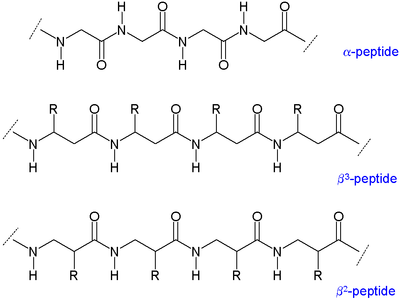
Beta-peptide
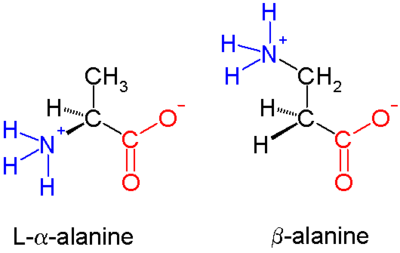
β-peptides consist of β amino acids, which have their amino group bonded to the β carbon rather than the α carbon as in the 20 standard biological amino acids. The only commonly naturally occurring β amino acid is β-alanine; although it is used as a component of larger bioactive molecules, β-peptides in general do not appear in nature. For this reason β-peptide-based antibiotics are being explored as ways of evading antibiotic resistance. Early studies in this field were published in 1996 by the group of Dieter Seebach[1] and that of Gellman[2].
Chemical structure and synthesis
In α amino acids (molecule at left), both the carboxylic acid group (red) and the amino group (blue) are bonded to the same carbon center, termed the α carbon () because it is one atom away from the carboxylate group. In β amino acids, the amino group is bonded to the β carbon (), which is found in most of the 20 standard amino acids. Only glycine lacks a β carbon, which means that β-glycine is not possible.
The chemical synthesis of β amino acids can be challenging, especially given the diversity of functional groups bonded to the β carbon and the necessity of maintaining chirality. In the alanine molecule shown, the β carbon is achiral; however, most larger amino acids have a chiral atom. A number of synthesis mechanisms have been introduced to efficiently form β amino acids and their derivatives[3][4] notably those based on the Arndt-Eistert synthesis.
Two main types of β-peptides exist: those with the organic residue (R) next to the amine are called β3-peptides and those with position next to the carbonyl group are called β2-peptides.[5]
Secondary structure
Because the backbones of β-peptides are longer than those of peptides that consist of α-amino acids, β-peptides form different secondary structures. The alkyl substituents at both the α and β positions in a β amino acid favor a gauche conformation about the bond between the α-carbon and β-carbon. This also affects the thermodynamic stability of the structure.
Many types of helix structures consisting of β-peptides have been reported. These conformation types are distinguished by the number of atoms in the hydrogen-bonded ring that is formed in solution; 8-helix, 10-helix, 12-helix, 14-helix, and 10/12-helix have been reported. Generally speaking, β-peptides form a more stable helix than α-peptides.[6]
Clinical potential
β-peptides are stable against proteolytic degradation in vitro and in vivo, an important advantage over natural peptides in the preparation of peptide-based drugs.[7] β-Peptides have been used to mimic natural peptide-based antibiotics such as magainins, which are highly potent but difficult to use as drugs because they are degraded by proteolytic enzymes in the body.[8]
References
- ^ β-Peptides: Synthesis by Arndt-Eistert homologation with concomitant peptide coupling. Structure determination by NMR and CD spectroscopy and by X-ray crystallography. Helical secondary structure of a -hexapeptide in solution and its stability towards pepsin Helvetica Chimica Acta Volume 79, Issue 4, Date: 26 Juni 1996, Pages: 913-941 Dieter Seebach, Mark Overhand, Florian N. M. Kühnle, Bruno Martinoni, Lukas Oberer, Ulrich Hommel, Hans Widmer doi:10.1002/hlca.19960790402
- ^ β-Peptide Foldamers: Robust Helix Formation in a New Family of -Amino Acid Oligomers Appella, D. H.; Christianson, L. A.; Karle, I. L.; Powell, D. R.; Gellman, S. H. J. Am. Chem. Soc. 1996; 118(51); 13071-13072. doi:10.1021/ja963290l
- ^ Basler B, Schuster O, Bach T. (2005). Conformationally constrained beta-amino acid derivatives by intramolecular [2 + 2]-photocycloaddition of a tetronic acid amide and subsequent lactone ring opening. J. Org. Chem. 70(24):9798-808. 2005 doi:10.1021/jo0515226 [1].
- ^ Murray JK, Farooqi B, Sadowsky JD, Scalf M, Freund WA, Smith LM, Chen J, Gellman SH. (2005). Efficient synthesis of a beta-peptide combinatorial library with microwave irradiation. J. Am. Chem. Soc. 127(38):13271-80. 2005 doi:10.1021/ja052733v[2]
- ^ β-Peptides: a surprise at every turn Dieter Seebach and Jennifer L. Matthews Chem. Commun., 1997, (21),2015-2022 doi:10.1039/a704933a
- ^ Gademann K, Hintermann T, Schreiber JV. (1999). "Beta-peptides: twisting and turning.", Curr Med Chem Oct;6(10):905-25. [3].
- ^ Beke T, Somlai C, Perczel A. (2006). "Toward a rational design of beta-peptide structures.", J Comp Chem Jan 15;27(1):20-38. [4].
- ^ Porter EA, Weisblum B, Gellman SH. (2002). Mimicry of host-defense peptides by unnatural oligomers: antimicrobial beta-peptides. J. Am. Chem. Soc. 124(25):7324-30. doi:10.1021/ja0260871