Chromatography

Chromatography (from Greek χρώμα:chroma, color and γραφειν:graphein to write) is the collective term for a set of laboratory techniques for the separation of mixtures. It involves passing a mixture dissolved in a "mobile phase" through a stationary phase, which separates the analyte to be measured from other molecules in the mixture based on differential partitioning between the mobile and stationary phases. Subtle differences in compounds partition coefficient results in differential retention on the stationary phase and thus changing the separation.
Chromatography may be preparative or analytical. The purpose of preparative chromatography is to separate the components of a mixture for further use (and is thus a form of purification). Analytical chromatography is done normally with smaller amounts of material and is for measuring the relative proportions of analytes in a mixture. The two are not mutually exclusive.
History
The history of chromatography begins during the mid-19th century. Chromatography, literally "color writing", was used—and named— in the first decade of the 20th century, primarily for the separation of plant pigments such as chlorophyll. New types of chromatography developed during the 1930s and 1940s made the technique useful for many types of separation process.
Some related techniques were developed during the 19th century (and even before), but the first true chromatography is usually attributed to Russian botanist Mikhail Semyonovich Tsvet, who used columns of calcium carbonate for separating plant pigments during the first decade of the 20th century during his research of chlorophyll.
Chromatography became developed substantially as a result of the work of Archer John Porter Martin and Richard Laurence Millington Synge during the 1940s and 1950s. They established the principles and basic techniques of partition chromatography, and their work encouraged the rapid development of several types of chromatography method: paper chromatography, gas chromatography, and what would become known as high performance liquid chromatography. Since then, the technology has advanced rapidly. Researchers found that the main principles of Tsvet's chromatography could be applied in many different ways, resulting in the different varieties of chromatography described below. Simultaneously, advances continually improved the technical performance of chromatography, allowing the separation of increasingly similar molecules.
Chromatography terms
- The analyte is the substance that is to be separated during chromatography.
- Analytical chromatography is used to determine the existence and possibly also the concentration of analyte(s) in a sample.
- A bonded phase is a stationary phase that is covalently bonded to the support particles or to the inside wall of the column tubing.
- A chromatogram is the visual output of the chromatograph. In the case of an optimal separation, different peaks or patterns on the chromatogram correspond to different components of the separated mixture.
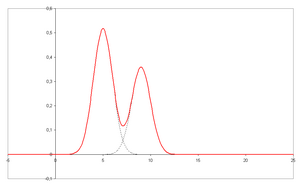
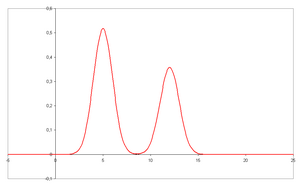
- Plotted on the x-axis is the retention time and plotted on the y-axis a signal (for example obtained by a spectrophotometer, mass spectrometer or a variety of other detectors) corresponding to the response created by the analytes exiting the system. In the case of an optimal system the signal is proportional to the concentration of the specific analyte separated.
- A chromatograph is equipment that enables a sophisticated separation e.g. gas chromatographic or liquid chromatographic separation.
- Chromatography is a physical method of separation in which the components to be separated are distributed between two phases, one of which is stationary (stationary phase) while the other (the mobile phase) moves in a definite direction.
- The effluent is the mobile phase leaving the column.
- An immobilized phase is a stationary phase which is immobilized on the support particles, or on the inner wall of the column tubing.
- The mobile phase is the phase which moves in a definite direction. It may be a liquid (LC and CEC), a gas (GC), or a supercritical fluid (supercritical-fluid chromatography, SFC). A better definition: The mobile phase consists of the sample being separated/analyzed and the solvent that moves the sample through the column. In the case of HPLC the mobile phase consists of a non-polar solvent(s) such as hexane in normal phase or polar solvents in reverse phase chromotagraphy and the sample being separated. The mobile phase moves through the chromatography column (the stationary phase) where the sample interacts with the stationary phase and is separated.
- Preparative chromatography is used to purify sufficient quantities of a substance for further use, rather than analysis.
- The retention time is the characteristic time it takes for a particular analyte to pass through the system (from the column inlet to the detector) under set conditions. See also: Kovat's retention index
- The sample is the matter analyzed in chromatography. It may consist of a single component or it may be a mixture of components. When the sample is treated in the course of an analysis, the phase or the phases containing the analytes of interest is/are referred to as the sample whereas everything out of interest separated from the sample before or in the course of the analysis is referred to as waste.
- The solute refers to the sample components in partition chromatography.
- The solvent refers to any substance capable of solubilizing other substance, and especially the liquid mobile phase in LC.
- The stationary phase is the substance which is fixed in place for the chromatography procedure. Examples include the silica layer in Chromatography#Thin layer chromatography Submitted by James Nguyen
Techniques by chromatographic bed shape
Column chromatography
Column chromatography is a separation technique in which the stationary bed is within a tube. The particles of the solid stationary phase or the support coated with a liquid stationary phase may fill the whole inside volume of the tube (packed column) or be concentrated on or along the inside tube wall leaving an open, unrestricted path for the mobile phase in the middle part of the tube (open tubular column). Differences in rates of movement through the medium are calculated to different retention times of the sample.[1]
In 1978, W. C. Still introduced a modified version of column chromatography called flash column chromatography (flash).[2][3] The technique is very similar to the traditional column chromatography, except for that the solvent is driven through the column by applying positive pressure. This allowed most separations to be performed in less than 20 minutes, with improved separations compared to the old method. Modern flash chromatography systems are sold as pre-packed plastic cartridges, and the solvent is pumped through the cartridge. Systems may also be linked with detectors and fraction collectors providing automation. The introduction of gradient pumps resulted in quicker separations and less solvent usage.
In expanded bed adsorption, a fluidized bed is used, rather than a solid phase made by a packed bed. This allows omission of initial clearing steps such as centrifugation and filtration, for culture broths or slurries of broken cells.
Planar chromatography

Planar chromatography is a separation technique in which the stationary phase is present as or on a plane. The plane can be a paper, serving as such or impregnated by a substance as the stationary bed (paper chromatography) or a layer of solid particles spread on a support such as a glass plate (thin layer chromatography). Different compounds in the sample mixture travel different distances according to how strongly they interact with the stationary phase as compared to the mobile phase. The specific Retardation factor (Rf) of each chemical can be used to aid in the identification of an unknown substance.
Paper chromatography
Paper chromatography is a technique that involves placing a small dot or line of sample solution onto a strip of chromatography paper. The paper is placed in a jar containing a shallow layer of solvent and sealed. As the solvent rises through the paper, it meets the sample mixture which starts to travel up the paper with the solvent. This paper is made of cellulose, a polar substance, and the compounds within the mixture travel farther if they are non-polar. More polar substances bond with the cellulose paper more quickly, and therefore do not travel as far.
Thin layer chromatography
Thin layer chromatography (TLC) is a widely employed laboratory technique and is similar to paper chromatography. However, instead of using a stationary phase of paper, it involves a stationary phase of a thin layer of adsorbent like silica gel, alumina, or cellulose on a flat, inert substrate. Compared to paper, it has the advantage of faster runs, better separations, and the choice between different adsorbents. For even better resolution and to allow for quantification, high-performance TLC can be used.
Displacement Chromatography
The basic principle of displacement chromatography is: A molecule with a high affinity for the chromatography matrix (the displacer) will compete effectively for binding sites, and thus displace all molecules with lesser affinities.[4] There are distinct differences between displacement and elution chromatography. In elution mode, substances typically emerge from a column in narrow, Gaussian peaks. Wide separation of peaks, preferably to baseline, is desired in order to achieve maximum purification. The speed at which any component of a mixture travels down the column in elution mode depends on many factors. But for two substances to travel at different speeds, and thereby be resolved, there must be substantial differences in some interaction between the biomolecules and the chromatography matrix. Operating parameters are adjusted to maximize the effect of this difference. In many cases, baseline separation of the peaks can be achieved only with gradient elution and low column loadings. Thus, two drawbacks to elution mode chromatography, especially at the preparative scale, are operational complexity, due to gradient solvent pumping, and low throughput, due to low column loadings. Displacement chromatography has advantages over elution chromatography in that components are resolved into consecutive zones of pure substances rather than “peaks”. Because the process takes advantage of the nonlinearity of the isotherms, a larger column feed can be separated on a given column with the purified components recovered at significantly higher concentrations.
Techniques by physical state of mobile phase
Gas chromatography
- ^ IUPAC Nomenclature for Chromatography IUPAC Recommendations 1993, Pure & Appl. Chem., Vol. 65, No. 4, pp.819-872, 1993.
- ^ Still, W. C.; Kahn, M.; Mitra, A. J. Org. Chem. 1978, 43(14), 2923-2925. doi:10.1021/jo00408a041
- ^ Laurence M. Harwood, Christopher J. Moody. Experimental organic chemistry: Principles and Practice (Illustrated edition ed.). pp. 180–185.
{{cite book}}:|edition=has extra text (help); Text "ISBN 0632020172" ignored (help); Text "ISBN 978-0632020171" ignored (help); Text "Number of Pages: 790" ignored (help); Text "Publisher: WileyBlackwell" ignored (help); Text "Publishing Date: 13 Jun 1989" ignored (help) - ^ Displacement Chromatography 101. Sachem, Inc. Austin, TX 78737
