Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. Inorganic phosphates are mined to obtain phosphorus for use in agriculture and industry.[1][2] In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry.
Chemical properties
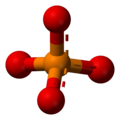
The phosphate ion is a polyatomic ion with the empirical formula PO43− and a molar mass of 94.973 g/mol; it consists of one central phosphorus atom surrounded by four identical oxygen atoms in a tetrahedral arrangement. The phosphate ion carries a negative three formal charge and is the conjugate base of the hydrogen phosphate ion, HPO42−, which is the conjugate base of H2PO4−, the dihydrogen phosphate ion, which in turn is the conjugate base of H3PO4, phosphoric acid. It is a hypervalent molecule (the phosphorus atom has 10 electrons in its valence shell). Phosphate is also an organophosphorus compound with the formula OP(OR)3 A phosphate salt forms when a positively-charged ion attaches to the negatively-charged oxygen atoms of the ion, forming an ionic compound. Many phosphates are not soluble in water at standard temperature and pressure. The sodium, potassium, rubidium, caesium and ammonium phosphates are all water soluble. Most other phosphates are only slightly soluble or are insoluble in water. As a rule, the hydrogenphosphates and the dihydrogenphosphates are slightly more soluble than the corresponding phosphates. The pyrophosphates are mostly water soluble.
In dilute aqueous solution, phosphate exists in four forms. In strongly-basic conditions, the phosphate ion (PO43−) predominates, whereas in weakly-basic conditions, the hydrogen phosphate ion (HPO42−) is prevalent. In weakly-acid conditions, the dihydrogen phosphate ion (H2PO4−) is most common. In strongly-acid conditions, aqueous phosphoric acid (H3PO4) is the main form.
-
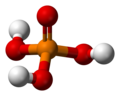
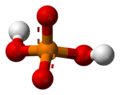
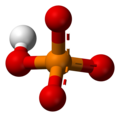
H3PO4 -
H2PO4− -
HPO42− -
PO43−
More precisely, considering the following three equilibrium reactions:
- H3PO4 ⇌ H+ + H2PO4−
- H2PO4− ⇌ H+ + HPO42−
- HPO42− ⇌ H+ + PO43−
the corresponding constants at 25°C (in mol/L) are (see phosphoric acid):
- (pKa1 2.16)
- (pKa2 7.21)
- (pKa3 12.32)
For a strongly-basic pH (pH=13), we find
showing that only PO43− and HPO42− are in significant amounts.
For a neutral pH (for example the cytosol pH=7.0), we find
so that only H2PO4− and HPO42− ions are in significant amounts (62% H2PO4−, 38% HPO42−). Note that in the extracellular fluid (pH=7.4), this proportion is inverted (61% HPO42−, 39% H2PO4−).
For a strongly-acid pH (pH=1), we find
showing that H3PO4 is dominant with respect to H2PO4−. HPO42− and PO43− are practically absent.
Phosphate can form many polymeric ions such as diphosphate (also pyrophosphate), P2O74−, and triphosphate, P3O105−. The various metaphosphate ions have an empirical formula of PO3− and are found in many compounds.
Phosphate deposits can contain significant amounts of naturally occurring uranium. Uptake of these substances by plants can lead to high uranium concentrations in crops.
Cellular function
Phosphate is useful in animal cells as a buffering agent. Phosphate salts that are commonly used for preparing buffer solutions at cell pHs include Na2HPO4 , NaH2PO4 , and the corresponding potassium salts.
Mining
Rock phosphate or phosphorite mines are primarily found in:
- North America
- United States of America, especially Florida, with lesser deposits in North Carolina, Idaho and Tennessee.
- Africa
- Morocco mainly near Khouribga and Youssoufia and Western Sahara
- Senegal
- Tunisia
- Oceania
See also
- Organophosphorus compounds
- Phosphate conversion coating
- Phosphine - PR3
- Phosphine oxide - OPR3
- Phosphinite - P(OR)R2
- Phosphonite - P(OR)2R
- Phosphite - P(OR)3
- Phosphinate - OP(OR)R2
- Phosphonate - OP(OR)2R
- Phosphate - OP(OR)3, such as triphenyl phosphate
- Polyphosphate - Pn
Further reading
This article needs attention from an expert in Chemicals. Please add a reason or a talk parameter to this template to explain the issue with the article. (November 2008) |
- Schmittner Karl-Erich and Giresse Pierre, 1999. Micro-environmental controls on biomineralization: superficial processes of apatite and calcite precipitation in Quaternary soils, Roussillon, France. Sedimentology 46/3: 463-476.
References
- ^ Phosphate Primer, website of the Florida Institute of Phosphate Research
- ^ "Figuring Out Phosphates," Food Product Design, June 2006, Lynn A. Kuntz
- ^ Campbell, Neil A. (2005). Biology (Seventh Edition ed.). San Francisco, California: Benjamin Cummings. p. 65. ISBN 0-8053-7171-0.
{{cite book}}:|edition=has extra text (help); Unknown parameter|coauthors=ignored (|author=suggested) (help)

![{\displaystyle K_{a1}={\frac {[{\mbox{H}}^{+}][{\mbox{H}}_{2}{\mbox{PO}}_{4}^{-}]}{[{\mbox{H}}_{3}{\mbox{PO}}_{4}]}}\simeq 6.92\times 10^{-3}}](https://wikimedia.org/enwiki/api/rest_v1/media/math/render/svg/b51cdf7bc855722ce8797a8f7493175cf1790f47)
![{\displaystyle K_{a2}={\frac {[{\mbox{H}}^{+}][{\mbox{HPO}}_{4}^{2-}]}{[{\mbox{H}}_{2}{\mbox{PO}}_{4}^{-}]}}\simeq 6.17\times 10^{-8}}](https://wikimedia.org/enwiki/api/rest_v1/media/math/render/svg/c7642278123aa8df8d41b29c3d50920bf0e70d37)
![{\displaystyle K_{a3}={\frac {[{\mbox{H}}^{+}][{\mbox{PO}}_{4}^{3-}]}{[{\mbox{HPO}}_{4}^{2-}]}}\simeq 4.79\times 10^{-13}}](https://wikimedia.org/enwiki/api/rest_v1/media/math/render/svg/0297e9288365bbb7eee61c09a4c4a8eb79c548f0)
![{\displaystyle {\frac {[{\mbox{H}}_{2}{\mbox{PO}}_{4}^{-}]}{[{\mbox{H}}_{3}{\mbox{PO}}_{4}]}}\simeq 7.5\times 10^{10}{\mbox{ , }}{\frac {[{\mbox{HPO}}_{4}^{2-}]}{[{\mbox{H}}_{2}{\mbox{PO}}_{4}^{-}]}}\simeq 6.2\times 10^{5}{\mbox{ , }}{\frac {[{\mbox{PO}}_{4}^{3-}]}{[{\mbox{HPO}}_{4}^{2-}]}}\simeq 2.14}](https://wikimedia.org/enwiki/api/rest_v1/media/math/render/svg/26667afe16154ca212a502b4cc1d7cd406af7acb)
![{\displaystyle {\frac {[{\mbox{H}}_{2}{\mbox{PO}}_{4}^{-}]}{[{\mbox{H}}_{3}{\mbox{PO}}_{4}]}}\simeq 7.5\times 10^{4}{\mbox{ , }}{\frac {[{\mbox{HPO}}_{4}^{2-}]}{[{\mbox{H}}_{2}{\mbox{PO}}_{4}^{-}]}}\simeq 0.62{\mbox{ , }}{\frac {[{\mbox{PO}}_{4}^{3-}]}{[{\mbox{HPO}}_{4}^{2-}]}}\simeq 2.14\times 10^{-6}}](https://wikimedia.org/enwiki/api/rest_v1/media/math/render/svg/45a9d19f1b88c87266cfed4a37fe44e445609353)
![{\displaystyle {\frac {[{\mbox{H}}_{2}{\mbox{PO}}_{4}^{-}]}{[{\mbox{H}}_{3}{\mbox{PO}}_{4}]}}\simeq 0.075{\mbox{ , }}{\frac {[{\mbox{HPO}}_{4}^{2-}]}{[{\mbox{H}}_{2}{\mbox{PO}}_{4}^{-}]}}\simeq 6.2\times 10^{-7}{\mbox{ , }}{\frac {[{\mbox{PO}}_{4}^{3-}]}{[{\mbox{HPO}}_{4}^{2-}]}}\simeq 2.14\times 10^{-12}}](https://wikimedia.org/enwiki/api/rest_v1/media/math/render/svg/5fce80104bde3a3449fba638dcdf1677e5f4a1ac)