petting and heavy petting? --Kurt Shaped Box (talk) 00:14, 2 September 2008 (UTC)
- LOL the only other place I've seen "heavy petting" used was in Ann Landers columns! :) (Well, that and other times - but I generally had my eyes closed or was way too close to actually see it.) Franamax (talk) 00:18, 2 September 2008 (UTC)
- Are you confusing petting and heavy petting? --Kurt Shaped Box (talk) 00:14, 2 September 2008 (UTC)
- There used to be a sign at my local swimming pool that had 'no heavy petting' listed amongst the obvious 'no smoking/no running/no food in the pool/no diving in the shallow end/no bombing/no balls or novelty inflatables' instructions. --Kurt Shaped Box (talk) 00:31, 2 September 2008 (UTC)
Human lungs
Can human lungs grow in response to intense use, like human muscles can? I mean, can they function better because one does a lot of physical exercise? Divers can learn to hold their breath for minutes, what happens to their lungs? Can the VO2 max of adult lungs increase? I realize that the question isn't worded accurately, but I hope I'm clear enough to get an answer. —Preceding unsigned comment added by 24.7.54.224 (talk) 22:20, 31 August 2008 (UTC)
- Yes, humans can train themselves to more efficiently take oxygen out of the air. I think Lance Armstrong has some kind of deformity that causes him to get more oxygen, but I could be wrong. --mboverload@ 22:41, 31 August 2008 (UTC)
- People who live very high in the mountains develop lungs that are a bit larger than normal. Andme2 (talk) 23:07, 31 August 2008 (UTC)
- Also, people that dwell in high elevations produce more red blood cells in order to transport more oxygen. I realize this borders on irrelevant.CalamusFortis 23:45, 31 August 2008 (UTC)
- Actually, I think the cell-count adaptation is the primary response. It's awfully hard to increase lung volume, what with the ribs and all. Franamax (talk) 01:28, 1 September 2008 (UTC)
- In addition, bigger lungs won't help, if the alveolar surface area is not increased. Oxygenation is primarily a problem of gas-exchange across membranes (and the carrying capacity of blood that has already been mentioned), not ventilation (movement of gas in and out of the alveoli). Scray (talk) 03:20, 1 September 2008 (UTC)
- Maybe we can't "improve" the lungs but we can do improve the diaphragm to improve the lung's performance.--Lenticel (talk) 00:54, 2 September 2008 (UTC)
- In addition, bigger lungs won't help, if the alveolar surface area is not increased. Oxygenation is primarily a problem of gas-exchange across membranes (and the carrying capacity of blood that has already been mentioned), not ventilation (movement of gas in and out of the alveoli). Scray (talk) 03:20, 1 September 2008 (UTC)
Sweet smell from blown up capacitors?
I've blown up various capacitors through various mishaps and I've noticed one thing: the electrolytic ones pop like pop corn and gives out a fairly sweet smell. Does anyone know what compound causes that smell? --antilivedT | C | G 23:12, 31 August 2008 (UTC)
- Are they on a circuit board? If so, it could be vapourized phenolic resin, which smells sweet but is not a good thing to smell a lot of. Franamax (talk) 23:37, 31 August 2008 (UTC)
- Yuck, that just led me through a series of stubby, badly written and unreferenced articles. C'mon science people, lets whip these things into shape! It's too much fun hanging out at the RefDesks... Franamax (talk) 23:42, 31 August 2008 (UTC)
- No the smell is still there if you pop a capacitor by itself, and is characteristic of electrolytic ones, so I'm guessing something in the electrolyte? --antilivedT | C | G 00:23, 1 September 2008 (UTC)
- Ethelyne Glycol is often used in the electrolyte, if I remember correctly. That would definitely give off a certain sweetish smell. ArakunemTalk 00:27, 1 September 2008 (UTC)
- I'm betting on Glycol too - that's the same stuff that's in antifreeze - it smells very sweet if your radiator hose splits and splashes coolant all over your engine. It's also pretty toxic...so you might want to try to avoid it where possible. SteveBaker (talk) 01:59, 1 September 2008 (UTC)
- What is the designation/manufacturer/part number on the specific capacitor you blew up? Many things have a safety data sheet - maybe we can trace it that way. Franamax (talk) 01:25, 1 September 2008 (UTC)
- Do you know the smell of Askarel (PCB)? I wouldn't call it sweet, but more like a dry-cleaning solvent. Old power correction capacitors and motor starting capacitors contained it. Considered hazardous.Edison2 (talk) 15:04, 1 September 2008 (UTC)
- I doubt it's PCB, since the capacitors are quite new. I don't remember the manufacturer of the capacitor but it's just one of the generic 47µF ones lying around, and a few more of various capacities. I guess it's glycol, unless there's a better guess somewhere. --antilivedT | C | G 05:11, 2 September 2008 (UTC)
- Do you know the smell of Askarel (PCB)? I wouldn't call it sweet, but more like a dry-cleaning solvent. Old power correction capacitors and motor starting capacitors contained it. Considered hazardous.Edison2 (talk) 15:04, 1 September 2008 (UTC)
- Ethelyne Glycol is often used in the electrolyte, if I remember correctly. That would definitely give off a certain sweetish smell. ArakunemTalk 00:27, 1 September 2008 (UTC)
Hydrogen Peroxide and Bacteria
What is the precise chemical reaction that occurs between hydrogen peroxide and bacteria that makes it so effective for killing them?CalamusFortis 23:45, 31 August 2008 (UTC)
- Hydrogen peroxide is a reactive oxygen species, which means it is willing to hand off an oxygen atom anywhere it has an excuse. Basically then, any oxidizing reaction will do. Franamax (talk) 23:46, 31 August 2008 (UTC)
- That I understand, but what exactly is it oxidizing? The cell walls of the bacteria? Proteins?CalamusFortis 23:48, 31 August 2008 (UTC)
- Initially, the cell walls. Oxidation ot the lipid membrane will affect the hydrophilic/hydrophobic balance that preserves the membrane structure and the cell will undergo lysis and spill out its contents. These will also be inactivated in the presence of such a strong oxidizing substance. Franamax (talk) 01:18, 1 September 2008 (UTC)
- Not only the cell walls. The article on ROS states, 1. damage of DNA; 2. oxidations of polydesaturated fatty acids in lipids; 3. oxidations of amino acids in proteins; 4. Oxidatively inactivate specific enzymes by oxidation of co-factors. --Ayacop (talk) 10:15, 1 September 2008 (UTC)
- Oh, OK. That was the specific answer I wanted.CalamusFortis 22:23, 1 September 2008 (UTC)
- You may also want to know that hydrogen peroxide is used by white blood cells to kill bacteria in several ways. H₂O₂ is deposited near bacteria within the leukocyte's phagocytic vacuoles where it reacts with the myeloperoxidase-H₂O₂-halide system to form hyperchlorous acid and/or singlet oxygen; H₂O₂ also reacts with oxygen or with lactoferrin or bacterial iron to form a hydroxyl radical (¹OH), the end products having a direct toxic effect on the bacteria. - Nunh-huh 03:31, 2 September 2008 (UTC)
- Oh, OK. That was the specific answer I wanted.CalamusFortis 22:23, 1 September 2008 (UTC)
- Not only the cell walls. The article on ROS states, 1. damage of DNA; 2. oxidations of polydesaturated fatty acids in lipids; 3. oxidations of amino acids in proteins; 4. Oxidatively inactivate specific enzymes by oxidation of co-factors. --Ayacop (talk) 10:15, 1 September 2008 (UTC)
- Initially, the cell walls. Oxidation ot the lipid membrane will affect the hydrophilic/hydrophobic balance that preserves the membrane structure and the cell will undergo lysis and spill out its contents. These will also be inactivated in the presence of such a strong oxidizing substance. Franamax (talk) 01:18, 1 September 2008 (UTC)
- That I understand, but what exactly is it oxidizing? The cell walls of the bacteria? Proteins?CalamusFortis 23:48, 31 August 2008 (UTC)
The Big Bang and Gravity
I know that this sounds like a dumb question, but could anyone tell me how planets, stars, etc. coalesced after the Big Bang? The universe is still expanding, and, in an explosion, matter does not coalesce; it keeps going outward. Hopefully there is a simple answer, and I thank you in advance for your patience. --Freiberg, Let's talk!, contribs 23:38, 31 August 2008 (UTC)
- I think it helps if you don't think of the Big Bang as an explosion per se, just the start of our universe. After matter formed, areas where there was more of it simply pulled themselves together. Paragon12321 23:42, 31 August 2008 (UTC)
- Hmm. OK, but wouldn´t that expansion of the universe itself cancel that out? Gravity, after all, is by far the weakest force. I could see individual atom forming through the strong and weak forces, but past that, it does not seem to make sense. --Freiberg, Let's talk!, contribs 23:53, 31 August 2008 (UTC)
- Gravity is weak, but over short enough distances, it's stronger than expansion. The rate of expansion is, roughly speaking, given by Hubble's Constant, which is about 70km/s/Megaparsec, that is two objects a megaparsec apart will move apart at 70km/s. Our galaxy is only 30 kiloparsecs in diameter, so the recession is far less, it's not that difficult for gravity to accelerate objects towards each other fast enough to overcome that recession. --Tango (talk) 00:20, 1 September 2008 (UTC)
- I think I get it now. So the inevitable variations in the initial Big Bang would be enough for various clumps of atoms to form. These clumps are now galaxies, with clumps within that becoming stars, etc. Alright! Thank you for your time.--Freiberg, Let's talk!, contribs 01:32, 1 September 2008 (UTC)
- Gravity is weak, but over short enough distances, it's stronger than expansion. The rate of expansion is, roughly speaking, given by Hubble's Constant, which is about 70km/s/Megaparsec, that is two objects a megaparsec apart will move apart at 70km/s. Our galaxy is only 30 kiloparsecs in diameter, so the recession is far less, it's not that difficult for gravity to accelerate objects towards each other fast enough to overcome that recession. --Tango (talk) 00:20, 1 September 2008 (UTC)
- Hmm. OK, but wouldn´t that expansion of the universe itself cancel that out? Gravity, after all, is by far the weakest force. I could see individual atom forming through the strong and weak forces, but past that, it does not seem to make sense. --Freiberg, Let's talk!, contribs 23:53, 31 August 2008 (UTC)
- What's really interesting (and bothers me a lot) is that if the universe started out as a zero sized singularity - then it was in all possible ways perfectly symmetrical...as only an infinitely small dot can be. So how did these initial clumps come about? Any distribution of forces, energy, velocity, matter or anything else you could imagine being able to measure should also have been perfectly symmetrical and perfectly smooth. Why did any clumpiness of any kind ever come about if every particle is being pulled in all directions identically? I'm guessing that the answer is of a quantum-mechanical/statistical nature...but it still bothers me that we just naturally assume that there would be even the slightest variation in density that would ultimately lead to bigger and bigger clumps - then stars and galaxies - when the source of all of this stuff was a 100% PERFECTLY symmetrical event - by definition. SteveBaker (talk) 03:53, 1 September 2008 (UTC)
- It's not an assumption; it's an observation. WMAP shows that the early universe had variations in density that exactly match the expectations based on quantum mechanical fluctuations in the first microsecond of the big bang. Dragons flight (talk) 04:47, 1 September 2008 (UTC)
- Ah - that's good. That was the only thing I could think of that it might be. I was aware that WMAP had shown variation that was sufficient to explain subsequent star/galaxy formation - I wasn't aware that it matched a quantum-statistical model. Well, that's very comforting. Living in a universe where everything was exactly the same shade of beige in every direction would have been less interesting! SteveBaker (talk) 05:56, 1 September 2008 (UTC)
- Our article on structure formation has a nice explanation of the end-of-end process. There are several stages. In outline:
- Cosmic inflation provides an almost-homogenous distribution of matter/energy in the early universe, with small inhomogeneities due to inflated quantum fluctuations.
- As the universe expands and cools it becomes dominated by matter - mostly dark matter - and gravitational attraction magnifies the inhomogeneities forming dark matter haloes.
- Radiative cooling and magnetohydrodynamic effects then accelerated the concentration process for ordinary baryonic matter, which forms gas clouds and eventually stars and planets. Gandalf61 (talk) 09:04, 1 September 2008 (UTC)
How did this compound get its name? I assume it was someone versed in Christian mythology, but who was it?CalamusFortis 00:02, 1 September 2008 (UTC)
- I can't give a who, but maybe a why. "Lucifer" simply means "light-bringer", which is what Luciferins do. The relation to the devil may be coincidental. Paragon12321 00:59, 1 September 2008 (UTC)
- Yes - I agree. Our article on Lucifer says that the name means "Light bringer" or "Morning Light" - originally meaning the planet Venus. It suggests that the use of the name to apply to Satan is a relatively modern confusion. Lucifer was originally some much hated tyrant King of Babylon...or perhaps Tyre, Lebanon...who subsequently was confused with the Devil. The article is hard-going...I don't recommend attempting to glean anything more meaningful from it unless you are already enough of a linguist to not need to read it! SteveBaker (talk) 03:47, 1 September 2008 (UTC)
- As for the who, according to the OED the word was first used (in the French form luciférine) in 1887 by one R. Dubois, in volume 105 of a journal identified by the abbreviation Compt. Rend., which I assume was Comptes Rendus de l'Académie des Sciences. And yes, as explained above, the word has nothing to do with the Devil. Deor (talk) 12:58, 1 September 2008 (UTC)
- Here is the link to the article of Dubois Recherches sur la fonction photogénique .--Stone (talk) 21:24, 1 September 2008 (UTC)
- Actually, I believe this is the article that the OED refers to. Dubois coins the name luciférine near the bottom of page 691. Deor (talk) 10:45, 2 September 2008 (UTC)
- Here is the link to the article of Dubois Recherches sur la fonction photogénique .--Stone (talk) 21:24, 1 September 2008 (UTC)
- As for the who, according to the OED the word was first used (in the French form luciférine) in 1887 by one R. Dubois, in volume 105 of a journal identified by the abbreviation Compt. Rend., which I assume was Comptes Rendus de l'Académie des Sciences. And yes, as explained above, the word has nothing to do with the Devil. Deor (talk) 12:58, 1 September 2008 (UTC)
- Yes - I agree. Our article on Lucifer says that the name means "Light bringer" or "Morning Light" - originally meaning the planet Venus. It suggests that the use of the name to apply to Satan is a relatively modern confusion. Lucifer was originally some much hated tyrant King of Babylon...or perhaps Tyre, Lebanon...who subsequently was confused with the Devil. The article is hard-going...I don't recommend attempting to glean anything more meaningful from it unless you are already enough of a linguist to not need to read it! SteveBaker (talk) 03:47, 1 September 2008 (UTC)
Amount of Potassium iodide in Iodized salt
From Potassium iodide they say that an adult should take 130 mg of it after being exposed to radiation. Hypothetically, how much iodized salt would be equivalent of 130 mg of Potassium Iodide? Would it matter whether it was Potassium iodide or Potassium iodate? From what I understand of Radiation poisoning this only protects the thyroid from Radioactive iodine, which makes me wonder: Would this kind of action really have much of an impact on survival percentages? Thanks. Anythingapplied (talk) 00:17, 1 September 2008 (UTC)
- My container of Morton's says that 1 serving (0.25 teaspoons) contains 130 micrograms of Potassium Iodide. So thats 1000 servings to get the thyroid blocking that I assume you're asking about. 1000 servings = 250 Teaspoons, or about 5 cups! I think the sodium would be more toxic than the radiation at that concentration. But yes, KI only protects the thyroid from uptake of radioactive iodine, which is a by-product of a nuclear chain reaction. ArakunemTalk 01:18, 1 September 2008 (UTC)
- Indeed the LD50 of salt is 3 g/kg in rats. Presuming it's fairly similar for humans, that's 300g for a 100kg human. Presuming you can actually consume that much salt I wouldn't think your chances of survival as very high Nil Einne (talk) 09:26, 1 September 2008 (UTC)
- Looks like 1 cups of salt is about 300g, so 5 would no doubt result in a very low survival probability. Anythingapplied (talk) —Preceding undated comment was added at 14:55, 2 September 2008 (UTC)
- Indeed the LD50 of salt is 3 g/kg in rats. Presuming it's fairly similar for humans, that's 300g for a 100kg human. Presuming you can actually consume that much salt I wouldn't think your chances of survival as very high Nil Einne (talk) 09:26, 1 September 2008 (UTC)
- According to one reference from the KI article [19] thyroid cancer rates increased 30- to 60-fold in very young children after the Chernobyl accident. Looking at statistics for thyroid cancer incidence in the UK [20] and using the general rate of 2.5 per 100,000 people, that would mean maybe 1 in 1,000 people would be protected from cancer. That gets a little confusing though when you look at the age-distribution graph, the normal thyroid cancer rate in children under 10 is close to zero. However, since radioactive iodine is known as a risk factor and has a short half-life, and it is very easy and cheap to give people near nuclear plants emergency kits with KI tablets, it makes sense to do so. Note though, the article doesn't say "after being exposed to radiation", KI is used before or during exposure to radioactive isotopes from an accidental release from a nuclear plant (or heaven forfend, bomb fallout) - the theory being to flood the body with stable iodine to prevent the radioactive iodine from being taken up by the thyroid. Franamax (talk) 11:32, 1 September 2008 (UTC)
- To answer you other question: yes it only protects against ragiation-induced thyroid cancer and not against cancers cause by any of the other isotopes that are released by a nuclear weapon or accident. However, since this one cnacer represents the biggest single health risk from such an incident, so yes it's worth tryin to defend against. Duringhte cold war, the US government seriously considered a law to require that all households keep a package of potassium iodide tablets (to be distributed by teh electric power company and taped to the fuse box, oddly enough.) The plan was abandoned due to concerns that more deaths would occur due to failure to follow the directions on the package than could be averted by proper use. -Arch dude (talk) 16:17, 1 September 2008 (UTC)
- Hmm...that distribution scheme actually makes a remarkable amount of sense. Every home should have a fuse box, and every homeowner ought to know where it is. having the electric company deliver the tablets ensures that every household will get some, and having the electric company staff tape the packet to the fuse box guarantees that the homeowner won't forget and misplace the tablets. Since KI tablets have an essentially unlimited shelf life, the job only has to be done once. Keeping them out of the medicine cabinet prevents them being thrown out accidentally when cleaning out the cabinet, and means that they won't get misplaced or damaged (transferred to a different drawer in the bathroom, moved to a first aid kit that gets left in the car or at the cottage, dropped in the sink and soaked, etc.). In homes with a basement, the fuse box is almost always located there rather than on the main floor of the house—if you've taken cover in your basement during a nuclear attack, you won't have to go upstairs to the bathroom – risking exposure to radiation and additional blasts – to find your KI tablets. TenOfAllTrades(talk) 16:44, 1 September 2008 (UTC)
- Potassium iodide tablets are available from internet sources by mail order [21] , with no prescription needed. Just keep'em near your radiation monitor [22] and hope you never need them. Edison2 (talk) 18:03, 1 September 2008 (UTC)
- Hmm...that distribution scheme actually makes a remarkable amount of sense. Every home should have a fuse box, and every homeowner ought to know where it is. having the electric company deliver the tablets ensures that every household will get some, and having the electric company staff tape the packet to the fuse box guarantees that the homeowner won't forget and misplace the tablets. Since KI tablets have an essentially unlimited shelf life, the job only has to be done once. Keeping them out of the medicine cabinet prevents them being thrown out accidentally when cleaning out the cabinet, and means that they won't get misplaced or damaged (transferred to a different drawer in the bathroom, moved to a first aid kit that gets left in the car or at the cottage, dropped in the sink and soaked, etc.). In homes with a basement, the fuse box is almost always located there rather than on the main floor of the house—if you've taken cover in your basement during a nuclear attack, you won't have to go upstairs to the bathroom – risking exposure to radiation and additional blasts – to find your KI tablets. TenOfAllTrades(talk) 16:44, 1 September 2008 (UTC)
September 1
Digital data over radio transmitter
Hello, can i KNow more about transmitting difital data using a Radio Transmitter? Thanks. Praveen Pkmchoudhari (talk) 02:02, 1 September 2008 (UTC)
- Well, that's a big subject. Television is about to be transmitted digitally in the USA, we have digital cell phones, we have WiFi connections between computers in our homes, some radio controlled toys use digital commands, radio is used to send commands to the two NASA rovers driving around on Mars...where would you like us to start? Essentially, we have a radio wave that's oscillating at some frequency ("pitch") and at some amplitude ("volume") - you can send digital data (which is basically just a string of 1's and 0's) by changing the frequency a little bit for a '1' and not changing it when there is a '0' (which would be like an FM radio station sends analog audio) - or you can sent the radio wave at full strength for a '1' and turn it way down (or even, off altogether) for a '0' (like an AM radio station sends analog audio by varying the "amplitude" of the radio wave). Do this very rapidly and you can send quite a bit of data in a short time. But there are lots of much more complicated and subtle ways that this can be done.
- Perhaps if you could be a bit more specific, we could help you out with a much more detailed explanation. SteveBaker (talk) 02:48, 1 September 2008 (UTC)
- The article Packet Radio may help. It's all about trasnmitting digital data using a Ham Radio. APL (talk) 03:07, 1 September 2008 (UTC)
(I don't know about this but I gave your question a title so it's easier to find and neater looking. Sorry for this is minor inconvenience! 88.211.96.3 (talk) 08:42, 1 September 2008 (UTC) )
The magical blue smoke
You know when you get a bit of electronic equipment such as a CRT monitor or a TV, and it dies and sometimes you get pale blue smoke (which my Dad jokingly tells me is the magic blue smoke that makes the TV work). What is this smoke made of and what causes it? Also is there a way to tell what things would produce said mystic smoke when they die, and under what circumstances? 88.211.96.3 (talk) 08:45, 1 September 2008 (UTC)
- Magic blue smoke. Yes, wiki has an article on everything. Dragons flight (talk) 09:06, 1 September 2008 (UTC)
applications of lagrangian dynamics
could you plz suggest a website where I can find problems and applications relating to the use of Euler-Lagrangian equations? I am still learning the elementary concepts in Lagrangian dynamics..so, plz see that the problems are not too complicated..thanx.. —Preceding unsigned comment added by 59.93.90.44 (talk) 08:59, 1 September 2008 (UTC)
thin layer chromatography
In TLC, I've always known of the Rf value as the retardation factor, but recently I've seen it referred to as "relative frontal mobility". Anyone else heard of this terminology? Is it correct/acceptable? --TomDæmon (talk) 09:31, 1 September 2008 (UTC)
- A google search would suggest it used at least by some. Retention factor is also possible, of course. --Cameron* 11:09, 1 September 2008 (UTC)
MRI machine
Hi
I wanted to know about the cryogen used in MRI machine and can liquid helium be replaced by liquid nitrogen? —Preceding unsigned comment added by Hetal R Shah (talk • contribs) 09:35, 1 September 2008 (UTC)
- This depends on the machine using high temperature superconductors. See MRI#Magnet: However, despite its cost, helium cooled superconducting magnets are the most common type found in MRI scanners today. --Ayacop (talk) 10:01, 1 September 2008 (UTC)
- The liquid Helium flask will be surrounded by liquid Nitrogen to maintain the Helium at its very low temperature. Liquid Nitrogen is much much cheaper than liquid Helium and is thus periodically topped up preventing the need to replace the expensive liquid Helium. Jdrewitt (talk) 10:26, 1 September 2008 (UTC)
- Additionally, no you cannot replace the Helium with Nitrogen because the superconducting coil must remain at a temperature below TC, the critical temperature where the superconductivity characteristics occur. If the magnet was cooled by liquid Helium originally (i.e. down to less than 4.2 K) then TC will be far too low for Nitrogen to achieve since Nitrogen freezes at 63 K. See Superconducting magnet. Jdrewitt (talk) 11:38, 1 September 2008 (UTC)
- Comercial MRI use Niobium-tin with critical temperature 18.3 kelvins. So Liquid nitrogen with 77K is not sufficient. With other superconducting material maybe but with the stuff maketed now. NO!--Stone (talk) 21:10, 1 September 2008 (UTC)
How can we calculate the chemical energy of a body?
How can we calculate the chemical energy of a body? Can we do that by using the calorific value of it and simply multiplying it by the mass?Anirban Chatterje (talk) 09:38, 1 September 2008 (UTC)
- Did you read the article on internal energy? --Ayacop (talk) 10:07, 1 September 2008 (UTC)
- Yes, I did.But the thing is that,only the Equation U=3RT/2 can be received.But how can I apply this in case of chemical compounds?Anirban Chatterje (talk) 18:40, 1 September 2008 (UTC)
Mortality of acute pancreatitis
Why is the mortality of acute pacreatitis so high? What are the mortality rates? I am not asking medical advice. —Preceding unsigned comment added by 217.227.75.39 (talk) 10:56, 1 September 2008 (UTC)
- The pancreas is a rather important organ, and you only have one of them. Is it really surprising that a major condition affecting it has a high mortality rate? Cardiac arrhythmias tend to be rather bad too Nil Einne (talk) 12:39, 1 September 2008 (UTC)
- The pancreas is fragile, and pretty damn important. Pancreatic cancer is a particlarly devastating condition, where Wikipedia states that there is roughly a 5% 5-year survival. Although, my Oxford Handbook of Medicine states that carcinoma of the pancreas carries a less than 2% 5-year survival with a mean survival of 6 months. That said, a procedure called pancreaticoduodenectomy can increase the prognosis to 5-14% —Cyclonenim (talk · contribs · email) 15:08, 1 September 2008 (UTC)
- The low survival rate for pancreatic cancer is because it doesn't have well-defined symptoms until the cancer has reached a very advanced stage. The survival rate for early-stage pancreatic cancer is similar to that of any other cancer. --Carnildo (talk) 22:41, 2 September 2008 (UTC)
- One way to think about it is as a positive-feedback loop. The pancreas produces zymogens that are usually involved in digesting our food after being pumped through the pancreatic duct into the duodenum (and then activated there, where the lining of the intestine keeps them from harming the host). In pancreatitis, these enzymes become active in the pancreas proper, and begin digesting - a bit like a fire at a fuel refinery. With the proximity of major arteries and bacteria-filled intestines, this makes for a lethal combination. Scray (talk) 15:06, 1 September 2008 (UTC)
regarding physics olympiad
what is the syllabus for physics olynpiad —Preceding unsigned comment added by 122.167.99.51 (talk) 13:29, 1 September 2008 (UTC)
- Assuming you're talking about the International Physics Olympiad, the syllabus is here. Algebraist 13:31, 1 September 2008 (UTC)
- There are more "Physics Olympiads". I know of two universities that host events with that specific name. -- kainaw™ 13:35, 1 September 2008 (UTC)
evolution
What is the benefit to male big cat reproductive success of leaving the area and pack. —Preceding unsigned comment added by 194.70.73.98 (talk) 15:44, 1 September 2008 (UTC)
- It varies from species to species - but what often happens is that young males are forced to leave the group - one older male remains. The benefit to the older male is obvious - he doesn't need help to impregnate multiple females - so less competition is good. The younger male is forced to leave or get into a fight with the leader which he'd surely lose due to smaller body size and inexperience. The young males go off by themselves for a while - those that survive may eventually return and kick out the old male after some kind of dominance battle. Evolutionary benefits are that weaker males don't get to spread their weak genetics to the next generation. Every animal born is a result of the best male genes available from the previous generation. SteveBaker (talk) 16:08, 1 September 2008 (UTC)
- I've been told that lions are the only social cats, having organized groups. What other species have pack dynamics? --Allen (talk) 16:56, 1 September 2008 (UTC)
- Define pack dynamics. I'd guess most Bovidae have a top dog and yes, many other mammals, too. --Ayacop (talk) 17:33, 1 September 2008 (UTC)
- I've been told that lions are the only social cats, having organized groups. What other species have pack dynamics? --Allen (talk) 16:56, 1 September 2008 (UTC)
- Sorry; I meant what other cat species. --Allen (talk) 19:38, 1 September 2008 (UTC)
- As far as I know, you're right. Lions are the only cat that exhibit pack dynamics. ScienceApe (talk) 21:48, 1 September 2008 (UTC)
- Hmm. See feral cat colony, for a counterexample. --Sean 22:26, 1 September 2008 (UTC)
- One benefit is to avoid inbreeding. I guess it's especially effective when only one sex is moving. 93.132.132.172 (talk) 18:54, 1 September 2008 (UTC)
Carotid artery
What is the best technique for determining the amount of plaque inside the carotid artery? —Preceding unsigned comment added by 79.76.154.239 (talk) 22:21, 1 September 2008 (UTC)
- The usual diagnostic test is carotid ultrasound, with the results reported as carotid plaque area. [23]. "Best" is a matter of opinion, of course. - Nunh-huh 22:27, 1 September 2008 (UTC)
- A carotid angiogram might give you more information. CT and MRI's are starting to show promising diagnostic results with the advantage of not being very invasive. Ultrasounds tend to be less exact. OrangeMarlin Talk• Contributions 02:23, 2 September 2008 (UTC)
- It rather depends on one's definition of 'best'. Using appropriate techniques, one can definitely get a very precise measurement (arguably better than ultrasound) using CT or (especially) MR methods. But a precise measurement isn't the only criterion. CT exposes the patient to (a fair bit of) ionizing radiation. Usually carotid occlusion is a problem in an older population, so cancer risk is low, but still worth considering. CT instrumentation is also relatively costly, and (with a few rare exceptions) non-portable. MR imaging equipment is extremely costly, entirely non-portable, and not suitable for patients with certain medical implants or prostheses. Ultrasound, on the other hand, is dirt cheap (compared to the other two, at least), portable, widely available, fast, and 'good enough' for most cases.
- Of course, for the absolute most accurate measurement, you have to wait for the patient to die, then cut out the carotid artery and have a look. While this is by far the least expensive and most direct method, it has certain drawbacks. TenOfAllTrades(talk) 02:48, 2 September 2008 (UTC)
- Back to the angiogram then, which balances risk to benefit. Of course, there would be other signs of vascular disease that would indicate the need for a carotid angiogram. OrangeMarlin Talk• Contributions 06:08, 2 September 2008 (UTC)
This question is in regard to galvanizing.
Presently I want to start a galvanizing company and during the research I was asking question about what materials is needed to galvanize a metal. Due to fact that businesses are scared of the competition they would not help me with all the information I was looking for.
I would like to know what is requite to melt a cutlery as in temp which should be applied and how would I be able to keep it into silver. More importantly which metal I should use while I’m still studying galvanizing?
My regards Christian —Preceding unsigned comment added by 41.208.48.160 (talk) 22:33, 1 September 2008 (UTC)
- Have you looked at galvanization and its links? Ζρς ι'β' ¡hábleme! 00:04, 2 September 2008 (UTC)
September 2
Solubility of Hydrocodone/Acetaminophen?
This question deals with possibly inappropriate use of controlled substances. Publishing an answer here would not be in the interests of Wikipedia or its readers. I have blanked this question, discussion here. Franamax (talk) 14:18, 2 September 2008 (UTC)
Word for a substance that makes you piss more?
Im pretty sure ive heard a word for substances that make you piss more, but i dont remember what it is. I believe caffeine is an example of such a substance. Anyone know what the word is? --212.120.246.239 (talk) 03:14, 2 September 2008 (UTC)
natural antibiotics?
I know there are several plants that are presumed to be "natural antibiotics", such as garlic. Is there any indication (journal articles) linking specific herbs with killing specific types of bacteria/viruses? Or it's all just "unproved naturopathy" Mathityahu (talk) 07:22, 2 September 2008 (UTC)
- It's very much proven. Instead of pointing you to a specific reference regarding a specific compound (like diallyl disulfide, or better de:Diallyldisulfid Refs 29-35 in the case of garlic) where you should find in the Reference Section links to clinical studies and other papers, I will just mention that plants produce such chemicals because, not having an immune system, they need such stuff to fight the steady attack of microorganisms, see secondary metabolite (which is sadly inadequate). In fact, even your normal antiobiotic comes from other organisms that had to fight bacteria: penicillin is a product of a fungus, and why should there be a difference in the general evolution of plants/fungi, just as there is a plant defense against herbivory?
- This does not mean you should abandon your doctor and treat yourself when you're ill. It also doesn't mean every plant antibiotic is suitable for human use, at all. Many fungal and bacterial antibiotics (yes bacteria against themselves) that were discovered have serious side effects, too. --Ayacop (talk) 10:12, 2 September 2008 (UTC)
- Thanks for the links, very interesting. Are there any other "proven" herbs? Mathityahu (talk) 19:10, 2 September 2008 (UTC)
metabolic functions of proteins from human/mouse
I'm puzzled. Every major protein in the Wikipedia lists lots of functions in its infobox, and also most of the time, an analogous protein from animals, mostly from the mouse. Now, did they find out all those functions from looking at humans or at mice? Do we generally transfer results directly from mouse to human? Shouldn't mouse results labeled as such? Because if the mouse were such a good model, we wouldn't need clinical studies, would we? --Ayacop (talk) 10:27, 2 September 2008 (UTC)
- Usually when you do genetic studies, you do them in a model organism, because it is exceedingly hard to get approval from an Institutional Review Board to do controlled crosses or make transgenics/knockouts with humans. Add to that the 10-20 year turn around for humans to reach sexual maturity, and such experiments on humans themselves become prohibitive. (Not to mention unethical.) To overcome these problems, researchers use model organisms. Mice and rats are usually used because they are mammals, easy to breed, have a (relatively) short life, and present less of an ethical/moral issue than, say, chimpanzees or dogs. For general information "protein X does Y", especially in a metabolic sense, the differences between mice and humans are vanishingly small. While we look vastly different, inside we're pretty darn close. In fact, a large amount of biochemical data has been first collected in yeast, flies or worms, and later been found to be applicable to humans. Now clinical studies are a different issue. When testing drugs small, seemingly innocuous changes, which may have no effect on the normal operation of the organism (such as single amino acid changes), can have a large effect on the efficacy of the drug. But note that there is usually a large body of model organism testing (usually on mice/rats) prior to the start of clinical trials - the in-human tests is to be doubly sure of the results. -- 128.104.112.147 (talk) 17:01, 2 September 2008 (UTC)
Political parties and teenage pregnancies
How would I get information on whether, for the same socio-economic class, Democrats or Republicans had a larger proportion of teenage pregnancies? I'm wondering about the influence of differing views on topics such as sex education and abstinence-only beliefs.
Thanks! — Sam 12:03, 2 September 2008 (UTC)
- The big problem here is that neither political alignment or teenage pregnancy status are statistics that are dependably tracked. The best you can hope for is a very small survey in a very small locality with a high rate of error. If you attempt to make a point using random statistics you find online, you will likely end up with a ridiculous point such as one I saw this past week... Rolla, MO has had a huge influx of frogs. In the last two years, more frog species are being discovered in Rolla than anywhere else in the world. Why are all the frogs suddenly being attracted to Rolla? ... That was taken from statistics showing a lot of frogs being identified in Rolla. Why? The local university started a class in biodiversity and one of the assignments is to locate and categorize frogs. So, in the last two years (since the class started), more frogs are being identified there than anywhere else. I hope that helps explain why you can't grab a statistic here and a statistic there and try to make some point about views on sex education. -- kainaw™ 12:16, 2 September 2008 (UTC)
- I don't know about Dems vs. Repubs, but there have been studies trying to determine whether sex-ed or abstinence-only is more effective at preventing teenage pregnancy. For example, this and this say that sex-ed is clearly superior at preventing teen pregnancy. (Incidentally, one of those reports notes that sex-ed also increases sexual activity, which of course some people view as unacceptable, even if that sex is safer.) Dragons flight (talk) 12:33, 2 September 2008 (UTC)
stripy babies
are lots of baby animals stripy? I know that tapirs have stripy babies to hide them in the dappled forest shade, but why would baby emus be stripy as they live on the plains? Bradley10 (talk) 12:42, 2 September 2008 (UTC)
- Plains or not, stripes still help to camouflage an animal. The point isn't so much to "look like your surroundings," but instead to break up the shape of the animal. Likewise a tiger can be very well camouflaged in the jungle, even though that might be surprising to anyone who had seen his bright orange (not very jungle-y) skin. — Sam 13:41, 2 September 2008 (UTC) —Preceding unsigned comment added by 63.138.152.238 (talk)
- Also, while it doesn't really apply in this case, zebras are also a plains animal that are very stripey.
Some zoologists believe that the stripes act as a camouflage mechanism. This is accomplished in several ways. First, the vertical striping helps the zebra hide in grass. While seeming absurd at first glance considering that grass is neither white nor black, it is supposed to be effective against the zebra's main predator, the lion, which is color blind. Theoretically a zebra standing still in tall grass may not be noticed at all by a lion. Additionally, since zebras are herd animals, the stripes may help to confuse predators - a number of zebras standing or moving close together may appear as one large animal, making it more difficult for the lion to pick out any single zebra to attack. A herd of zebras scattering to avoid a predator will also represent to that predator a confused mass of vertical stripes travelling in multiple directions making it difficult for the predator to track an individual visually as it separates from its herdmates, although biologists have never observed lions appearing confused by zebra stripes.
- -- MacAddct 1984 (talk • contribs) 13:54, 2 September 2008 (UTC)
- Also, while it doesn't really apply in this case, zebras are also a plains animal that are very stripey.
- It's a popular misconception that camouflage is most effective by making an animal look like the surroundings. Instead most "blending in" is caused by disruption of the shape of the animal. The brain does most of the work of camouflage—if we don't see something that looks like an animal, we don't see it at all. --98.217.8.46 (talk) 23:26, 2 September 2008 (UTC)
Do the hurricanes have anything to do with global warming?
I've been wondering lately about these hurricanes and cyclones that we hear about all the time in recent years (Katrina, Cyclone Nargis in Burma, and now Gustav). Is there any science that points to these being somehow the result of global warming? It seems to me that they are worse than they used to be, but that might just be confirmation bias screwing with my head. Are they worse than they used to be? More frequent? (I guess the sample size is pretty small, even if they are more frequent it could just be a conincidence).
By the way, I don't have a political agenda here, I'm really just curious, and I haven't heard anyone explain it properly to me. 83.250.202.36 (talk) 14:54, 2 September 2008 (UTC)
I have no idea of the science but the articles Effects of global warming and this part in particular (Effects_of_global_warming#Effects_on_weather) consider the effect that global-warming has on 'extreme weather'. I suspect you will suffer a bit of bias - I think the term is something like time-memory bias or something silly ilke that (look for article list of biases) - essentially the recent past may seem larger/more important in your mind than the distant past, even if both events were of equal size. 194.221.133.226 (talk) 15:15, 2 September 2008 (UTC)
- I'm no expert, but I think it's extremely tricky to try to point to a single cause of a hurricane. One thing we know- many of these storms feed on warmth- see Warm core. So, if there are higher air or water temperatures in the areas that have them, I think this will tend to lead to increased storm activity. It's all very complex and unpredictable though - a general trend of bigger storms doesn't mean any particular hurricane season will be bigger. Friday (talk) 15:20, 2 September 2008 (UTC)
- Note in the warm core link above that warm water is only half of the story. You need high-level condensation - so you need cooler air on top. If the water is warmer and the air is warmer, it won't help much. If the water is warmer and the air is cooler, you get more power out of the warm core. As for hurricanes, the frequency and strength have not had any significant increase. Much more powerful storms existed in the past and they came in both greater and fewer numbers, varying from year to year. In the recent past (15-20 years), two big changes happened. First, we have a huge number of people moving to the southeast coast so the population of people expecting to be hit is increased. Second, we have multiple 24-hour weather news services letting us know every minor detail of every storm. Combined, we know a hell of a lot more about more storms that are doing more financial damage. -- kainaw™ 15:56, 2 September 2008 (UTC)
- The articles mentioned above are all good sources of science, and are most neutral. Check out the top editors of the articles, and you might also post your question to their user talk pages, and they'll point you in the right direction. Always read the citations and/or sources for anything in any Wikipedia article, so that you can dig deeper in the topic. OrangeMarlin Talk• Contributions 16:18, 2 September 2008 (UTC)
- I finally found a rather unbiased graph of the number of hurricanes per year. This one actually counts tropical storms, not just hurricanes. It shows the slight upwards trend in frequency that has been attributed by many sources as a normal cycle between high frequency and low frequency. It does not show the hockey-stick curve projected by alarmists. Also, you can see the most expensive hurricanes in List of costliest Atlantic hurricanes. Ensure you look at the lower table where costs are adjusted for inflation. -- kainaw™ 16:50, 2 September 2008 (UTC)
Hurricane question
Is it possible for two hurricanes to collide or would they repel each other? Thanks, Alex--AlexSuricata (talk) 17:24, 2 September 2008 (UTC)
- See Fujiwhara effect. They can get close and circle one another, but not truly collide or merge. -- kainaw™ 17:31, 2 September 2008 (UTC)
- Of note -- our article claims that they will eventually merge if they don't separate. I've always heard it explained that one storm will weaken the other to the point that it goes away, leaving only the stronger of the two. So, it is up to you to decide if that means they collided and merged. -- kainaw™ 17:32, 2 September 2008 (UTC)
Blindness
Can people who have been blind from birth dream or picture things in their minds eye ? —Preceding unsigned comment added by 80.5.198.130 (talk) 18:38, 2 September 2008 (UTC)
Air-water experiment
This is about a simple experiment which can be considered as a thought experiment or could actually be performed. (This is not a homework assignment, just a question I dreamt up.)
The experiment requires a closed container full of air at atmospheric pressure, and another container of hot water. The closed container has a gauge that measures the difference in pressure between the inside and the outside of the container.
The container is quickly opened and some water is "instantaneously" injected. The water displaces a volume of air equal to the volume of water. Then the container is quickly closed and shaken vigourously, bringing air and water to the same temperature. As the air is heated in the process, it expands increasing the pressure in the container.
Suppose the container volume is V, the initial air temperature is TA, the initial water temperature is TW, and the volume of water injected is VW.
TW is greater than TA, and VW is less than or equal to V.
If VW is zero, the pressure will NOT increase because no hot water is injected. If VW equals V, the pressure will NOT increase because all the air is displaced and none is left to expand.
The tentative conclusion is that somewhere between VW = 0 and VW = V there is a volume of water that will cause the maximum pressure rise.
What ratio of VW/V will give the largest pressure rise? Does this answer depend on the temperatures of the air and the water? Are there other factors that need to be known to work this out?
Wanderer57 (talk) 20:33, 2 September 2008 (UTC)
- Water has pressure too, unfortunately for this experiment. Someguy1221 (talk) 20:53, 2 September 2008 (UTC)
cellulose
How does cellulose contribute to the cell wall? —Preceding unsigned comment added by 76.18.248.143 (talk) 21:02, 2 September 2008 (UTC)
- Have you read cell wall? Specifically, The composition section answers your question. Someguy1221 (talk) 21:13, 2 September 2008 (UTC)
Genes for Skin
Approximately how many genes make up the appearance/type of our human skin including the color? —Preceding unsigned comment added by 70.136.156.69 (talk) 21:39, 2 September 2008 (UTC)
What kind of moth?!?!
I have searched but i cannot find what kind of moth, (if it even is a moth,) this is. I found in a public laundry room in Northwest Louisiana. If anyone knows it would awesome to know! Thanks ahead of time for any time taken to find out what kind it is. Nick910 (talk) 21:45, 2 September 2008 (UTC)

Poppy Seeds
I understand that opium can be extracted from poppy seeds. I was in the store today, saw a 60g container of poppy seeds, and couldn't help but wonder how much opium that could produce (though I'd imagine it's not very much at all). In light of a recent question, I'll be perfectly clear that I have no intention to refine opium, and my questions stems purely from curiosity. Thanks!--El aprendelenguas (talk) 22:37, 2 September 2008 (UTC)
- According to this page [24], the alkaloid content of poppy seeds is about 50 parts per million, which cannot have any pharmaceutical effect. DuncanHill (talk) 22:41, 2 September 2008 (UTC)
- So does that mean that out of 60g of poppy seeds you could hypothetically extract 3mg of alkaloids, of which about 360 micrograms of which would be morphine? Which would be an incredibly uneconomical way to go about producing opium. (I'm dubious it would work, anyway, since my understanding of opium production is that you need the entire unripe seed pod, and many of them, to produce even just a little bit of the stuff. I'm suspicious that the dried seeds alone could produce anything.) --98.217.8.46 (talk) 23:23, 2 September 2008 (UTC)
- Man, where did I read about the guy who ended up in intensive care after he bought several pounds of poppy seeds, boiled them, mashed them up, then shot the mush into his arm with a large bore needle several times, under the mistaken assumption that poppy seeds = opium? It was reasonably recent too. --Kurt Shaped Box (talk) 23:29, 2 September 2008 (UTC)
2 granny smiths
How many women can take 2 granny smiths without their eyes watering? For the avoidance of doubt, Im talking vaginal capacity of course!
















