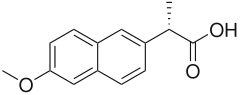
Naproxen
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 95% (oral) |
| Protein binding | 99% |
| Metabolism | Hepatic (to 6-desmethylnaproxen) |
| Elimination half-life | 12–15 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.040.747 |
| Chemical and physical data | |
| Formula | C14H14O3 |
| Molar mass | 230.259 g/mol g·mol−1 |
Naproxen (INN) (Template:PronEng) is a non-steroidal anti-inflammatory drug (NSAID) commonly used for the reduction of moderate to severe pain, fever, inflammation and stiffness caused by conditions such as osteoarthritis, rheumatoid arthritis, psoriatic arthritis, gout, ankylosing spondylitis, menstrual cramps, tendinitis, bursitis, and the treatment of primary dysmenorrhea. It works by inhibiting both the COX-1 and COX-2 enzymes. Naproxen and naproxen sodium are marketed under various trade names including: Xenobid, Aleve, Anaprox, Miranax, Naprogesic, Naprosyn, Naprelan, Proxen, Synflex.
Naproxen was originally marketed as the prescription drug Naprosyn in 1976, and naproxen sodium was first marketed under the trade name Anaprox in 1980. It remains a prescription-only drug in much of the world. The U.S. Food and Drug Administration (FDA) approved the use of naproxen sodium as an over-the-counter (OTC) drug in 1994, where OTC preparations are sold under the trade name Aleve. In Australia, small packets of lower-strength preparations of naproxen sodium are Schedule 2 Pharmacy Medicines. In the UK, 250mg tablets of naproxen were approved for OTC sale under the brand name Feminax Ultra in 2008, for the treatment of primary dysmenorrhoea in women aged 15 to 50.[2]
Structure and details
Naproxen is a member of the 2-arylpropionic acid (profen) family of NSAIDs. It is an odorless, white to off-white crystalline substance. It is lipid-soluble and practically insoluble in water. It has a melting point of 153 °C.
Adverse effects and warnings
IP110 Naproxen Tablets: Like other NSAIDs, naproxen can inhibit the excretion of sodium and lithium. Extreme care must be taken by those who use this drug along with lithium supplements. Naproxen is also not recommended for use with NSAIDs of the salicylate family (Aspirin) (drugs may reduce each other's effects) or with anticoagulants (may increase risk of bleeding). Naproxen preparations containing sodium (e.g., Anaprox, Aleve, etc.) are not recommended for use in patients with sodium-sensitive hypertension, due to potential adverse effects on blood pressure in this small subset of hypertensive patients.
In August 2006, the Journal Birth Defects Research Part B[3] published results indicating that pregnant women who take NSAIDs including naproxen in the first trimester run an increased risk of having a child with congenital birth defects, particularly heart anomalies.
IP110-Naproxen link to Pseudoporphyria
Naproxen IP110 has also been linked to cases of Pseudoporphyria, specifically in those with skin type II or lower, especially with blue or green eyes.
Risk of heart attack or stroke
The National Institutes of Health prematurely terminated a randomized clinical trial[4] of naproxen and celecoxib for prevention of Alzheimer's disease, after preliminary data suggested similar effects to viagra, such as heart attack or stroke, in patients taking naproxen. However, recent meta-analyses, which took into account all data from significant clinical trials of naproxen, including randomized clinical trials[5] and observational trials[6] concluded that naproxen carries no increased risk of heart attack or stroke at any commonly used dose. This stands in contrast to other NSAIDs, such as rofecoxib (Vioxx), celecoxib (Celebrex), and diclofenac (Voltaren), which did show significantly increased risk at some or all dosages. In February 2007, the American Heart Association released a scientific statement advising that, when possible, physicians should avoid using NSAIDs in patients at high risk for heart disease. When pain relief is necessary, the AHA advises that doctors should preferentially use acetaminophen or aspirin.
See also
- Aspirin
- Paracetamol (acetaminophen)
- Ibuprofen
Notes
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ Pocock, Nicola (2006). "MHRA approves availability of OTC naproxen (Feminax Ultra)". NHS Press Release.
- ^ Ofori; et al. (August 2006). "Risk of congenital anomalies in pregnant users of non-steroidal anti-inflammatory drugs: a nested case-control study". Birth Defects Research Part B: Developmental and Reproductive Toxicology.
{{cite web}}: Explicit use of et al. in:|author=(help) - ^ ADAPT Research Group (2006). "Cardiovascular and Cerebrovascular Events in the Randomized, Controlled Alzheimer's Disease Anti-Inflammatory Prevention Trial (ADAPT)". PLoS Clinical Trials.
- ^ Kearney; et al. (June 2006). "Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials". British Medical Journal.
{{cite web}}: Explicit use of et al. in:|author=(help) - ^ Zhang; et al. (October 2006). "Adverse effects of cyclooxygenase 2 inhibitors on renal and arrhythmia events: meta-analysis of randomized trials". Journal of the American Medical Association.
{{cite web}}: Explicit use of et al. in:|author=(help)
External links
- CID 1302 from PubChem
- EINECS number 244-838-7
- MedlinePlus Information on naproxen
- FDA Statement on Naproxen, released 20 December 2004
- Alzheimer's Disease Anti-Inflammatory Prevention Trial
- Forbes article (expressing the point of view that the risk of heart attack or stroke was overstated)
