Tris
| |
| Names | |
|---|---|
| IUPAC name
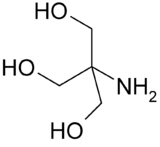
2-Amino-2-hydroxymethyl-propane-1,3-diol
| |
| Other names
TRIS, Tris, Tris base, Tris buffer,
TrizmaTM, Trisamine, THAM, Tromethamine, Trometamol, Tromethane | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.000.969 |
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C4H11NO3 | |
| Molar mass | 121.136 g·mol−1 |
| Appearance | White crystalline powder |
| Melting point | >175-176°C (448-449 K) |
| Boiling point | 219°C (492 K) |
| ~50 g/100 ml (25°C) in water | |
| Acidity (pKa) | 8.06 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tris (also known as THAM) is an abbreviation of the organic compound known as tris(hydroxymethyl)aminomethane, with the formula (HOCH2)3CNH2. Tris is extensively used in biochemistry and molecular biology.[1] In biochemistry, tris is widely used as a component of buffer solutions, such as in TAE and TBE buffer, especially for solutions of nucleic acids. It is a primary amine and thus undergoes the reactions associated with typical amines, e.g. condensations with aldehydes.
Buffering features
Tris has a pKa of 8.06, which implies that the buffer has an effective pH range between 7.0 and 9.2.
Buffer details
- The pKa declines approximately 0.03 units per degree Celsius rise in temperature.[2] [3]
- Silver-containing single-junction pH electrodes (e.g., silver chloride electrode) are incompatible with Tris (Ag-tris precipitation clogs the junction). Double-junction electrodes are resistant to this problem, and non-silver containing electrodes are immune.
- It is toxic to mammalian cells.
- A common variant of tris (aka tris base) is tris-HCl, the acid salt. When titrated to a specific pH with the corresponding counterion (OH- for tris-HCl, H+ for tris base) they are equivalent. However, the molecular weights are different and must be correctly accounted for in order to arrive at the expected buffer strength.
Buffer inhibition
- It is reported that Tris inhibits a number of enzymes [4][5], and therefore, it should be used with care when studying proteins.
Preparation
Tris is prepared in two steps from nitromethane via the intermediate (HOCH2)3CNO2 . Reduction of the latter gives tris(hydroxymethyl)aminomethane.[6]
Uses
The useful buffer range for tris (7-9) coincides with the typical physiological pH of most living organisms. This, and its low cost, make tris one of the most common buffers used in the biology/biochemistry lab.
Medical
Tris (usually known as THAM in this context) is used as alternative to sodium bicarbonate in the treatment of metabolic acidosis.[7]
See also
References
- ^ Gomori, G., Preparation of Buffers for Use in Enzyme Studies. Methods Enzymology., 1, 138-146 (1955).
- ^ El-Harakany, A.A. (1984). "Dissociation constants and related thermodynamic quantities of the protonated acid form of tris-(hydroxymethyl)-aminomethane in mixtures of 2-methoxyethanol and water at different temperatures" (PDF). J. Electroanal. Chem. 162 (1–2): 285–305.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Vega, C.A. (1985). "Thermodynamics of the Dissociation of Protonated Tris(hydroxymethy1)aminomethane in 25 and 50 wt % 2-Propanol from 5 to 45 O C". J. Chem. Eng. Data. 30: 376–379.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Desmarais, WT (2002). "The 1.20 Å resolution crystal structure of the aminopeptidase from Aeromonas proteolytica complexed with Tris: A tale of buffer inhibition
". Structure. 10: 1063–1072. PMID 12176384.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help); line feed character in|title=at position 139 (help) - ^ Ghalanbor, Z (2008). "Binding of Tris to Bacillus licheniformis alpha-amylase can affect its starch hydrolysis activity". Protein Peptide Lett. 15: 212–214. PMID 18289113.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Sheldon B. Markofsky “Nitro Compounds, Aliphatic” Ullmann's Encyclopedia of Industrial Chemistry 2002 by Wiley-VCH, Wienheim, 2002. DOI: 10.1002/14356007.a17_401.
- ^ Kallet, RH (2000). "The treatment of acidosis in acute lung injury with tris-hydroxymethyl aminomethane (THAM)". American Journal of Respiratory and Critical Care Medicine. 161 (4): 1149–1153. PMID 10764304.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)


