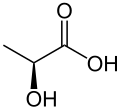
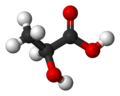
Lactic acid
This article needs additional citations for verification. (July 2008) |
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-hydroxypropanoic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.000.017 | ||
| E number | E270 (preservatives) | ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C3H6O3 | |||
| Molar mass | 90.08 g/mol | ||
| Melting point | L: 53 °C D: 53 °C D/L: 16.8 °C | ||
| Boiling point | 122 °C @ 12 mmHg | ||
| Acidity (pKa) | 3.86 at 25 °C | ||
| Related compounds | |||
Other anions
|
lactate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Lactic acid (IUPAC systematic name: 2-hydroxypropanoic acid), also known as milk acid, is a chemical compound that plays a role in several biochemical processes. It was first isolated in 1780 by a Swedish chemist, Carl Wilhelm Scheele, and is a carboxylic acid with a chemical formula of C3H6O3. It has a hydroxyl group adjacent to the carboxyl group, making it an alpha hydroxy acid (AHA). In solution, it can lose a proton from the acidic group, producing the lactate ion CH3CH(OH)COO−. It is miscible with water or ethanol, and is hygroscopic.
Lactic acid is chiral and has two optical isomers. One is known as L-(+)-lactic acid or (S)-lactic acid and the other, its mirror image, is D-(−)-lactic acid or (R)-lactic acid. L-(+)-Lactic acid is the biologically important isomer.
In animals, L-lactate is constantly produced from pyruvate via the enzyme lactate dehydrogenase (LDH) in a process of fermentation during normal metabolism and exercise. It does not increase in concentration until the rate of lactate production exceeds the rate of lactate removal which is governed by a number of factors including: monocarboxylate transporters, concentration and isoform of LDH and oxidative capacity of tissues. The concentration of blood lactate is usually 1–2 mmol/L at rest, but can rise to over 20 mmol/L during intense exertion.
Industrially, lactic acid fermentation is performed by Lactobacillus bacteria, among others. These bacteria can operate in the mouth; the acid they produce is responsible for the tooth decay known as caries{[fact|date=February 2010}}.
In medicine, lactate is one of the main components of Ringer's lactate or lactated Ringer's solution (Compound Sodium Lactate or Hartmann's Solution in the UK). This intravenous fluid consists of sodium and potassium cations, with lactate and chloride anions, in solution with distilled water in concentration so as to be isotonic compared to human blood. It is most commonly used for fluid resuscitation after blood loss due to trauma, surgery, or a burn injury.
Exercise and lactate
During power exercises such as sprinting, when the rate of demand for energy is high, lactate is produced faster than the ability of the tissues to remove it and lactate concentration begins to rise. This is a beneficial process since the regeneration of NAD+ ensures that energy production is maintained and exercise can continue. The increased lactate produced can be removed in a number of ways including
- oxidation to pyruvate by well-oxygenated muscle cells which is then directly used to fuel the Krebs cycle,
- conversion to glucose via gluconeogenesis in the liver and release back into the circulation, see the Cori cycle.
Contrary to popular belief, this increased concentration of lactate does not directly cause acidosis, nor is it responsible for delayed onset muscle soreness.[1] This is because lactate itself is not capable of releasing a proton, and secondly, the acidic form of lactate, lactic acid, cannot be formed under normal circumstances in human tissues.[citation needed] Analysis of the glycolytic pathway in humans indicates that there are not enough hydrogen ions present in the glycolytic intermediates to produce lactic or any other acid.
The acidosis that is associated with increases in lactate concentration during heavy exercise arises from a separate reaction. When ATP is hydrolysed, a hydrogen ion is released. ATP-derived hydrogen ions are primarily responsible for the decrease in pH. During intense exercise, aerobic metabolism cannot produce ATP quickly enough to supply the demands of the muscle. As a result, anaerobic metabolism becomes the dominant energy producing pathway as it can form ATP at high rates. Due to the large amounts of ATP being produced and hydrolysed in a short period of time, the buffering systems of the tissues are overcome, causing pH to fall and creating a state of acidosis, a natural process which facilitates the easier dissociation of Oxyhaemoglobin and allows easier transfer of oxygen from the blood[2]. This may be one factor, among many, that contributes to the acute muscular discomfort experienced shortly after intense exercise.[citation needed]
The effect of lactate on acidosis has been the topic of many recent conferences in the field of exercise physiology. Robergs et al. have accurately chased the proton movement that occurs during glycolysis. However, in doing so, they have suggested that [H+] is an independent variable that determines its own concentration. A recent review by Lindinger et al.[3] has been written to rebut the stoichiometric approach used by Robergs et al. (2004).[1] In using this stoichiometric process, Robergs et al. have ignored the causative factors (independent variables) of the concentration of hydrogen ions (denoted [H+]). These factors are strong ion difference [SID], PCO2, and weak acid buffers. Lactate is a strong anion, and causes a reduction in [SID] which causes an increase in [H+] to maintain electroneutrality. PCO2 also causes an increase in [H+]. During exercise, the intramuscular lactate concentration and PCO2 increase, causing an increase in [H+], and thus a decrease in pH. (See Le Chatelier's principle)

Lactic acid as a polymer precursor
Two molecules of lactic acid can be dehydrated to lactide, a cyclic lactone. A variety of catalysts can polymerise lactide to either heterotactic or syndiotactic polylactide, which as biodegradable polyesters with valuable (inter alia) medical properties are currently attracting much attention.
Nowadays, lactic acid is used as a monomer for producing polylactic acid (PLA) which later has application as biodegradable plastic. This kind of plastic is a good option for substituting conventional plastic produced from petroleum oil because of low emission of carbon dioxide. The commonly used process in producing lactic acid is via fermentation, and later to obtain the polylactic acid, the polymerization process follows.
Lactic acid in foods
Lactic acid is primarily found in sour milk products, such as: koumiss, leban, yogurt, kefir, and some cottage cheeses. The casein in fermented milk is coagulated (curdled) by lactic acid.
Sauerkraut contains lactic acid, formed when lactic acid bacteria ferment the sugars in finely shredded cabbage. Lactic acid is an ingedient in a corn based plastic that does not have a very large environmental footprint.
Lactic acid in detergents
Lactic acid has gained importance in the detergents industry the last decade. Being a good descaler, soap-scum remover and being a registered anti-bacterial agent - an economically beneficial as well as environmentally beneficial trend toward safer and natural ingredients has also contributed.
See also
References
- ^ a b R. Robergs, F. Ghiasvand, D. Parker (2004). "Biochemistry of exercise-induced metabolic acidosis". Am J Physiol Regul Integr Comp Physiol. 287 (3): R502–16. doi:10.1152/ajpregu.00114.2004. PMID 15308499.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1111/j.1399-6576.1995.tb04325.x, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1111/j.1399-6576.1995.tb04325.xinstead. - ^ Michael I. Lindinger, John M. Kowalchuk, and George J. F. Heigenhauser (2005). "Applying physicochemical principles to skeletal muscle acid-base status". Am J Physiol Regul Integr Comp Physiol. 289 (3): R891 – R894. doi:10.1152/ajpregu.00225.2005.
{{cite journal}}: CS1 maint: multiple names: authors list (link)