Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
May 6
epson salts
how come the epson salts article dosent explain how it helps sore joints. —Preceding unsigned comment added by Tom12350 (talk • contribs) 00:57, 6 May 2010 (UTC)
- It's sort of mentioned in the talk page... Maybe it's because no one can find a good reliable source to make it worth including? Vespine (talk) 06:20, 6 May 2010 (UTC)
- Perhaps because it does not generally help sore joints. I know it's personal research but I have never heard that Epsom salts relieves sore joints. However if you have a reliable source for that claim then you might consider adding it to the article. Be bold! Caesar's Daddy (talk) 06:21, 6 May 2010 (UTC)
- They're a fairly standard ingredient for many bath salts - which are the standard home remedy for sore joints. http://www.google.co.uk/search?q=epsom+salts+sore+joints&hl=en&start=0&sa=N
- Perhaps because it does not generally help sore joints. I know it's personal research but I have never heard that Epsom salts relieves sore joints. However if you have a reliable source for that claim then you might consider adding it to the article. Be bold! Caesar's Daddy (talk) 06:21, 6 May 2010 (UTC)
- The epsom salt council suggests that it is magnesium that is effective. http://www.epsomsaltcouncil.org/about_better_health_through_soaking.htm 77.86.68.186 (talk) 17:01, 6 May 2010 (UTC)
- Is that source to be trusted, though? I've read that it is not very effective. 67.243.7.245 (talk) 19:16, 6 May 2010 (UTC)
- Could be a placebo effect - there's a vast amount of people claiming they are good though. Who knows? It's an unwanted product of the chemical industry.. Conspiracy theory perhaps??77.86.68.186 (talk) 21:03, 6 May 2010 (UTC)
- Is that source to be trusted, though? I've read that it is not very effective. 67.243.7.245 (talk) 19:16, 6 May 2010 (UTC)
- I could certainly believe that soaking in warm water could help - relaxing the muscles - taking the weight off the joint and allow neutral bouyancy to support the limb, calming and mentally relaxing the patient because while in the bath they aren't being hassled by the perils of daily life. So it wouldn't surprise me if the whole business of using epsom salts had nothing whatever to do with it! If this hypothesis is correct then people will doubtless claim that soaking in an epsom salt bath helps their ailment - not realising that it's the bath - not the salts - that's really doing the job. Without a proper double-blind trial, I don't think we could say for sure...but the idea that magnesium salts migrate through our skins (which are pretty water-tight stuff!) through layers of fat and muscle to reach the knee - and then somehow acts to help the pain without all sorts of dangerous chemical imbalance side-effects - is one hell of a stretch! SteveBaker (talk) 01:59, 7 May 2010 (UTC)
- There's a study here http://www.epsomsaltcouncil.org/articles/Report_on_Absorption_of_magnesium_sulfate.pdf
- If you don't like the idea - how about a Magnesium salt lick , or just eat your greens :)
- 77.86.68.186 (talk) 11:01, 7 May 2010 (UTC)
- That is one seriously flawed study!
- It was funded by the "Epsom Salt Council" who are hardly an unbiassed source of information.
- They only used 19 test subjects. That's not a good enough statistical sample.
- Their test subjects were all young and healthy without joint problems. Is it not possible (if magnesium is indeed implicated in joint pain) that the entire reason older and less healthy individuals suffer joint pain is because whatever mechanism for magnesium uptake is responsible for the rises measured in this experiment is broken? That would completely invalidate the entire set of conclusions.
- Their findings for one or two baths are less than their error bars and only after weeks of this treatment did they get an actual, measurable effect. An occasional bath in this stuff is useless - even by their own evidence. Do they tell people to bath in the stuff for weeks at a time? No.
- They did no placebo controls and their "control" experiments are not explained. That's a huge "no-no".
- The experiment was not done 'blind' - much less 'double-blind' - so some kind of placebo effect is a possibility here.
- The amount of Epsom salts they needed to use to produce a measurable effect were over half a kilogram per bath! So two or three bottles of the pretty scented stuff you can buy in big glass jars or ten packets of the stuff you buy in a boring cardboard box at the supermarket in each bath - for weeks and weeks! Their main web page says to use two cupfulls per bath - which is below the level that this study says has any measurable effect! So one major conclusion should be "In normal amounts, Epsom salts have no measurable effect on magnesium levels in the blood of young, healthy individuals even after two weeks of use".
- The "paper" hasn't been peer-reviewed, the experimental results have not been duplicated and no major scientific journal has published the study. They have not released the raw data for other people to analyse with their own statistical tools. So it's clearly not acceptable science yet.
- Then we have to ask how long the stuff stays in the blood and whether increased magnesium levels in the blood is even any good for you in the first place! Their own article points out that the body normally carefully regulates magnesium levels in the blood - there is probably a very good reason our bodies do that!
- They explain that the test subjects found bathing in the required high concentrations of epsom salts to be unpleasant. What happened to the "warm relaxing bath" thing?
- They conclude "Bathing in Epsom salts is a safe and easy way to increase sulfate and magnesium levels in the body" - but none of those conclusions are actually found in the body of the paper. Where did they follow the subsequent health of these volunteers? Maybe they all dropped dead right after the study - or will have long-term health problems as a consequence.
- This is a classic example of "junk science" and it should be ignored. SteveBaker (talk) 14:42, 7 May 2010 (UTC)
- That is one seriously flawed study!
Curvature of the earth
Not a big deal, but I was wondering just now if it is known how much a certain area of earth's surface (assuming a perfectly flat area) is curved. Is there any measurable curvature within, say a mile? –Juliancolton | Talk 01:19, 6 May 2010 (UTC)
- Divide the number of miles in the circumference, about 24000, into 360°. This will tell you how many miles it takes to produce one degree of curvature. --Chemicalinterest (talk) 01:29, 6 May 2010 (UTC)
- That makes sense. Thanks! –Juliancolton | Talk 01:39, 6 May 2010 (UTC)
- Not quite. That assumes the Earth is perfectly spherical, but it is actually a bit of a flattened sphere. Over a small distance like a mile, this imperfection can be discounted, but on a large scale it has to be considered. 76.199.153.83 (talk) 01:49, 6 May 2010 (UTC)
- Not quite. If you wish to take flattening of the ellipsoid into account, then variations in distance per unit curvature as a function of latitude is as (or slightly more) noticeable for small distances then it is for large distances. 58.147.58.122 (talk) 15:50, 6 May 2010 (UTC)
- Yes. The earth's diameter is about 24 miles bigger at the equator than from pole to pole because of centrifrugal force. --Chemicalinterest (talk) 15:40, 6 May 2010 (UTC)
- Not quite. That assumes the Earth is perfectly spherical, but it is actually a bit of a flattened sphere. Over a small distance like a mile, this imperfection can be discounted, but on a large scale it has to be considered. 76.199.153.83 (talk) 01:49, 6 May 2010 (UTC)
- That makes sense. Thanks! –Juliancolton | Talk 01:39, 6 May 2010 (UTC)
Look at a map of one of the plains states in the US, like North Dakota or Nebraska, and you will see lots of places where a road, or a series of successive county boundaries, forms a straight north-south line that jogs to the west every so often as you go north. In Canada the boundary between Manitoba and Saskatchewan is an even better example, having the same sort of shape for hundreds of miles with no further irregularities. The reason for this is that both the Public Land Survey System in the US and the Dominion Land Survey System in Canada were based on dividing the land into squares with specific sizes -- squares whose boundaries run north-south and east-west. For example, a 1-mile square on this system was called a section.
But of course that's impossible on a curved planet. The way it was really done was that they made the shapes almost square, with true north-south sides, running north and/or south from an east-west baseline until they got too far from the desired size; then they jogged all the boundaries to one side (by an amount that depended on how far they were from a principal meridian) and continued north or south from there. So for example each of the stairstep points in the Saskatchewan-Manitoba border is the same distance west of a principal meridian that runs north and south more or less through Winnipeg. The jogs in this case are about 24 miles apart. Look at a map of Manitoba and you can see how much the curvature of the Earth affects things. --Anonymous, 02:15 UTC, May 6, 2010.
- To really get a gut feel for it - imagine you're standing on the end of a long, straight, "flat" road (we have to put "flat" in quotes - because it follows the earth's curvature). Over the distance between where you're standing and where the horizon is - the difference between a 'flat earth' and the real curved earth is exactly the height of your eyes from the ground. Within a mile, this "curvature" distance is about 8 inches...which is quite measurable. It gets bigger fast though! Within 3 miles, it's about 6 feet of curvature...which is why (if you happen to be a little over 6' tall) the horizon is about 3 miles away on dead flat, level ground. SteveBaker (talk) 03:18, 6 May 2010 (UTC)
- Pages 774-779 of an old science book from 1887 give an approchable understanding of the Earth's curvature. If the diameter is 8000 miles, then in 1 mile there is 8 inches of curvature. The "drop" increase more than 8 inches in the 2nd mile,to 32 inches, and to 72 inches at 3 miles, and 128 inches at 4 miles, 66 feet at 10 miles. Observers watching a ship see it go "hull down" with only the mast or superstructure visible, through a telescope, when it is several miles away. One old source claims "optical depression" of only 6 inches in the first mile at sea, with similar geometric increases in dip per mile. Only the top of a tall hill or mountain will be visible from a distance. If you note the setting of the sun from the ground floor of a skyscraper, then ride the elevator to the top floor, you might see it once again partly above the horizon. Edison (talk) 03:38, 6 May 2010 (UTC)
- Another example of this effect is to look at a city skyline from across a wide body of water; if you're familiar with the relative heights of the different buildings, you can tell that their bottoms are missing. I have noticed this when riding toward Hamilton over the Garden City Skyway on a day when the air was clear, and observing the Toronto skyline across part of Lake Ontario. --Anonymous, 04:20 UTC, May 6, 2010.
- Fascinating stuff. Please excuse my stupidity - why are the "drop" numbers not increasing at a constant rate? Steve said '"It gets bigger fast, though!"' -- why? 218.25.32.210 (talk) 05:46, 6 May 2010 (UTC)
- Think of a ball. Put your finger on the center top. Move it sideways and watch as it also moves down. At the beginning it moves down slowly, but as you get closer to the edge it moves down faster and faster. Ariel. (talk) 07:10, 6 May 2010 (UTC)
- Ahhh... most helpful! I see now that the constant rate drop I was expecting would mean I was standing on a planar surface - such as the hypotenuse of a triangle (if imagined in 2D). Thank you! 218.25.32.210 (talk) 07:54, 6 May 2010 (UTC)
- Civil engineering surveyors also notice on very large man made structures such as the Humber Bridge ”The towers, although both vertical, are not parallel, being 36 millimetres (1.4 in) farther apart at the top than the bottom as a result of the curvature of the earth.”--Aspro (talk) 11:28, 6 May 2010 (UTC)
- Thanks for the responses; Steve's explanation is particularly enlightening. –Juliancolton | Talk 18:41, 6 May 2010 (UTC)
- Some of the old books say the curvature can be noted in the plains of the US, but when I am driving on a long straight highway and see things behind falling below the horizon, I always figure it could be due to rising and falling terrain. As a child I saw a book showing a man watching through a telescope as a ship sailed out of sight, gradually disappearing below the water, and wondered how he knew the ship was disappearing over the horizon and not simply sinking. Edison (talk) 19:13, 6 May 2010 (UTC)
- Well, the idea is that you look for ships that are sailing towards you - then, when they reach land you can ask them whether they were miraculously recovered from sinking or merely hiding behind the planet! :-) The ocean (or, better still, a nice calm lake to avoid big waves getting in the way) is a better place for testing these ideas than on land because you can be sure that there aren't any hills worth mentioning! SteveBaker (talk) 01:50, 7 May 2010 (UTC)
- "How do you know it's not sinking?" Maybe it is! When the Titanic was sinking, there was actually another ship within sight, the Californian, which had stopped for the night due to the ice hazard. Its radioman had already gone to bed when the Titanic hit the iceberg, but its crewmen saw the Titanic's distress rockets. However, its captain said, in effect, "Well, maybe they're not distress rockets," and decided to do nothing. When the Titanic could no longer be seen, they didn't know it had sunk, because it could have just sailed away. They found out what had happened when the radioman got up. (At least, this is the most widely accepted version of the story, but it certainly has been disputed. Wikipedia has something at RMS Titanic#SS Californian inquiry and SS Californian#Captain Stanley Lord and other places.) --Anonymous, 06:37 UTC, May 7, 2010.
can Blue cheese produce penicillin?
Just curious. If the cheese could produce penicillin then it might be bad to those that are allergic to penicillin. --121.54.2.188 (talk) 05:20, 6 May 2010 (UTC)
- I'll leave it to a biologist rather than speculate, but I can link you to Penicillium roqueforti, the fungus used in the making of blue cheese; Penicillium, our article on the whole genus; and this Straight Dope column from 2004, which claims that "most cheeses contain relatively small levels of antibiotic mold relative to that found in concentrated pharmaceuticals". Comet Tuttle (talk) 06:47, 6 May 2010 (UTC)
- Nobody has mentioned the traditional folk medical practice of using mouldy bread or grain in poultices. Bread supports some penicillium varieties. The practice of using mould has been recorded way back, including of course -mouldy cheese. Moulds in folk medicine--Aspro (talk) 17:30, 6 May 2010 (UTC)
inverse square law and electromagnetic force
If the distance between two positively charged nuclei is halved, the inverse square (of Newton's law of gravity) says that the gravitational force will increase (by a factor of four) (i.e. become more positive/stronger) and the inverse square (of Coulomb's law) says that the electrical force will decrease (also by a factor of four) (i.e. become more positive/weaker). Is this correct? I am having trouble with the semantics of saying that something (electrical force) which becomes more positive is actually decreasing in force/strength. This also doesn't seem to fit with my (fairly poor) understanding of magnets, whereby the force required to bring two repelling magnets together seems to increase (my numbers aren't bearing this out?).
The more I think about it, the more it appears illogical that a decrease in separation also equals a decrease in the net force. But my head gets so screwed up with these negative numbers. I've done the calculations for the original distance and the halved distance, and my net force has moved from a –x10-27 to a –x10-26. Can that be right?
Does this mean the electric force can eventually become a net positive? Why do I think this should all be working the other way around? Any tidbits, filling in the gaps, overviews, corrections, numbers, calculations, examples, etc very welcome. Thank you. Differentially (talk) 06:48, 6 May 2010 (UTC)
Thanks to Ariel for an answer, but I've just finally realised that –x10-27 is a smaller repulsive force than –x10-26. As soon as it's that way around, it makes sense! Differentially (talk) 08:19, 6 May 2010 (UTC)
- Force is not positive or negative. Force is force, and it has a direction (it's a vector, not a scalar). A negative force is a positive force pointing in the other direction. Don't think of negative forces, just think of the direction of the force. Ariel. (talk) 07:08, 6 May 2010 (UTC)
Who's on first? ("Extraterrestrial life(forms)") (done)
Greetings Earthlings! The article Extraterrestrial life mentions people and schools who thought first about life beyond earth but there is no informationen who coined (used for the first time) the English expression "Extraterrestrial life(forms)". So - please no Greek, no other language - who (which book, which article) used this term first? I do not know the answer myself, but I have a bet going on, that it was rather a literary person than a person of science. Am I right? I appreciate any clues (going back in time). Grey Geezer 07:36, 6 May 2010 (UTC) —Preceding unsigned comment added by Grey Geezer (talk • contribs)
- This would probably do better in the language reference desk, but I found this published 1870. And this in 1854. Yet this claims 1868, which is clearly wrong. I think you won't find a definitive answer. Google scanning books has actually caused a revolution in etymology finding much earlier uses that were currently known for many many words. Edit: Even earlier 1848 Ariel. (talk) 09:06, 6 May 2010 (UTC)
- I searched GB before and got unsatisfactory results. It is the combination of life and extraterrestrial. I think I will move to "Languages". Thanks! Grey Geezer 12:03, 6 May 2010 (UTC)
- Google Books is absolutely unreliable in their claimed publication dates, since their system may report the first date found in a work. If it is the 2010 volume from some organization founded in 1660, it usually cites the publication as being from 1660, so it is essential to page back to the title page or equivalent to determine the publication date. They do not even have a channel for reporting incorrect date attribution. Edison (talk) 19:21, 6 May 2010 (UTC)
Mathematics of Kepler's laws
Hi,
I have the following comments about the mathematics of Kepler's laws.In this concern the original paragraphs were edited as follows: Please let me appreciate your editing for this article.Thanks.
TASDELEN's arugments for why Kepler's Laws are wrong
|
|---|
Kepler’s laws are wrong.Kepler’s laws do not explain why the celestial bodies cycle around a barycenter, while Newton’s mechanical laws explain this cycling and give the shape of the orbits. GeneralWhen two bodies are in the empty universe (even n-body in a system) m1*r1=m2*r2 where (r1+r2)=d F=G*m1*m2/d^2 means F*d^2=Ct (this is an hyperbola on Cartesian) Transporting the axes, we write: (f+f0)*(d^2+do^2)=Ct When f=0 dmax^2=(Ct/f0-d0^2) and then the attraction force=centrifugal force So, dmax has a limited, fixed value. Also the barycenter is fixed. First law: The orbits are not elliptical.Why the bodies cycle around a barycenter? Consider the velocities of the bodies in the attraction field V^2=Vp^2+Vr^2 where Vr is the radial velocity and Vp is the tangential (perpendicular) velocity Consider Newton’s law F=G*m1*m2/d^2.We write F=G*m1*m2/d^2=m1*Vp1^2/d=m2*Vp2^2/d then, m1*Vp1^2=m2*Vp2^2 where (d) is eliminated. For the equilibrium of the bodies in motion we see that: Vp1 and Vp2 should exist. This explains the cycling around a barycenter.
Consider Newton’s F*dt=m*dv.This is, F*r*dt=m*r*dv (energy conservation equation), then we write 1/2*m*Vr^2+m*gr*r+1/2*I*w^2=m*r*dVr (total energy with g variable) This is a differential equation dr^2+K*dt^2=2*r*d(dr) with solution r=-a*t*(t*tmax)+K where K=2*gr*r+I*w^2/m=-a^2*tmax^2/(1+4*a) On Cartesian, the graph of (r) is a parabola. On Polar this graph is a cardioidal looking spiral: billions of spirals. Expanding then after compressing; with a max.point, only one max.point. Located on a paraboloid surface along the orbit of the Sun in its galaxy.(our Milky Way) This is the shape of the orbits. No sign of ellipse, no sign of aphelion, no sign of perihelion. See the spirals on Nasa’s galaxies photos. The barycenter is not at one focus of an ellipse. Second law: the areas swept out in equal time are not equalKepler says the Vp velocities are variable, the areal velocities are constant. No.Vp velocities are constant since the existence of the body. This is an innate velocity. Consider the mass m1,and the velocities Vp11,Vp12,…Vp1n for different values of r1: From V^2=Vr^2+Vp^2 we write V11^2+Vp11^2+2*g11*r11=Ct (energy conservation). (I*w^2 is invariable, is innate) V11^2+Vp11^2+2*g11*r11=V1n^2+Vp1n^2+2*g1n*r1n=Ct In an attraction field, Vp doesn’t matter. So, Vr11^2+2*g11*r11=Vr1n^2+2*g1n*r1n (energy conservation, with g variable),therefore Vp11=Vp12=Vp13…..=Vp1n= Ct That is to say: Vp at dmax has the same value as at d0: invariable until the body reaches to the barycenter. Same reasoning for m2. Third law: periods (P1/P2)^2=(r1/r2)^3 is correct.In 1609 Kepler said, orbits are elliptical. Then in 1618 he gave the law of periods. But this last law is valid only and only when the orbits are circular. How Keplerian solid elliptical orbits could be transformed to circular orbit according this law of periods? Simply the orbits were not elliptical. Kepler himself has pronounced his orbital reasoning change with this period’s law: the actual orbits were not elliptical but another shape which could reach to circular orbits. These are spiraled orbits. Sun near the barycenter, no elliptical focus, no closest farthest point in one cycle of the body around the Sun, no aphelion, no perihelion, no equality of swept out areas. |
TASDELEN (talk) 17:47, 8 May 2010 (UTC)=== Consequences: ===
As (r) is variable in the spiraled orbits theory:
P^2/r^2 =Ct is no more valid. It is variable
If today 1 year=365 days, billion years ago it was for example 15 days.
Light-year distance has no sense. Years have different quantity of days.
Light-day or 1000 LD has a meaning, since I*w^2 is constant, is innate.
Newton do not confirm Kepler as say the mathematicians.Etc,…TASDELEN (talk) 08:34, 6 May 2010 (UTC)
- I've taken the liberty of reformatting your post and adding a collapse section so it doesn't take up a large portion of the page.
- To be blunt, almost every bit of mathematics you try to derive above is wrong. Kepler's Laws are a strict mathematical consequence of Newton's Law of Gravitation when one has only two point masses. A complete derivation is given in the article at Kepler's laws#Derivation from Newton's laws, though I don't really expect that you will be able to follow it. Dragons flight (talk) 09:16, 6 May 2010 (UTC)
- Without doubt, TASDELEN's mathematics are incorrect. Probably the first issue I spot is his first line, an equation for what appears to be a ... moment of inertia, or something ... but does not define distance from any particular location (presumably he meant to specify the center of mass, but critical steps are missing and/or rely on erroneous, unstated assumptions). The next line introduces a value, Ct, without explanation or justification. These sorts of skips and jumps are not permissible in a proper physics derivation. For a correct version of these derivations see our article on the two-body problem, specifically how to reduce it to a 1-body problem. Once you have mastered these equations, which are indisputably correct and have been verified by thousands of mathematicians and physicists during the last four centuries, you may be able to expand the theory; but the current work you have presented above is full of physics and mathematical error. Nimur (talk) 15:01, 6 May 2010 (UTC)
- I agree - I'm sorry but what you've presented above is wrong - both physics-wise and mathematically. You can't just make stuff up - that's not how science works! If you attempted to add this to the article, it would be reverted instantly. Heck - I'll personally revert it if you did that!
- But it doesn't actually matter whether your ideas are right or wrong. We simply don't write Wikipedia by having clever, original ideas and writing articles about them - that's just not how Wikipedia operates. In fact, we have a specific rule: No Original Research which actively prohibits you from writing about your own ideas...right or wrong. Instead, we have to write about things for which solid, reliable outside references can be found. Third party, peer-reviewed scientific papers, published in reputable journals are required for science articles like this one. So if you look at the bottom of the Kepler's laws article - you'll see all of the learned scientific papers that were referred to when writing that article.
- So to get your ideas into Wikipedia - you'd first have to write this up as a formal scientific paper. Then you'd need to present your paper for publication at (let's say) "The Journal of Celestial Mechanics and Dynamical Astronomy" (that's a real journal by the way). They would examine your paper, have several other celestial mechanics experts read it - and only if they agreed with your findings and found the math plausible - would they allow it to be published. That's called "peer review" and it's a tough test to pass. If you did get published - then it would be appropriate to go to the talk page of the Kepler's Laws article and suggest a revision to include these new findings - along with a proper link to your article in the journal.
- But the problem with doing that is that your math and science are hopelessly, naively, catastrophically wrong! (I'm sorry - but there is no polite way to express just how wrong it truly is!) Hence, the journal of celestial mechanics are going to write you a polite letter telling you in no uncertain terms that your paper is worthless and they won't publish it. That means that Wikipedians couldn't write about it - even if we agreed that the guys at that journal were clueless and you were the next Einstein (which we don't!).
- So, no - there is no possibility whatever of your change being kept in the Kepler's laws article - or anyplace else in Wikipedia for that matter.
- If you are unconvinced by what I say - please read WP:FRINGE - which covers how we handle these kinds of 'fringe theory'.
Hi,
Many thanks for the comments.Dear commentors:
Dragons flight (talk) 09:16, 6 May 2010 (UTC)
Nimur (talk) 15:01, 6 May 2010 (UTC)
SteveBaker (talk) 15:19, 6 May 2010 (UTC)
“…almost every bit of mathematics you try to derive above is wrong. Kepler's Laws are a strict mathematical consequence of Newton's……”
If I say 2*2=7, then you say “No.You are wrong; 2*2=4”. So, you prove that I am wrong. Otherwise I must ask you “where is the wrong point ?”.Please, show me the wrong point of my mathematics.
“…Derivation from Newton's laws, though I don't really expect that you will be able to follow it.”
Thanks for the compliment. Really I am unable to follow this. This why, I have to repeat my Newton’s derivation so that you can point out the wrong expressions. I think you are mathematicians, dealing with physics, or astronomer. I am a mechanic, diesel engine repairer.
Newton’s F=G*M*m/d^2
is explaining,why the masses have to orbit around their barycenter. Vpm and VpM (the perpendicular velocities to the attraction direction) should EXIST for cycling. In fact F=G*M*m/d^2= m*Vpm^2/d= M*VpM^2/d, that is Attraction Force = Centrifugal force: m*Vpm^2=M*VpM^2, So the cycling is a MUST, due to these velocities.
Newton’s F*dt=m*dv gives
the shape of the orbits: Consider F*r*dt=m*r*dv
F*r*dt is the energy conservation expression’s left side .This is
F*r*dt=1/2*m*Vr^2+m*gr*r+1/2*I*w^2 (total energy, where Vr is the radial velocity).Then
1/2*m*Vr^2+m*gr*r+1/2*I*w^2=m*r*dv (we assume g variable,gr)
Vr=dr/dt and dVr= d(dr/dt)/dt
1/2*m*(dr/dt)^2+m*gr*r+1/2*I/w^2=m*r*d(dr/dt)/dt simplifiying
(dr)^2+ K*dt^2=2*r*d(dr) a differential equation, with solution
r=-a*t*(t*tmax)+K where K=2*gr*r+I*w^2/m=-a^2*tmax^2/(1+4*a)
On Cartesian, the graph of (r) is a parabola. On Polar, this graph is a cardioidal looking spiral: billions of spirals. No sign of ellipse! And this is due to Newton’s laws. Please edit and show the wrong points, before saying “…almost every bit of mathematics you try to derive above is wrong. Kepler's Laws are a strict mathematical consequence of Newton's……”
When speaking of areas
and considering the mass m, the total energy of the body is constant due to energy conservation law of Newton. That is:
Assuming V^2=Vr^2+Vp^2 (radial and perpendicular components of the velocity V)
1/2*m*Vr1^2+1/2*m*Vp1^2+m*g1*r1+1/2*I*w1^2=Constant=C1 (conservation)
1/2*m*Vrn^2+1/2*m*Vpn^2+m*gn*rn+1/2*I*wn^2=Constant=C1 (conservation)
1/2*I*w1^2=1/2*I*wn^2 ( as innate=nothing added to the mass,no modification of w)
In an attraction field a work is done ONLY in the direction of attraction. So,
1/2*m*Vr1^2+m*g1*r1=1/2*m*Vrn^2+m*gn*rn , then
Vp1=Vpn=Constant =C2
That is:
Vp perpendicular, to Vr, is constant at every level of the distance (r).Vr is variable.
When Vp is constant (that is the Vx in Cartesian) no equality of swept out areas in equal interval of time.So, what about Newton’s confirmation of the old mathematicians? Is this is a correct confirmation or a tricky confirmation? Have you controlled this confirmation yourself or just copy-pasted the confirmation of the old mathemeticians? Try it yourself.
“Once you have mastered these equations, which are indisputably correct and have been verified by thousands of mathematicians and physicists during the last four centuries, you may be able to expand the theory; but the current work you have presented above is full of physics and mathematical error.” Where are this errors?
I know it is difficult
to change the perception of the community with such contradictory arguments.And no one of the Keplerian religion will agree with these last Newtonian religion.
“Third party, peer-reviewed scientific papers, published in reputable journals are required for science articles like this one. So if you look at the bottom of the Kepler's laws article - you'll see all of the learned scientific papers that were referred to when writing that article.”
This is not
a scientific article.I do not know how to design a scientific article.I am sure Peer-reviewers will prefer to keep their position in Keplerian religion.But if you start to believe to my mathematics,then someone should help me write a scientific version of my evaluations.
Regards.TASDELEN (talk) 17:47, 8 May 2010 (UTC)
- I did read much of your derivation, so I'll just point out the first couple of mistakes. First, you said that the gravitational attraction is equal to centripetal force (actually, you said centrifugal force, but you meant centripetal) - that is only true for circular orbits where d is constant. In general (and in reality) Keplerian orbits are non-circular ellipses. Second, you said "F*r*dt=1/2*m*Vr^2+m*gr*r+1/2*I*w^2". That equation has to be wrong because one side has a differential in it and the other doesn't - every term needs to be of the same order for it to make sense. You clearly don't understand differentials, so I suggest you avoid them. They are very confusing and completely unnecessary for this. Stick to derivatives (ie. don't say F*dt=m*dv, say F=m*dv/dt). --Tango (talk) 18:20, 8 May 2010 (UTC)
Dear Tango (talk) 18:20, 8 May 2010 (UTC)
By attraction force I mean "attraction towards the barycenter" and by centrifugal force I mean "the force in the contary direction".
When you make the unit analysis of the equation's terms
"F*r*dt" =1/2*m*Vr^2+m*gr*r+1/2*I*w^2 = m*r*dv you have
(dr)^2+ K*dt^2=2*r*d(dr) that is
(metre)^2+K*dt^2= metre*metre= metre^2 which means "terms are of the same order,on left side and on rigth side"
and K=2*gr*r+I*w^2/m=-a^2*tmax^2/(1+4*a)=metre/sec^2*metre= metre^2/sec^2
therefore K*dt^2= metre^2
So,the terms are of the same order.No discrepancies.
"You clearly don't understand differentials, so I suggest you avoid them. They are very confusing and completely unnecessary for this. Stick to derivatives (ie. don't say F*dt=m*dv, say ...." is not valid.I am not mathematician but I know to make "unit analisis".I hope you will appreciate my knowledge.ThanksTASDELEN (talk) 15:50, 9 May 2010 (UTC)
- There is no force in the opposite direction. There is gravity pulling the objects together, that is it. I'm not talking about units, I'm talking about the differential order - basically, count the d's. F*r*dt has one d. 1/2*m*Vr^2 has no d's. That means having both terms in the same equation doesn't make sense. --Tango (talk) 22:30, 9 May 2010 (UTC)
- Dear TASDELEN, I wonder if you have read our article on Johannes Kepler. It explains that he chose his mathematical model (after several failed models) to match the observations of Tycho Brahe. Good scientists always choose a model to match observations. If you can come up with an orbital equation based on spirals that comes as near to matching observations as does the standard Newtonian derivation of Kepler's model, then perhaps someone will take your theory seriously. It is always interesting to read new viewpoints and alternative theories, but they have to be based on real mathematics, and they have to match observations. I suggest that you take another unbiased look at your theory, check it with any mathematicians or physicists that you know, and then find a more profitable outlet for your abilities. Sorry to be so blunt, but I think you are wasting your time on this. Dbfirs 17:34, 10 May 2010 (UTC)
living species which appear to have stopped evolving
Is their a list of species such as sharks, crocodiles, palmetto bugs and ants which are not extinct but which appear to have evolving or which appear to have reached an apex of evolution, excluding my neighbor Billy Bob? :-} 71.100.0.29 (talk) 09:55, 6 May 2010 (UTC)
- There is no such concept as "apex of evolution", evolution doesn't work that way. Living fossil may be of interest. How can you tell if an animal "stopped evolving" without being able to predict the future? Ariel. (talk) 09:59, 6 May 2010 (UTC)
- Silly person. Because you do not need to predict the future to answer the question since the question only concerns itself with the present and the past. In fact even if you have a crystal ball I'm not interested in what it shows you. 11:45, 6 May 2010 (UTC) —Preceding unsigned comment added by 71.100.0.29 (talk)
- The closest sensible version of the question might be: What species appear to have changed the least in the last 100 million years? Is that what you are asking? alteripse (talk) 10:33, 6 May 2010 (UTC)
- It could simply be that they fill their ecological niche so well. Their present morphology is undoubtedly successful at preventing other species from taking over due to Competitive exclusion.--Aspro (talk) 10:41, 6 May 2010 (UTC)
- Exactly. If the environment doesn't change, then the species won't change either. That's why Australian species have been preserved unchanged for so long: there were no changes in the enviromental condition, and there was no external influences that changed them (like new species that migrated from a different environment). Then the European arrived to Australia and introduced new species (rats and rabbits, among other), and existing species had to change or die. --Enric Naval (talk) 11:02, 6 May 2010 (UTC)
- It could simply be that they fill their ecological niche so well. Their present morphology is undoubtedly successful at preventing other species from taking over due to Competitive exclusion.--Aspro (talk) 10:41, 6 May 2010 (UTC)
- "That's why Australian species have been preserved unchanged for so long" [citation needed]. Who says they were unchanged? Do you have any evidence that the rate of speciation in Australia was markedly different than similar areas? Australian species are different because they evolved independently for a very long time, but on average they most definitely evolved. It just happened that evolution often took different paths than in other parts of the world. Even in the absence of migration the continent has still experienced large climate shifts (along with the rest of the globe) since it became an isolated island, and that is plenty of impetus for evolution. And even in the absence of external pressure genetic mutations still sometimes create new traits that are so successful they can disrupt established ecosystems anyway. Dragons flight (talk) 11:52, 6 May 2010 (UTC)
- (edit conflict) Minor correction - it is probable that humans had a major impact on the Australian environment well before the arrival of Europeans - see our article on Australian megafauna. More significant correction - the engine room of evloution is random mutation, and there is no reason why this should stop or slow down just because a species has filled a comfortable niche in a stable environment. Even in a stable environment, species will tend to differentiate and specialise. Gandalf61 (talk) 11:57, 6 May 2010 (UTC)
- 100 million years is an exceptionally long time. Almost no species last that long. Either they die out entirely, or they evolve sufficiently new traits that they come to be labeled as a new species. The typical duration of a species level taxon is only a few million years. Higher level categories like families and classes are more robust, but individual species are usually ephemeral. Probably less than 1% of species would be expected to persist for 100 million years. Dragons flight (talk) 11:52, 6 May 2010 (UTC)
So if the environment is really changing then you are likely to see a reflection of those changes if species that have stopped evolving all of the sudden begen to change again. geez. 71.100.0.29 (talk) 11:49, 6 May 2010 (UTC)
- You can't tell if a species stopped evolving, because you don't know what it will do in the future. Living fossil is probably the best answer available for your question. Ariel. (talk) 12:02, 6 May 2010 (UTC)
- Reading some of the examples should also help. An example I like to use is the tuatara. As our article notes:
- Tuatara have been referred to as living fossils,[2] which means their group retains many basal characteristics from around the time of the squamate - rhynchocephalian split (220 MYA).[19] However, taxonomic work[20] on Sphenodontia has shown that this group has undergone a variety of changes throughout the Mesozoic, and a recent molecular study showed that their rate of molecular evolution is faster than of any other animal so far examined.[21][22] Many of the niches occupied by lizards today were then held by sphenodontians. There was even a successful group of aquatic sphenodontians known as pleurosaurs, which differed markedly from living tuatara. Tuatara show cold weather adaptations that allow them to thrive on the islands of New Zealand; these adaptations may be unique to tuatara since their sphenodontian ancestors lived in the much warmer climates of the Mesozoic.
- Although as a caveat to the rate of evolution I'll add [1] [2] [3] [4] [5].
- Another interesting example is the case of the Wollemia (of which the location of the only known wild specimens is still being kept secret AFAIK [6], again the article helpful as are the references and external links e.g. [7]
- Speaking more generally, if subject to a major environmental change, the rate of evolution may change (or more likely the species will just die off) but it doesn't mean that they ever 'stopped evolving'. That's primarily a simplistic and flawed creationist idea which doesn't get much consideration from evolutionary biologists.
- Nil Einne (talk) 14:23, 6 May 2010 (UTC)
- Reading some of the examples should also help. An example I like to use is the tuatara. As our article notes:
- 100 million years is really just a blink of the eye. Bus stop (talk) 14:28, 6 May 2010 (UTC)
- No, it isn't. 100 million years is 100 millions years, which is a significant amount of time, even speaking geologically. The earth is about 4.5 billion years old, which makes 100 millions years a span of slightly more than 2% of the total - if each blink of your eyes took 2% of your life, you'd miss out on quite a bit, I think. And multicellular life is "only" about a billion years old - of which 100 million years would represent 10% of the span. It's important to emphasize the realities of deep time, but exaggerating it does no service. Matt Deres (talk) 16:37, 6 May 2010 (UTC)
- Well, the age of the universe is 13.75 billion years. That's about 3 times older than the age of the Earth. Bus stop (talk) 17:04, 6 May 2010 (UTC)
- True, but what's your point? The discussion at hand is about rates of evolution and speciation and so on, so the age of the universe is pretty much irrelevant. 100 million years is a very long time indeed and you're not doing anybody a service by telling them otherwise. Matt Deres (talk) 20:26, 6 May 2010 (UTC)
- In a sense, these animals haven't stopped evolving at all. They will still be getting genetic change from mutation and the general genetic diversity within the species - but what is happening is that any changes that happen are having a negative effect - so the mutated animals are not surviving as well as the unmutated population.
- What we need here is a good analogy - and the one I'm about to give is a classic. Imagine all possible sets of genes as being laid out on a large map with the similar gene makeups close to each other and the less similar ones further away - and imagine that the reproductive success/survival rate of a hypothetical animal with a particular genetic makeup to be represented by the elevation of this 'genetic terrain'. Then there will be hills and valleys all over the place on this map - with many of the hilltops being populated by particular species of real animals that have that particular makeup...but with other hilltops representing possible kinds of successful animals that don't happen to exist in the real world.
- As particular animals mutate - their genes change which "moves them" to a slightly different location on the map...typically not far from their parents. Hence, individual animals appear that are a little way away from the others of their species - but because all of the members of the species share most of their genes, they come out as a small 'fuzzy' group of dots on this landscape. If some members of the species are positioned at a lower elevation (ie less reproductively successful) than the others higher up the hill - then they'll be out-bred and eventually die out leaving the ones on the higher elevations who survive. If the species is not quite at the top of the hill yet - then a random change could produce an animal that's higher up the hill (ie more successful at breeding and surviving) - and that animals' ancestors will come to dominate the population. Over many generations, you'd therefore see the members of the species gradually climbing to the top of the hills and then staying there because "when you're at the top, the only way is down".
- So after enough time has passed, you'd see little groups of animals huddled together at the tops of their respective hills - having evolved to be there and all having genetic makeups that can't be improved upon.
- Now, the nature of this process is that once on a 'hillside' the population will climb to the top of that hill. That doesn't mean that the hill is the highest one around. If there is a species in the same environment that's managed to get to the top of a higher hill - then the lesser species will be out-competed and die off - being unable to evolve to be any better without getting worse first.
- It's also possible that there may be other hilltops nearby that are higher than the one they are currently sitting on. But they can't evolve over to that higher hill because any small genetic change just moves the animal off to lower ground where it gets out-bred by the ones higher up in the genetic landscape. A little further away, there will almost certainly be some higher mountains - sets of genes that are much better for survival than the present makeup of these animals. However, if those higher peaks are further away than a few generations of genetic change will reach - then the animals are effectively trapped with their present genes. Any small genetic change makes them worse off - but the huge genetic changes that would get them to a higher hill and allow them to evolve up that new slope are too far away.
- If the slope of the local landscape is shallow enough - then it may be possible for a few generations of mutated animals to survive despite not being so great as the others and drift across that shallow genetic valley onto the slopes of the higher peak. When that happens, they'll rapidly climb that peak and suddenly you have a new species that's much better than the old one.
- But if the slopes are steep then any animal that shifts it's genetic makeup far from the local peak will end up in a deep valley and will die off before it can get onto higher genetic terrain.
- However, this is a statistical matter - it's possible that some really unlikely mutation could change the animal enough to transport it's genetic makeup to the slopes of a quite distant and much taller peak - and then you'd find a sudden shift in the population to a significantly different animal...probably a different species. But if it happens that these animals are on a really steep-sided hill in the middle of a vast plain - then it's possible that no 'reasonable' amount of genetic change will get them to a higher peak - and they will (in effect) have stopped evolving. That's evidently what's happened with these 'static' species. Any small genetic change in a crocodile makes a worse crocodile - and the necessary change to have six legs (or whatever it would take to be a better crocodile) is too large to happen in a single generation. So they are stuck on their little steep-sided hill - unable to evolve in any direction.
- But the thing is that the world changes. If the environment changes (due to global warming, for example) - then that re-prints the map! Genetic positions that used to be hills could now become valleys, other genetic hills will get taller - and the animals will spontaneously start evolving to find the new hilltops. This sudden 'scrambling' to get to the higher genetic peaks is what drives these sudden bursts of new species that pop up after a major environmental change.
- There are other places in this genetic landscape where there are relatively flat, high plateaus. In that case, there are lots of small changes that can happen to the species which are neither better nor worse than their present genes. The animals spread over the plateau because no place is particularly better than any other - and you see considerable diversity within the species. Humans are kinda like that - we have genes for different hair, eye and skin color - genes for lactose tolerance and intolerance - all sorts of variations - but none of those are having much of an impact on our survival rates - so you see people with all kinds of different skin/hair/eye colors surviving equally well.
- If you take another situation, the dark skinned people of Africa had a problem with malaria - and a local 'peak' in the genetic landscape corresponded to having a particular gene that conferred protection against the problem...albeit at the cost of some individuals getting two copies of the gene and dying young with sickle-cell disease. However, this was a local peak and evolution took those people up to it. Now, transport those same people to a region where there is no malaria, or add medical treatment that makes the value of natural malaria immunity 'go away' - and the genetic landscape changes. The peak caused by the malaria problem goes away - revealing a valley due to the sickle-cell issue. Logic says that we should see this gene becoming less common over coming generations as those groups of people evolve back up the sides of that new valley to the top of whatever is the nearest local peak.
- For people who wish to do further research, does that classic analogy have a name? Does Wikipedia have an article about it?
- -- Wavelength (talk) 15:38, 6 May 2010 (UTC)
- That's a good analogy, but it would be better if you turned it upside-down. Better genes should be lower down. That reflects the fact that species will naturally move towards them, just as objects in real space naturally move downwards. They then reach the bottom of a local depression and are stuck there. Getting to a deeper depression would involve climbing over a ridge, which is difficult. --Tango (talk) 17:02, 6 May 2010 (UTC)
- It sounds like something used in The Blind Watchmaker by Richard Dawkins, but with a bit of chemistry/physics thinking mixed in. The analogy lacks enough of a genetic drift element, to my mind. Even without the landscape changing, populations evolve through genetic drift. Not just a few places on the landscape having plateaus (and anyway, this gets a bit weird because we don't have enough dimensions to keep track of all the variables we're talking about), but almost all the places have plateaus for some parts of the genome. And this genetic drift can even alter the shape of the landscape without anything happening externally, leading to selection pressure on this species and others. This is why species continue to evolve, even when conditions don't change. 86.178.228.18 (talk) 19:29, 6 May 2010 (UTC)
- You're close - it comes from Dawkins - but you got the wrong book: "Climbing Mount Improbable". But Dawkins isn't really the originator of this analogy. The general idea of mathematical optimisation is usually visualised that way - and optimising algorithms are sometimes called Hill climbing for that very reason. The way genes evolve is a lot like that mathematical process. When you do optimisation using 'hill climbing' math inside a computer program, you 'climb' a slope until you hit a local maxima...and you have the same problem that an evolving species has - that you may successfully reach a local maxima - yet miss a much better optimisation that's further away - but an overly rigorous algorithm might never be able to descend into the valley and cross the intervening space to find a higher peak. Computers can be programmed to do smarter things like "Shotgun hill climbing" and "Random restart hill climbing" - which involves occasionally injecting some randomness into the process to hopefully jump you over to somewhere on the map where you'll find a better hill. Real world species fail to do that - so it's interesting to note that evolution isn't the perfect algorithm that some people think it is...without the ability to somehow look for yet higher mountain on the other side of nearby valleys - you'll rarely hit upon the best possible solution. Genetics can't do that...computer programs can! SteveBaker (talk) 01:34, 7 May 2010 (UTC)
Okay, so we are talking about a landscape called an environment and organisms on that landscape. I mean this analogy seems to have paralleled the real world exactly. As for dimensions we have all sorts of things, temperature and light and moisture level and turbulence and you name it all of which can change, with the organisms themselves in this terrain representing the genetic code they contain. The mountains or valleys are just places where the environment comes closest to fulfilling the requirements of the genes and vice versa. So if the environment does not change and a change in the genes occurs that does not bring the organism closer to matching the environment then its numbers will decrease due to reproduction. It all makes sense that while a palmetto bug might do better outside due to temperature and rotten vegetation and dead worms, etc. except for spiders and birds and lizards it still comes inside to find enough hidden crud or crumbs to do well. What I'm looking for, however, even though genes will change and the environment will change is a list of organisms which have adapted to both through all kinds of changes and yet remains essentially the same as it always was. If I were designing a tank this is the tank I would want to design. One that is exposed to all sorts of change but is still functioning in the end due to the flexibility or stamina or shape, etc. of the initial design. I just need a list of organisms that are living today and the amount of time since their last notable change or origination as a new species. 71.100.0.29 (talk) 21:04, 6 May 2010 (UTC)
- The original question was sensible, except that it should have used a term such as "local optimum" instead of "apex of evolution". There are a few species such as the cockroach, horseshoe crab, and coelecanth that appear to have changed very little over the last 100 million years, but I'm not aware of a list of such species. (Not that I would know, not being an evolutionary biologist.) Looie496 (talk) 21:39, 6 May 2010 (UTC)
- I've wondered about this--so modern horseshoe crabs resemble fossils from hundreds of millions of years ago, so one could say their appearance has not changed much, right? But couldn't there have been all kinds of change that does not get fossilized? Our page says they use Hemocyanin in their blood to carry oxygen. Would one be able to tell, from fossils, when the use of Hemocyanin in horseshoe crabs began? Was it there all along? ..there must be all kinds of important stuff that is lost in the fossil record. Wouldn't it be impossible to make any claims about the actual genetics of fossils? Or when a new species evolved? Like, a modern horseshoe crab could breed with an ancient one from how long ago? Seems like the best that could be done is making statements about appearance, no? Pfly (talk) 02:17, 7 May 2010 (UTC)
- The entire thread appears to be a tl;dr situation, although the above two posts try to give an answer. My two cents: Triops. 220 million years. ~ Amory (u • t • c) 03:43, 7 May 2010 (UTC)
- I've wondered about this--so modern horseshoe crabs resemble fossils from hundreds of millions of years ago, so one could say their appearance has not changed much, right? But couldn't there have been all kinds of change that does not get fossilized? Our page says they use Hemocyanin in their blood to carry oxygen. Would one be able to tell, from fossils, when the use of Hemocyanin in horseshoe crabs began? Was it there all along? ..there must be all kinds of important stuff that is lost in the fossil record. Wouldn't it be impossible to make any claims about the actual genetics of fossils? Or when a new species evolved? Like, a modern horseshoe crab could breed with an ancient one from how long ago? Seems like the best that could be done is making statements about appearance, no? Pfly (talk) 02:17, 7 May 2010 (UTC)
Chemical explosive rail-gun-better than electric?
If a massive gun had a series of explosive chambers at right angles all the way along it's length, and after a projectile (a manned rocket perhaps) was set moving with an initial explosion at the start of the barrel, would a series of timed chemical explosions in the side chambers speed the projectile up even more, enough to escape Earth orbit without the G-force killing the astronaut, or could it be a more compact and powerful equivalent to an electric rail-gun for military use? Would the speed be higher than any other method? [Trevor Loughlin]80.1.80.12 (talk) 11:43, 6 May 2010 (UTC)
- You are describing something very similar to the Nazi V3 weapon of WW2 [8]--Aspro (talk) 11:58, 6 May 2010 (UTC)
- It's better than one large explosion, but not as good as electric. Electric is constant acceleration, this would be a series of jerks. Humans wouldn't like that. The force is lower for each explosion, but now you are shaking the person violently - this might actually damage them more than a single larger explosion. You could maybe add a buffer to stretch out the force for each explosion - a heavy pusher plate on a spring maybe, with the explosions carefully timed for when the pusher is just about to switch from compressing the spring to stretching it. But an explosion is not really the most efficient way to accelerate something, a constant fire would be better. Ariel. (talk) 12:08, 6 May 2010 (UTC)
- We have, of course, an article at V-3 cannon. To escape from Earth, the projectile needs to reach the second cosmic velocity or escape velocity, which for Earth is 11.2 km/s (call it 10km/s). Assuming perfectly constant acceleration, at 1 g (call it 10m/s), you need to accelerate for roughly 1000 seconds to reach escape velocity. At 10 g, 100 seconds. In that time, your projectile will have traveled approximately 500 km. That's a mighty long barrel for a gun... --Stephan Schulz (talk) 12:16, 6 May 2010 (UTC)
- It's better than one large explosion, but not as good as electric. Electric is constant acceleration, this would be a series of jerks. Humans wouldn't like that. The force is lower for each explosion, but now you are shaking the person violently - this might actually damage them more than a single larger explosion. You could maybe add a buffer to stretch out the force for each explosion - a heavy pusher plate on a spring maybe, with the explosions carefully timed for when the pusher is just about to switch from compressing the spring to stretching it. But an explosion is not really the most efficient way to accelerate something, a constant fire would be better. Ariel. (talk) 12:08, 6 May 2010 (UTC)
- The idea reminds me of Project Orion which was to be a spaceship powered by detonating atomic bombs behind a large 'pusher plate' behind the craft. The issue of peak versus average acceleration was handled by large shock-absorbers. In the case of our OP's idea, you could use a larger number of smaller charges to even out the acceleration still more - and even approximate continuous thrust that way. The difficulty (as others have pointed out) is that you effectively need the rail to be as long as distance travelled by a conventional rocket while under power - and that's going to be a very long 'rail'. Rail guns are a more practical proposition for unmanned launches where the g-forces can be higher...but their main advantage is re-use and the fact that they could be powered by electricity - making them feasible for things like doing mining operations out on the asteroids or on the moon where solar power is cheap but rocket fuel (or explosives) might be unobtainable - and the escape velocities are much lower. SteveBaker (talk) 14:02, 6 May 2010 (UTC)
- Another problem with relying on a series of explosive chambers is the choice of propellant. The lower the molecular mass of the combustion products the faster it will expand (and push on the projectile). The railgun avoids this issue completely and can already achieve (so we are told) 3,600 meters per second (with a hydrogen filled barrel). Railgun#Tests. Faster speeds are theoretically possible to 7,000 m/s. Light gas guns can already achieve this using pure high pressure hydrogen -the lightest of the lot. Using multiple chambers on their own therefore, would not I think, ever be capable of beating this. So, I suppose the answer to the OP's question is no. --Aspro (talk) 15:00, 6 May 2010 (UTC)
I should point out that there are theoretical limits to how fast a projectile can be fired out of a cannon with smokeless powder. The limit is around 1600 m/sec. The Paris Gun came about as fast as a projectile is going to get when fired from a classic style cannon. A railgun on the other hand has a theoretical limit of 6 KILOMETERS/sec. So to answer your question, even with multiple charges, there's no way you can compete with railguns. Besides, having multiple charges in a cannon would only work on one projectile instead of multiple ones, and that's assuming it would even work at all, which I doubt. ScienceApe (talk) 01:12, 12 May 2010 (UTC)
Electrolysis of CuSO4
Is sulfate oxidized to SO4 when CuSO4 is electrolyzed? My chemistry teacher in 11th grade keeps saying that, but I don't think it does. Thanks. --Chemicalinterest (talk) 13:04, 6 May 2010 (UTC)
- persulfate is a possibility SO52−. But SO4 is not realistic. But I do not know if it is formed this way. Graeme Bartlett (talk) 13:21, 6 May 2010 (UTC)
- I think that the reaction is: 2 CuSO4 + 2 H2O → 2 Cu + O2 + 2 H2SO4 The sulfuric acid may react with additional copper sulfate to form copper bisulfate. They said that the reaction was: CuSO4 → Cu + SO4 --Chemicalinterest (talk) 13:36, 6 May 2010 (UTC)
Here is how you would figure this out. You have three species in the beaker: Cu2+, SO42-, and H2O. Figuring out the anodic and the cathodic reactions is actually very easy. Just look on any table of standard reduction potentials, like Standard electrode potential (data page) and then look up the potentials for each reaction. Possible cathodic reactions are:
- SO42− + 4 H+ + 2 e− ⇌ SO2(aq) + 2 H2O Eo = +.17
- Cu2+ + 2 e− ⇌ Cu(s) Eo = +.340
- 2 H2O + 2 e− ⇌ H2(g) + 2 OH− Eo = -.8277
Possible anodic reaction are:
You need to choose a reaction for each electrode. You always choose the one with the highest (most positive) electrode potential, that gives us a cathodic reaction of:
- Cu2+ + 2 e− ⇌ Cu(s) Eo = +.340
and an anodic reaction of
- 2 H2O ⇌ O2(g) + 4 H+ + 4 e− Eo = -1.23
So, we double the first, and combine to get an overall reaction.
- 2 Cu2+ + 2 H2O ⇌ 2 Cu(s) + O2(g) + 4 H+ Eo = -.89
Meaning that, assuming 1 molar concentrations you'd need a minimum of 0.89 V to make the electrolysis extensive. Where your teacher messed up is that they forgot that water was present; in any electrolysis, you need to consider whether or not water is more likely to electrolyze than your other reactants. At the anode, it turns out that the water is far more likely than the sulfate to electrolyze.
This assumes aqueous-phase electrolysis. You can also do liquid-phase electrolysis, but that requires liquid copper(II) sulfate, which isn't usually done in your high school chem lab. In that case, you'd need the liquid-phase electrode potential data; I don't have that at my finger tips, but the method is identical, except you don't have water present, so you'd need a liquid-phase half reaction for the oxidation of the sulfate ion into sulfur trioxide gas and oxygen gas. --Jayron32 15:40, 6 May 2010 (UTC)
- CuSO4 decomposes before it melts into SO3 and CuO. Thanks. Another questionable reaction was that: Zinc and copper are placed in a sodium chloride solution. The sodium ions accept electrons to produce sodium, and the chloride ions give away electrons to produce chlorine. --Chemicalinterest (talk) 15:47, 6 May 2010 (UTC)
- Bullshit. There's nothing you could do to an aqueous solution of sodium chloride to produce sodium metal or chlorine gas, especially not with Zinc metal or Copper metal. Sodium is far more active a metal than Zinc or Copper. You can compare the ionization energy for the metals, or you can look at the electrode potentials for either of them. Putting zinc metal in a salt solution will get you a wet, salty piece of zinc metal. There are corrosion reactions that can occur here, but these involve oxygen and water. Sodium ions can act as a catalyst for these reaction via ion exchange, but you never get sodium metal in the presence of water. It just doesn't happen. Oh, and I looked over your reaction; its pretty much the same as the one I came up with; just that sulfate is a spectator ion to the overall reaction, so I left it out of my analysis, and you kept it in yours. Either way, its correct and your teacher is incorrect. --Jayron32 15:53, 6 May 2010 (UTC)
Planets in Universe
why is it important to find out if there is anther planets in the universt ? —Preceding unsigned comment added by 90.149.184.157 (talk) 14:47, 6 May 2010 (UTC)
- People like to see whether there is other life in space. See SETI. --Chemicalinterest (talk) 15:22, 6 May 2010 (UTC)
- A lot of people consider understanding the universe to be important. There isn't necessarily any practical reason to do it, it's just human nature to want to understand things. Finding out about planets around other stars may help us understand our own solar system better (which may or may not be actually useful). --Tango (talk) 15:34, 6 May 2010 (UTC)
- Pragmatism. The planet cannot sustain the current population growth levels of humans. At some point, either human population growth is going to have to level off (and the mechanisms for that will involve aggessive competition for resources, i.e. wars where lots of people are killed) OR we're going to have to find some other place to live. One of the things about finding life outside of the earth is it allows us to understand how to survive in other environments, either in our own solar system (like Mars) or, how life can be made to work in completely different environments. Furthermore, the prospect of finding a peaceful, but technologically advanced alien race would be quite helpful. Learning how to effectively travel interstellarly is something that seems impossible given our current understanding of the universe, but if another alien race has figured it out, we could too, and that would open up MANY possibilities for human colonization of the galaxy. Even if we were to figure out how to do that on our own, knowing what life, if any, existed outside of our solar system is a handy bit of knowledge. Trying to colonize a planet whose already intelligent inhabitants aren't interested in us doing so is something we'd want to know before we showed up. --Jayron32 15:58, 6 May 2010 (UTC)
- I disagree with your parenthetical assertion. There seems to be a natural reduction in birthrate as standard of living increases (most of the developed world would be showing population decline if it weren't for immigration from the rest of the world). If we can increase the standard of living in the rest of the world, we may well find our population problems disappear. --Tango (talk) 16:53, 6 May 2010 (UTC)
- Oh, yes, if we could raise the standard of living in the rest of the world, that would go a long way towards ameliorating overpopulation problems. How's that been going so far? --Jayron32 17:06, 6 May 2010 (UTC)
- Well, we solved world hunger, then we solved global warming. What's next? Vimescarrot (talk) 18:30, 6 May 2010 (UTC)
- Oh, yes, if we could raise the standard of living in the rest of the world, that would go a long way towards ameliorating overpopulation problems. How's that been going so far? --Jayron32 17:06, 6 May 2010 (UTC)
- I disagree with your parenthetical assertion. There seems to be a natural reduction in birthrate as standard of living increases (most of the developed world would be showing population decline if it weren't for immigration from the rest of the world). If we can increase the standard of living in the rest of the world, we may well find our population problems disappear. --Tango (talk) 16:53, 6 May 2010 (UTC)
- Pragmatism. The planet cannot sustain the current population growth levels of humans. At some point, either human population growth is going to have to level off (and the mechanisms for that will involve aggessive competition for resources, i.e. wars where lots of people are killed) OR we're going to have to find some other place to live. One of the things about finding life outside of the earth is it allows us to understand how to survive in other environments, either in our own solar system (like Mars) or, how life can be made to work in completely different environments. Furthermore, the prospect of finding a peaceful, but technologically advanced alien race would be quite helpful. Learning how to effectively travel interstellarly is something that seems impossible given our current understanding of the universe, but if another alien race has figured it out, we could too, and that would open up MANY possibilities for human colonization of the galaxy. Even if we were to figure out how to do that on our own, knowing what life, if any, existed outside of our solar system is a handy bit of knowledge. Trying to colonize a planet whose already intelligent inhabitants aren't interested in us doing so is something we'd want to know before we showed up. --Jayron32 15:58, 6 May 2010 (UTC)
- Jayron, you laugh condescendingly at the idea of raising the standard of living in the third world, but you think colonizing other planets can solve overpopulation? -- BenRG (talk) 00:25, 7 May 2010 (UTC)
- With any forseeable technology, of course, there is no way to send enough folks to another planet to make even a dent in population. What it is just barely conceivable that we might be able to do, though, is create a self-sustaining population somewhere else, in such a way that if worse comes to worst on this planet, the species will nevertheless survive.
- But a flip side of that has occurred to me. It is possible that, on one planet, no one will deploy (or at least detonate) a weapon that would make the planet uninhabitable, because they themselves would have nowhere to go. With two planets, they could fire them at each other.... --Trovatore (talk) 00:31, 7 May 2010 (UTC)
- No ecological catastrophe could make Earth nearly as uninhabitable as the Moon or Mars are already. Whatever technology might allow people to survive there long-term, you might as well just deploy it here. Put it underground or at the bottom of the ocean if you have to; that's still much, much easier than putting it on Mars. -- BenRG (talk) 03:48, 7 May 2010 (UTC)
- Well, there's something to that argument, but I don't completely buy it. A sufficiently large body colliding with the Earth could indeed make it more uninhabitable, for one thing. Granted that impacts that large are pretty rare. But even in the case of a less-badly-wrecked Earth, one thing you could have here that you don't have there are the people who were left out of the ark. They might be tempted to attack it. --Trovatore (talk) 08:25, 7 May 2010 (UTC)
- A large impact might make the Earth less inhabitable than the Moon for a short period of time (a few years, maybe) but after that time it would go back to being easier to inhabit. Really, the only thing that might be better about the Moon than the Earth is the absence of a dust cloud blocking out the sun making solar power impossible. As long as you have some other way of generating power, the Earth will probably be easier to inhabit within a couple of days of the impact (once the shockwaves have dissipated, for example), and generating power is easier on the Earth than the Moon (you can use geothermal energy, wind energy, tidal power, etc. on Earth, even after a big impact, but not on the Moon). --Tango (talk) 18:29, 8 May 2010 (UTC)
- You won't live long enough to get to the time when the Earth is easier to inhabit. I'm talking about really big impacts, say of the sort that split the Moon off in the first place. --Trovatore (talk) 19:52, 8 May 2010 (UTC)
- Well, I wouldn't use the word "uninhabitable" to describe that situation. I would say that just a collision is fatal, not that it makes the Earth uninhabitable. Habitability is a long term thing, not an instantaneous one. There aren't going to be any collisions as big as the one that created the Moon anyway - that object is believed to have been about the size of Mars. There are only 8 objects that big in the Solar System (including the Earth and Sun) and none of them is going to collide with us before the Sun renders the Earth uninhabitable in about a billion years. --Tango (talk) 03:10, 9 May 2010 (UTC)
- You won't live long enough to get to the time when the Earth is easier to inhabit. I'm talking about really big impacts, say of the sort that split the Moon off in the first place. --Trovatore (talk) 19:52, 8 May 2010 (UTC)
- A large impact might make the Earth less inhabitable than the Moon for a short period of time (a few years, maybe) but after that time it would go back to being easier to inhabit. Really, the only thing that might be better about the Moon than the Earth is the absence of a dust cloud blocking out the sun making solar power impossible. As long as you have some other way of generating power, the Earth will probably be easier to inhabit within a couple of days of the impact (once the shockwaves have dissipated, for example), and generating power is easier on the Earth than the Moon (you can use geothermal energy, wind energy, tidal power, etc. on Earth, even after a big impact, but not on the Moon). --Tango (talk) 18:29, 8 May 2010 (UTC)
- Well, there's something to that argument, but I don't completely buy it. A sufficiently large body colliding with the Earth could indeed make it more uninhabitable, for one thing. Granted that impacts that large are pretty rare. But even in the case of a less-badly-wrecked Earth, one thing you could have here that you don't have there are the people who were left out of the ark. They might be tempted to attack it. --Trovatore (talk) 08:25, 7 May 2010 (UTC)
- No ecological catastrophe could make Earth nearly as uninhabitable as the Moon or Mars are already. Whatever technology might allow people to survive there long-term, you might as well just deploy it here. Put it underground or at the bottom of the ocean if you have to; that's still much, much easier than putting it on Mars. -- BenRG (talk) 03:48, 7 May 2010 (UTC)
- Jayron, you laugh condescendingly at the idea of raising the standard of living in the third world, but you think colonizing other planets can solve overpopulation? -- BenRG (talk) 00:25, 7 May 2010 (UTC)
To understand our local solar system. We have a sample size of 1. This is not enough to know if our solar system is unique, unlikely, or common. So we look for others and try to get more information about solar systems. Ariel. (talk) 20:10, 6 May 2010 (UTC)
- Incidentally, the wording of your question is a little hard to understand, but suggests that you may not realise that we have already found out if there are other planets in the universe (beyond the other 7 in our own Solar system); so far we have detected over 450 Extrasolar planets, and the number is climbing as fast as we can improve our instruments and methods. 87.81.230.195 (talk) 21:10, 6 May 2010 (UTC)
Do airlines use ground length measurements for their mile programs, or air length?
since airplanes travel at an altitude of somewhere near 30,000 feet, wouldn't that add a few miles to the length when compared from measuring from the ground? I know 30,000 feet is insignificant to the radius of the earth, and would only add a couple miles on really long trips, but i like to argue with airline companies. —Preceding unsigned comment added by 76.21.237.247 (talk) 16:05, 6 May 2010 (UTC)
- They use "marketing-ese" to measure the mileage. I think they will tell you this in blunt terms, if you try to dispute a mileage number. They have a standard to determine how many "miles" a particular flight is worth to a frequent-flier program. Think of those as "points", not "miles" in the true sense. The pilots have much more accurate measures of the flight distance than the airline reports to you, the lowly customer; see air speed and ground speed. Integrating either air speed or ground speed will give you a different measure of the total trip-length; air-speed does account for the vertical distance, but is much more significantly affected by wind. Nimur (talk) 16:44, 6 May 2010 (UTC)
- Back when I flew United constantly and obsessed over miles, I found that their FF mileages were extremely close to those from this great circle calculator. That would correspond to the shortest path, along the ground. But in terms of arguing with them, I agree with Nimur: They disclose how many miles the trip gets you, and that's it. They're more of an abstraction than an actual distance. -- Coneslayer (talk) 16:51, 6 May 2010 (UTC)
- From the point of view of the customer, mileage should be calculated as shortest distance between points of departure and arival. The actual path taken, and height travelled, is somewhat irrelevent for your calculations since frequent flier miles are calculated by great-circle distances between airports. And for pilots, the air-mileage is also largely irrelevent. Far more important, for calculation of fuel consumption, is the intergrated air speed, as noted by Nimur. Fuel consumption will be directly related to integrated air speed, which is "virtual air miles travelled", basically the sum of the actual air miles traveled combined with the effect of headwinds or tailwinds. I doubt that anyone has any practical use for calculating the actual miles traveled through the air. It is trivial to calculate it, its just not very useful for either the customer or the airline to know it. --Jayron32 17:04, 6 May 2010 (UTC)
- Here's the thing: Suppose you took a piece of string, long enough to stretch tightly all around the equator. If you then wanted to lift it up and put it on top of 10' poles all around the world - how much more string would you need? It sounds at first like it would be a lot - but in fact, it's pi times 10' - so it's just 31.4 feet extra. So for your airplane at 30,000 feet - the total extra distance if it flew all around the world would be pi times 30,000 feet - so 93,000 feet extra. That's a little over 17 miles. But if you're only flying (at most) halfway around - the difference is only around 8 miles - and for most flights the difference between "air distance" and "ground distance" is going to be just one or two miles. It's really completely irrelevent which they choose. When you consider that the plane is probably flying at between 300 and 400 mph - it's covering a mile every
secondten seconds! You'd only have to be off-course bya few secondshalf a minute to wipe out the error! SteveBaker (talk) 00:04, 7 May 2010 (UTC)- Steve, I think you need to re-check the number of seconds in an hour :-).
- By the way, most commercial flights are traveling over 400 mph, and often as much as 600 mph, at cruising speed. --Trovatore (talk) 02:26, 7 May 2010 (UTC)
- Oopsie! Thanks...fixed it. SteveBaker (talk) 03:35, 7 May 2010 (UTC)
- Also, the height you give is added to the radius of the earth, not the diameter. Therefore, a plane flying at 30,000 ft would add 188,500 ft to an around the equator trip, for a total addition of 36 miles. 36 miles of 25,000 is still pretty much negligible for the purposes of the original inquiry, especially since the longest non-stop flight I know of is from LAX to Sydney, which is under 1/3 of the earth circumference, and would thus add less then 12 miles. Googlemeister (talk) 13:29, 7 May 2010 (UTC)
- Oopsie! Thanks...fixed it. SteveBaker (talk) 03:35, 7 May 2010 (UTC)
Snow accumulating in above-freezing temperatures?
A friend in Bethel, Alaska tells me that they had an accumulation of 5 inches (130 mm) of snow a few days ago, even though the temperature was above freezing. How is this possible? I understand that snow can fall when it's above freezing, but (1) how can it possibly accumulate so much when the air is above freezing, or (2) if enough is falling to accumulate this much snow, how doesn't the large amount of snow cool the air to a temperature below freezing? I should note that my friend is trustworthy; I have no reason to believe that she's making this up. Nyttend (talk) 17:10, 6 May 2010 (UTC)
- It could simply be that the latent heat heat that the snow needed in order to melt was in sort supply due to the specific heat capacity of the air being so low (maybe it was dryish air too). The only other place the heat could come from is the ground and maybe that was very chilly as well. In other words the heat gradient was very small. Also, in the absence of wind there could very well be a laminar boundary layer of much colder dense air close to freezing - covering the snow. Just like the open top freezers in supermarkets.--Aspro (talk) 17:52, 6 May 2010 (UTC)
- Additionally (expand above) if it had been very cold previously the ground itself could still be sub-zero - but the atmosphere above zero - which would delay melting, and cause the near ground temp to be lower..77.86.68.186 (talk) 19:26, 6 May 2010 (UTC)
- Also, it is easy to overlook the amount of heat required to melt five inches of snow. It equals about half an inch of solid ice. To melt that, you need about the same number of calories as you need to bring half an inch of water up to boiling point. Being snow, it also reflects most infra-red heat and the air above it is an insulator. It is going to take time.--Aspro (talk) 19:48, 6 May 2010 (UTC)
- The heat content of air is very small, but snow is wetter when it falls in warmer temperatures. It forms bigger flakes. --Chemicalinterest (talk) 20:44, 6 May 2010 (UTC)
- Isn't snow stable up to about 4 °C, as long as it is kept out of direct sunlight? —Preceding unsigned comment added by Csmiller (talk • contribs) 21:29, 6 May 2010 (UTC)
- The heat content of air is very small, but snow is wetter when it falls in warmer temperatures. It forms bigger flakes. --Chemicalinterest (talk) 20:44, 6 May 2010 (UTC)
- If the frozen precipitation is heavy enough, it can accumulate when the surface temperature is well above freezing. Of course, lighter showers will melt at more than a few degrees above freezing. It doesn't surprise me that 5 inches fell, but it would be good to know how much above freezing the temperature was. –Juliancolton | Talk 02:32, 8 May 2010 (UTC)
- If I remember rightly (can't access it anymore, since this was several days ago now), Weather.com said that it was in the high 30s °F. Nyttend (talk) 02:50, 8 May 2010 (UTC)
- That's definitely possible, then. Snow can fall at temperatures exceeding 40° given the right conditions. –Juliancolton | Talk 03:20, 8 May 2010 (UTC)
- If I remember rightly (can't access it anymore, since this was several days ago now), Weather.com said that it was in the high 30s °F. Nyttend (talk) 02:50, 8 May 2010 (UTC)
metal differentiation
I am having some difficulties differentiating two metal samples. They have the same dimensions and density, though the metal alloys are quite different, with one sample containing nickel, and the other sample does not. Neither sample is magnetic. I can not damage the samples, so using chemicals that would react to nickel would not be feasible. For the same reason, I can not test the hardness or tensile strength of the alloys. I tried using a multimeter to determine if there is a difference in the resistance, however since both are metal, the multimeter gives only a very low Ω value which is lower then the multimeter can use. Are there any other ideas? Googlemeister (talk) 18:38, 6 May 2010 (UTC)
Have you considered X-ray fluorescence analysis?--Aspro (talk) 19:19, 6 May 2010 (UTC)
- Have you got the composition of the two alloys and have to tell A from B, or is the only info you have is that one contains nickel.?
- There are surface tests for nickel (specifically usually for jewelry for people are allergic) - this would not substantially damage the sample. ie you just wipe a swap on the surface...77.86.68.186 (talk) 19:20, 6 May 2010 (UTC)
- Sadly, I do not have equipment for XRF available to me, though I would love such a device. Also, the surface test mentioned is not a good idea since even minor damage would be unacceptable. How about thermo effects on the material? Perhaps I could determine one sample heats faster then the other under the same circumstance, or one is a better conductor of heat? Googlemeister (talk) 19:31, 6 May 2010 (UTC)
- I usually find that if I need a bit of equipment, somebody else has needed it so bad that they have actually purchased it. If they have already purchased it, I can always think of lots of reasons why they should allow me use it. So just because you can't afford one, is not what I consider a legitimate excuse ;-) Aspro (talk) 20:39, 6 May 2010 (UTC)
- Sample A is 75% copper and 25% nickel, and sample B is 56% copper, 35% silver and the balance in Manganese. Googlemeister (talk) 19:33, 6 May 2010 (UTC)
- You could measure the specific heat of the metals. Heat them both to identical temperatures, drop them into identical, insulated containers filled with the same amount of the same temperature water. Watch for the final temperature of the water. You wouldn't even need to calculate the final specific heat, since you know the identity of the two samples (but not which is which), you would just need to know which had the higher specific heat, and compare to the expected values. The one with the higher specific heat will heat the water to the higher temperature. --Jayron32 19:41, 6 May 2010 (UTC)
- Sadly, I do not have equipment for XRF available to me, though I would love such a device. Also, the surface test mentioned is not a good idea since even minor damage would be unacceptable. How about thermo effects on the material? Perhaps I could determine one sample heats faster then the other under the same circumstance, or one is a better conductor of heat? Googlemeister (talk) 19:31, 6 May 2010 (UTC)
- I would expect B to be yellow or yellowish in color - if one is more yellow/orange then that is definately B. (or maybe not): At the risk of appearing facetious it sounds like you are trying to tell apart nickel coins.. Nickel_(United_States_coin)#Wartime_nickels - if so - would the date on the coin be a give away? :) 77.86.68.186 (talk) 19:50, 6 May 2010 (UTC)
- Technically - measuring compressive
tensilestrength is non destructive too... the silver allow should be weaker. or not.. really need a table of values for both.77.86.68.186 (talk) 19:46, 6 May 2010 (UTC)- Not if you suspect that they used the incorrect materials when they made the coin. Googlemeister (talk) 19:53, 6 May 2010 (UTC)
- Are the coins 'as new'.. if not a photo would help - I claim to be able to spot silver patina at long range.77.86.68.186 (talk) 20:00, 6 May 2010 (UTC)
- Not if you suspect that they used the incorrect materials when they made the coin. Googlemeister (talk) 19:53, 6 May 2010 (UTC)
- You said the density of both is the same, yet my simplistic calculation[9][10] shows sample A to have a density of 8.917 g/cm^3 and B is 9.339 g/cm^3. (Does alloying metal change the density, like dissolving salts does?) Ariel. (talk) 20:30, 6 May 2010 (UTC)
- Not sure. All I know is that the weight is identical, and the external dimensions are identical. Googlemeister (talk) 20:33, 6 May 2010 (UTC)
- If the weight is identical to the level of accuracy of the scales you've got, but isn't accurate enough to distinguish the above method ... you can use a simple balance (diy) - it's easy (use a knife edge as the pivot) - a 10cm arm will easily distinguish weight differences of 1mg. —Preceding unsigned comment added by 77.86.68.186 (talk) 20:51, 6 May 2010 (UTC)
- Not sure. All I know is that the weight is identical, and the external dimensions are identical. Googlemeister (talk) 20:33, 6 May 2010 (UTC)
- You said the density of both is the same, yet my simplistic calculation[9][10] shows sample A to have a density of 8.917 g/cm^3 and B is 9.339 g/cm^3. (Does alloying metal change the density, like dissolving salts does?) Ariel. (talk) 20:30, 6 May 2010 (UTC)
- If you've got an ideal material, you can find the Young's modulus non-destructively, but finding the yield strength and failure strength is a destructive process. In practice, most metals behave non-ideally, so even measuring the Young's modulus is a destructive test. (When I worked in a mechanical testing lab, our compressive test specimens would typically be 1/40" shorter after testing). --Carnildo (talk) 01:54, 7 May 2010 (UTC)
Alloys have density as a weighted average of the individual metals composing it. --Chemicalinterest (talk) 20:48, 6 May 2010 (UTC)
- According to http://www.ukcoinpics.co.uk/metal.html the two allows also have similar electric resistances. I can't find any physical data for the Mn alloy - presumably because it was a one off.77.86.68.186 (talk)
- The US national mint probably has the data - should be obtainable since USA has freedom of information acts. Not sure exactly where you would write to? 77.86.68.186 (talk) 21:36, 6 May 2010 (UTC)
- What about microscopy - comparing them against known control samples?77.86.68.186 (talk) 20:52, 6 May 2010 (UTC)
- I don't think density is a weighted average (by %composition) of the component densities unless the volumes literally add. But alloys aren't individual blocks of the components, more like a solution where atoms of one can fit in spaces between others in a packing different than either one alone (for example, nonideal Volume of mixing, so the volume of the alloy is not exactly the volume of the two pure metals being mixed). DMacks (talk) 21:59, 6 May 2010 (UTC)
- Yes you may be able to discern crystal structure or corrosion products with a microscope. Another test is the speed of sound. This will change the pitch of the clink when you tap the sample, and if they are truly the same dimensions should differentiate. This can reveal the compressibility since you know the density. Graeme Bartlett (talk) 22:11, 6 May 2010 (UTC)
- Besides specific heat, you can measure thermal conductivity. That should differ between different alloys. Electrical conductivity might also differ, but you need to be more creative than to just use a multimeter to measure resistance. Rather than just asserting "Neither sample is magnetic" you need to characterize their exact degree of Paramagnetism, Diamagnetism or Ferromagnetism. Copper and silver are diamagnetic, nickel is ferromagnetic, manganese is paramagnetic (no idea how alloys will come out). Such testing goes far beyond trying to pick them up with a magnet. A powerful electromagnetic field and sensitive measurements of effect are needed, but it should be a good test. Getting or making a known standard sample of one of the possible alloys would be very useful if that is possible. Edison (talk) 15:55, 7 May 2010 (UTC)
Cold, flu, etc season/s
Is there one cold, flu, allergy or etc for the whole world or 2 separate ones for all of them?
Basically, I always wonder about this.--Jessica A Bruno (talk) 22:14, 6 May 2010 (UTC)
- I'm not sure I understand what you are asking? They are all 3 different medical conditions, with different causes, though their symptoms can overlap:
- The common cold is caused by a number of different viruses which usually infect the nose and upper respiratory tract, called rhinoviruses. Rhinovirus meaning "virus that infects your nose".
- The Flu, or Influenza, is caused by one of the group of influenza viruses, usually Influenza A or Influenza B. There are other medical conditions which get called flu (such as a "stomach flu") which are unrelated. Commonly (but not always) flu symptoms are more intense than cold symptoms (though sometimes a cold can be really bad, confusing the two). The other difference is that the Flu can be vaccinated against via the flu vaccine and there are antiviral drugs, like Tamiflu, which can fight the infection. The types of viruses that cause a cold are too varied for effective vaccination and treatment under current technology.
- Upper respiratory allergies, sometimes called "Hayfever", properly termed Allergic rhinitis, which is doctor speak for "allergy that causes stuffy nose", is caused by allergens (usually small little bits of dust or pollen) that iritate the nose and trigger a histamine response; such histamine responses usually cause similar symptoms to a cold or flu, however usually absent the fever.
- Hope that helps. --Jayron32 21:12, 6 May 2010 (UTC)
- The article Vitamin D and influenza suggests that there are two seasons, one for each hemisphere. Pollen allergy obviously oscillate between the two hemispheres. Colds? Pass! A submariner told me that for the first couple of weeks of a new tour, everyone has the sniffles. This can not be due to the lack of vitamin D as the body can store quite a bit and so it wont run out so suddenly. However, they are in a very enclosed space. The carbon/ electrostatic filtration system is obviously of little help in this regard. UV lamp sterilizers might help though.--Aspro (talk) 21:14, 6 May 2010 (UTC)
Thank you and interesting.--Jessica A Bruno (talk) 22:14, 6 May 2010 (UTC)
Night Vision
Is it possible to read a book in the dark with night vision goggles? —Preceding unsigned comment added by 71.104.119.240 (talk) 21:13, 6 May 2010 (UTC)
- I don't know why it wouldn't be possible. --Chemicalinterest (talk) 21:16, 6 May 2010 (UTC)
- The output of image intensifiers suffer from scintillation (speckling) , which may make it hard to make out fine details, which is needed for reading. However active IR night vision will probably work, as long as paper is IR reflective, and the ink absorbs IR. CS Miller (talk) 21:27, 6 May 2010 (UTC)
- As above. Yes, if it is not active IR it will need some other light source (unless you have a generation 3½ device or something). --Aspro (talk) 21:33, 6 May 2010 (UTC)
- IIRC, the first image intensifiers (in passive mode) need moonlight to work, the latest will work with starlight on a cloudless night. I'm not sure if the resolution of these devices is enough to read standard size print.
- It goes with out saying that the body-heat night vision systems won't let you read a book. CS Miller (talk) 21:46, 6 May 2010 (UTC)
- As above. Yes, if it is not active IR it will need some other light source (unless you have a generation 3½ device or something). --Aspro (talk) 21:33, 6 May 2010 (UTC)
- I've just tried out a class one device (i.e., cheap and cheerful) on a newspaper and I can easily read the news print. The thing I did notice, was that the optics are so poor that only the centre of the image was in sharp focus. This would make reading slow. Whilst it's possible to buy a reasonably good pair of binoculars for under a hundred dollars these days, if you want a good monocular night scope you might need to spend a thousand or so.--Aspro (talk) 21:57, 6 May 2010 (UTC)
Thank you, everyone - special thanks to Aspro for doing the experiment. Where do you go to buy them, anyway? I know they can be ordered online but it would be nice to try before I buy. 71.104.119.240 (talk) 22:45, 6 May 2010 (UTC)
- I used a Tchibo nachtsichtgerät (night vision aid) tonight. This is a German company (the Nazis had nachtsichtgerät way back during the second world war) that buys job lots and markets them under its own brand. Good enough, if one wants to protect one's night adapted vision, (which would be washed out by the use of an electric lantern). Unfourtunatly, it does not respond fast enough to be able to follow bats and other critters that move quickly. They just don't show up! If you live in Virginia I don't know where you could try some out ( Norfolk Naval Base perhaps). I'd recommend getting one that has adaptations so that you can stick it on your SLR camera/ video camera. It is really nice to be able to record some of the things you see -just as you would, if they happened in daytime. Google around until you find an emporium that stocks them. If you have a specific use, then Google around for other people that would use them for the same thing. They will know which are the best ones to buy. Note: they are very sensitive to light, so it is not practical to try one out in a retail store. You will wreak it! If your just curious to try one out, buy a cheap one. A big benefit, that I have already mentioned is that you can still see ( it takes two hours for your eyes to become fully night adapted). Where as, using a torch to ensure you don't fall a*** over t** into the nearest ditch, would also alert any critters of you presents. Military scopes are great, but not worth the money IMHO for just exploring the back yard. --Aspro (talk) 00:11, 7 May 2010 (UTC)
Hmmm, if there is moonlight you can read a newspaper at night without any device (I would guess the Moon needs to be more than half full). Even on moonless skies, you can read newspaper headlines if there is significant light pollution, see here
Class 8: City sky. The sky glows whitish gray or orangish, and you can read newspaper headlines without difficulty.
Count Iblis (talk) 23:24, 6 May 2010 (UTC)
- I too have read using one of these. There are really two problems - one is that they have a very narrow field of view - the other is that they are generally focussed out to infinity (or at least beyond a few tens of feet) - so it's hard to have things be in focus close-up. SteveBaker (talk) 23:54, 6 May 2010 (UTC)
Longevity of a species
Perhaps I failed to make my previous question clear so I apologize. Now what I am asking is where can I find a list of species and the amount of time the species existed or the age of the species. In other words if man is a species than how old the species of man is.
For example:
| Man | 1 million years |
| Palmetto Bug | 350 million years |
Thanks in advance. 71.100.0.29 (talk) 21:36, 6 May 2010 (UTC)
- For old ones - Category:Living fossils (you'll need to get the dates yourself - and it's not exactly what you asked - but it's a start...) (misc. Anenomes are immortal).77.86.68.186 (talk) 21:44, 6 May 2010 (UTC)
- Are you talking about the plants, the animals, the song or the actress? 71.100.0.29 (talk) 22:24, 6 May 2010 (UTC)
- The rock pool animal.
- For a overview (and earliest possible date for classes of species) Timeline of evolution is a good start.77.86.68.186 (talk) 22:39, 6 May 2010 (UTC)
- It does not appear capable of surviving an oil spill. 71.100.0.29 (talk) 22:46, 6 May 2010 (UTC)
- link ? 77.86.68.186 (talk) 23:10, 6 May 2010 (UTC)
- Only if it could digest bunker c would I call it immortal. 71.100.0.29 (talk) 00:22, 7 May 2010 (UTC)
- link ? 77.86.68.186 (talk) 23:10, 6 May 2010 (UTC)
- It does not appear capable of surviving an oil spill. 71.100.0.29 (talk) 22:46, 6 May 2010 (UTC)
- Are you talking about the plants, the animals, the song or the actress? 71.100.0.29 (talk) 22:24, 6 May 2010 (UTC)
classification characteristics
Is there a table of characteristics which define Phylums within Kingdoms, Classes within Phylums, Orders within Classes, Families within orders, Genus within families, and Species within Genus such that one could determine by querying the table what sort of species they had in hand? 71.100.0.29 (talk) 22:21, 6 May 2010 (UTC)
For all species? Do you know how big that would be? 71.104.119.240 (talk) 22:42, 6 May 2010 (UTC)
- Computers now-a-days should be able to handle not only the table but the queries. 71.100.0.29 (talk) 22:48, 6 May 2010 (UTC)
- The key word is "taxonomic key" or "dichotomous key" or "identification key" - I've not seen one that goes all the way down in one book.
- If there are 2 million species that would be about 22 questions in depth to get to a single species.77.86.68.186 (talk) 22:57, 6 May 2010 (UTC)
- No problem. In fact it could probably be pre-programmed and made into a child's game here: http://www.20q.net/ 71.100.0.29 (talk) 23:02, 6 May 2010 (UTC)
- Try the "Tree of Life Web Project" http://tolweb.org/tree/ - it has many keys for different groups - I don't know if it is complete.77.86.68.186 (talk) 23:04, 6 May 2010 (UTC)
- I see the project and the structure, the names and the individuals that have been included... but alas, not the criteria or the characteristics. 71.100.0.29 (talk) 23:21, 6 May 2010 (UTC)
- Another incomplete key here http://en.wikibooks.org/wiki/Dichotomous_Key/Start 77.86.68.186 (talk) 23:36, 6 May 2010 (UTC)
- That's more like what I had in mind but I fear biological taxonomists will see it as a threat to the realm of biological taxonomy and as an act of biological taxonomic terror. 71.100.0.29 (talk) 00:04, 7 May 2010 (UTC)
- Another incomplete key here http://en.wikibooks.org/wiki/Dichotomous_Key/Start 77.86.68.186 (talk) 23:36, 6 May 2010 (UTC)
- I see the project and the structure, the names and the individuals that have been included... but alas, not the criteria or the characteristics. 71.100.0.29 (talk) 23:21, 6 May 2010 (UTC)
- I'm surprised that nobody mentioned our very own WikiSpecies over on http://species.wikimedia.org/wiki/Main_Page - it has the taxonomy for about a quarter million species. Of course that's really only a drop in the bucket. SteveBaker (talk) 00:36, 7 May 2010 (UTC)
- Unfortunately the wikispecies project has the same problem as the tree of life web project. One must already be an expert to use it. Using characteristics you need not be an expert in the field but instead rely upon the expertise built into the system to which your job is to present measurements or observations. Why are these projects duplicating taxonomic systems only experts can use instead of making the system the expert that everyone can use? What unmitigated and self-serving hypocrisy I observe. 71.100.0.29 (talk) 01:45, 7 May 2010 (UTC)
- I think that's a bit over-the-top. Bear in mind that Wikispecies is not a stand-alone project - like Wiktionary and other such projects, it's designed to complement Wikipedia. You can do formal navigation of the taxonomy tree using Wiktionary - and get to articles written in Wikipedia...or vice-versa. If you want to find an article about Giraffe - use Wikipedia - if you want to find where Giraffa camelopardalis rothschildi fits into the taxonomy tree - use Wikispecies. The reason it uses cold scientific latin terms throughout is that there are no language-specifiec Wikispecies branches as there are for Wikipedia. A German reader should be able to use WikiSpecies with little or no trouble. You can't traverse a modern taxonomy with 'characteristics' because there can be similar - or even identical features in unrelated species that happen to have evolved some similar characteristic. The characteristic: "Has simple eyes" would find the branch of the tree with fish and mammals in it - but it would also have to include cephalopods, annelids, crustacea and cubozoa - which all have eyes that evolved in completely separate ways. The most recent common ancestor of (say) humans and squid didn't have eyes at all. So there is no single point in a taxonomic tree where "has eyes" is a distinguishing characteristic. So a modern evolutionary-based tree can't be navigated that way. About the closest you could come to that would be to query genetic sequences - but that would be even tougher than the latin names! SteveBaker (talk) 02:18, 7 May 2010 (UTC)
- The points you make support the need to ultimately replace the current taxonomic system with DNA sequence classification for animals and plants. (Show me a plant or animal that does not contain minerals or the non-existence of an animal which can photosynthesize.) Regardless, DNA sequences classification will still require classification of associated characteristics that can be measured, observed and described. To prepare for such a system we need to start now by developing a 30 characteristics based query system to permit identification by measurement, observation and description of what characteristics we have already observe. 71.100.0.29 (talk) 06:48, 7 May 2010 (UTC)
- The taxonomic system (you mean 'shared characteristics' or Phenetics) is being replaced by cladistics or phylogenetic classification (as you request)..
- Nevertheless it's still possible to create a decision tree system to result in a phylogenetic classification - in recent times species are being re-assessed to place them in the tree according to their evolutionary heritage (derived from DNA mostly) - more info Biological_classification#Modern_systems. 77.86.67.180 (talk) 11:16, 7 May 2010 (UTC)
- I think there is a problem with attempting to make a single global decision tree for classification (which might still exist) - in that classification isn't a completely precise science - and that classifications are based on a number of factors per branch rather than a single binary decision (ie the decision tree isn't in itself the classification system..)- maybe that's why the biologists don't seem to have produced such a thing. 77.86.67.180 (talk) 11:53, 7 May 2010 (UTC)
- I agree to the extent that:
- query order be dynamic and use many-valued independent variables
- emphasis be on keys which provide the greatest separatory value
- multiple systems, each designed with respective users in mind
- Humans
- robotic probes
- Biologist have had computer technology such as:
- I agree to the extent that:
- The points you make support the need to ultimately replace the current taxonomic system with DNA sequence classification for animals and plants. (Show me a plant or animal that does not contain minerals or the non-existence of an animal which can photosynthesize.) Regardless, DNA sequences classification will still require classification of associated characteristics that can be measured, observed and described. To prepare for such a system we need to start now by developing a 30 characteristics based query system to permit identification by measurement, observation and description of what characteristics we have already observe. 71.100.0.29 (talk) 06:48, 7 May 2010 (UTC)
- I think that's a bit over-the-top. Bear in mind that Wikispecies is not a stand-alone project - like Wiktionary and other such projects, it's designed to complement Wikipedia. You can do formal navigation of the taxonomy tree using Wiktionary - and get to articles written in Wikipedia...or vice-versa. If you want to find an article about Giraffe - use Wikipedia - if you want to find where Giraffa camelopardalis rothschildi fits into the taxonomy tree - use Wikispecies. The reason it uses cold scientific latin terms throughout is that there are no language-specifiec Wikispecies branches as there are for Wikipedia. A German reader should be able to use WikiSpecies with little or no trouble. You can't traverse a modern taxonomy with 'characteristics' because there can be similar - or even identical features in unrelated species that happen to have evolved some similar characteristic. The characteristic: "Has simple eyes" would find the branch of the tree with fish and mammals in it - but it would also have to include cephalopods, annelids, crustacea and cubozoa - which all have eyes that evolved in completely separate ways. The most recent common ancestor of (say) humans and squid didn't have eyes at all. So there is no single point in a taxonomic tree where "has eyes" is a distinguishing characteristic. So a modern evolutionary-based tree can't be navigated that way. About the closest you could come to that would be to query genetic sequences - but that would be even tougher than the latin names! SteveBaker (talk) 02:18, 7 May 2010 (UTC)
- Unfortunately the wikispecies project has the same problem as the tree of life web project. One must already be an expert to use it. Using characteristics you need not be an expert in the field but instead rely upon the expertise built into the system to which your job is to present measurements or observations. Why are these projects duplicating taxonomic systems only experts can use instead of making the system the expert that everyone can use? What unmitigated and self-serving hypocrisy I observe. 71.100.0.29 (talk) 01:45, 7 May 2010 (UTC)
- available to them for only a (relatively) short period of time. 71.100.0.29 (talk) 12:43, 7 May 2010 (UTC)
How many humans a asteroid would be able to have?
How large an asteroid population would be able to be?
I tried to find this info on net but was not able to. —Preceding unsigned comment added by 187.116.80.68 (talk) 23:10, 6 May 2010 (UTC)
- Zero. Except for Ceres, the largest known asteroid, none are large enough for humans to live on. Ceres appears to have enough mass that you won't drift off into space if you try to walk around, but its is leagues away the largest asteroid, so much so that some people have started characterizing it as a "dwarf planet" due to its size. For the "average" asteroid, most are no more than a kilometer or two across, and the simple act of walking on them would generate more than enough escape velocity to send you drifting off into space. --Jayron32 23:16, 6 May 2010 (UTC)
- I think it depends on your imagination. Colonization of the asteroids covers this topic nicely. You might have a hard time living on the outside of one - but you could hollow a small one out, fill it full of air and spin it to make artificial gravity. A C-type asteroid would contain maybe 10% water, plus all sorts of useful organic compounds. It could be hollowed out while mining the ice for drinking water, oxygen for breathing and oxygen/hydrogen rocket fuel. You'd just need a heck of a lot of solar panels to make electricity to first melt the ice, then convert to oxygen & hydrogen for fuel and breathing. As you dig, you'd fill the resulting chamber with air and use the rocket fuel to start spinning it up to speed - a slow process, to be sure - but maybe you can have robots do that part for you and you can just move in when it's all ready. The rock and dirt that comes out of the 'dirty snowball' would be what you'd need for growing food in and for processing into silicon (or whatever) to make more solar panels and other things. The organic compounds would also be good for food production. It's doubtful that you could get one full 'g' of gravity by spinning it because it would fall apart - but something much less than that might be enough for comfortable living conditions. If you picked your target asteroid well, you might find several in similar orbits - the low gravity would make finding and mining other asteroids for metals and other useful substances attractive. When your population gets too big - you can just move to another one. I think asteroids make pretty good homes for technologically advanced humans. SteveBaker (talk) 23:43, 6 May 2010 (UTC)
- Oh - yes...and actually answer the question! It's hard to estimate how many people you could house this way - it depends too much on the actual resource availability and the size of rock you pick. But hollowing out the asteroid into swiss-cheese-like holes with tunnels between them, the surface area of the interior (being three-dimensional) could be much larger than the surface area. But the sustainability issue becomes a problem with having to grow food using predominantly artificial light generated from solar power (or maybe nuclear power if you can find the right materials locally). The acreage of solar panels would have to be many times greater than the acreage of crops planted (because you're essentially condensing large areas of sunlight to account for the fact that the asteroid belt is so far from the sun. So the actual farmable space might be ten times less than the outside surface because of that. Asteroids come in all shapes and sizes - so it's tough to know which size you'd choose. I'd guess a thousand people on a reasonably sized (say 20 mile across) asteroid. SteveBaker (talk) 23:50, 6 May 2010 (UTC)
- As for the solar panels, under the feeble gravity of an asteroid, they could be extended great distances into space, couldn't they ? If the asteroid is spinning, that might present more of a problem, but perhaps light energy could be beamed down to receiver stations on the asteroid, by solar panel satellites orbiting it. StuRat (talk) 21:50, 7 May 2010 (UTC)
- If you imagine something like The Little Prince then it's fiction and doesn't work for a number of reasons. For one thing, there wouldn't be enough gravity to hold on to an atmosphere so you would need to keep air in a closed space like a spacesuit, a building or a hole inside the asteroid. If humans ever get on to an asteroid then it may be a very expensive science, mining or diversion operation relying on supplies from Earth, and not a permanent settlement. The number of people would likely be determined by the task and how many supplies you want to spend resources bringing in, and not on what the asteroid could theoretically support. PrimeHunter (talk) 00:19, 7 May 2010 (UTC)
- The prime examples of fictional hollowed-out asteroids would be Fred Pohl's Gateway and Star Trek: The Original Series's "For the World Is Hollow and I Have Touched the Sky". Clarityfiend (talk) 00:45, 7 May 2010 (UTC)
- Or for a total nut-job take on the idea, it's hard to beat Hollow Earth! SteveBaker (talk) 02:02, 7 May 2010 (UTC)
dinosaur o2 requirements versus mammals and others
In a previous question no one seemed to think that the extinction of the dinosaurs 65 million years ago was due to asphyxiation by carbon dioxide even owing to the deaths of animals and humans known to have died after collapsing within seconds of entering a ravine or other enclosure or depression where co2 has accumulated. Since the avail dinosaurs (birds) survived, however, I was wondering whether or not extinction of 65 million years ago may have been due not to a low-to-ground atmosphere of pure co2 but simply the absence of sufficient mixture of oxygen which the grounded dinosaurs could not overcome. Of course this idea might also account for the fact that some dinosaurs occupied mountainous areas high enough to escape the co2 but too high to provide enough o2. In other words a layer between the area too high in co2 and too low in o2 for a dinosaur to survive. Could then the lack of sufficient oxygen be the cause of mass dinosaur extinction whereas what was available was enough for avail dinosaurs, insects, mammals and the rest? 71.100.0.29 (talk) 23:56, 6 May 2010 (UTC)
- I'm sure toxic gases played a role after the impact, but large levels of O2 would have been required for the growth of the dinosaurs. In fact, high levels of diatomic oxygen gas are required for all of the fabulous processes of mammals, and while early mammals may have needed far less than we do now their requirements surely could be on par with current rodents. ~ Amory (u • t • c) 03:33, 7 May 2010 (UTC)
- I think the extinction was more likely due to the impact sending huge amounts of water vapor and particulates into the air, causing worldwide nuclear winter: those creatures that could adapt to the cold temperatures survived, whereas dinosaurs, being essentially huge two-legged cold-blooded lizards, could not adapt (lizards need outside heat for their bodies to function, and dinosaurs need a lot of heat because of their size), so they died out. FWiW 67.170.215.166 (talk) 05:29, 8 May 2010 (UTC)
- Actually the reverse is true. The amount of energy required to support a large animal's BMR is less: e=m^(3/4). 71.100.0.29 (talk) 20:21, 8 May 2010 (UTC)
- I think the extinction was more likely due to the impact sending huge amounts of water vapor and particulates into the air, causing worldwide nuclear winter: those creatures that could adapt to the cold temperatures survived, whereas dinosaurs, being essentially huge two-legged cold-blooded lizards, could not adapt (lizards need outside heat for their bodies to function, and dinosaurs need a lot of heat because of their size), so they died out. FWiW 67.170.215.166 (talk) 05:29, 8 May 2010 (UTC)
May 7
humanure
i knew a guy in the usa who got committed for 5 days because he practiced humanure. there was nothing wrong with him and he was eventually released. a) how can they do that and b) can he sue them and under what grounds? —Preceding unsigned comment added by Tom12350 (talk • contribs) 02:19, 7 May 2010 (UTC)
- That's odd...how'd anyone know? DRosenbach (Talk | Contribs) 02:31, 7 May 2010 (UTC)
- A) There may be some local laws to keep people from using human excrement as compost. Composting toilets may not be allowed where he lives due to the availability of citywide sewage systems or laws which require septic systems. Dismas|(talk) 02:40, 7 May 2010 (UTC)
i think it was legal since he lived in the country side but thats irrelevant. if it was illegal they should have arrested him no t committed him. clearly they had no cause or legal right to commit him.
—Preceding unsigned comment added by Tom12350 (talk • contribs) 03:09, 7 May 2010 (UTC)
Sounds like we aren't getting all the facts here. Also be aware that there is a difference in Humanure and Night soil. Beach drifter (talk) 03:13, 7 May 2010 (UTC)
what more facts do you need? —Preceding unsigned comment added by Tom12350 (talk • contribs) 16:27, 7 May 2010 (UTC)
- If you are claiming that someone was involuntarily committed to a psychiatric facility because he was putting his excrement in a garden or yard, I do not believe you. You either do not know all the facts or are withholding or misrepresenting some of them. alteripse (talk) 16:44, 7 May 2010 (UTC)
that is really what happened. he also only bathed once a week, which they also held against him. thats all he did. they said it was "abnormal behavior" and claimed it was enough to commit him. he has the paperwork to prove it which he showed me. —Preceding unsigned comment added by Tom12350 (talk • contribs) 04:08, 8 May 2010 (UTC)
- See involuntary commitment for a discussion of the rules on this in the US. Even for a brief commitment of 2 days, someone would have had to persuade the police and an admitting psychiatrist that he was a danger to himself or someone else. You dont have the whole story or you are not giving us the whole story. alteripse (talk) 04:28, 8 May 2010 (UTC)
they specifically said he was NOT a danger to himself or someone else. they claimed that because he was bathing once a week and storing urine and feces (for humanure) that he was "unable or unwilling to care for himself" despite showing the doctors he could they still kept him for 5 days. —Preceding unsigned comment added by Tom12350 (talk • contribs) 19:51, 8 May 2010 (UTC)
What did he grow with his humanure? If the answer is nothing then forget about a case. 71.100.0.29 (talk) 21:14, 8 May 2010 (UTC)
- This is the sort of question that changes a LOT depending on the details. Does he live out in a farm and was using a modified outhouse to properly compost and sterelize human wastes? Or was he a city dweller keeping his crap in jars who ranted about saving the environment anytime someone questioned him about it? What you described could easily go either way.
- They don't commit people "For" doing some particular thought crime, the person's mental state has to be evaluated. In that sense, it's not really about the humanure, they could commit him 'for' filling out a crossword puzzle if he was somehow doing it in a way that led people to think he was badly insane.APL (talk) 22:52, 8 May 2010 (UTC)
- Good grief APL. You dont do the ref desk or the questioners any favor by giving answers about things you know nothing about. Didnt you even read the involuntary commitment article referenced above? "Insane" is neither a legal nor a medical terms and certainly not a criterion for commitment. American cities are full of people who are not just diagnosable with a serious mental illness but cannot take care of themselves, yet our laws forbid involuntary commitment unless they seem to be posing an imminent threat to themselves or others. They are called "homeless". alteripse (talk) 13:18, 9 May 2010 (UTC)
- Having read the article I stand by my basic point that the question as-asked, is unanswerable because people are not committed for doing specific "things" (Like composting human waste), but because someone believes they have a dangerous state of mind. That's what makes the whole process different than arresting people on criminal grounds where there really is a giant list of specific things you can't do. APL (talk) 18:24, 9 May 2010 (UTC)
- Good grief APL. You dont do the ref desk or the questioners any favor by giving answers about things you know nothing about. Didnt you even read the involuntary commitment article referenced above? "Insane" is neither a legal nor a medical terms and certainly not a criterion for commitment. American cities are full of people who are not just diagnosable with a serious mental illness but cannot take care of themselves, yet our laws forbid involuntary commitment unless they seem to be posing an imminent threat to themselves or others. They are called "homeless". alteripse (talk) 13:18, 9 May 2010 (UTC)
so does this guy have a case to sue them or not? he was using the humanure in his garden. —Preceding unsigned comment added by Tom12350 (talk • contribs) 18:34, 9 May 2010 (UTC)
- (1) Anyone can sue anyone for anything-- that is not the same as having a good case that a lawyer would take. (2)We cannot give legal advice here and IANAL. (3) Not even the best lawyer in the country would give you a yes or no answer to your question without wanting to ask your friend a whole lot of questions. (4) I think we have about exhausted this question. alteripse (talk) 23:46, 9 May 2010 (UTC)
Don't hold your breath
I was able to hold my breath with no distress to 1:45 and after beginning to increasingly suffer, I made it to 2 minutes before I had to exhale and then inhale. Is that normal for a 28 yo healthy white male? I was very surprised, assuming that humans couldn't hold their breath for that long. Granted it's not the 30 minutes-plus of a marine mammal, but is 2 minutes for a human while sitting quietly normal? DRosenbach (Talk | Contribs) 02:30, 7 May 2010 (UTC)
- Normal? Maybe. With practice, a person can train themselves to hold their breath for longer and longer times. Have you been practicing? Dismas|(talk) 02:44, 7 May 2010 (UTC)
- PS I'm in my mid-30s and was just able to make it to 1:30 without too much strain. Dismas|(talk) 02:50, 7 May 2010 (UTC)
- 2 minutes is not that big a deal especially if you hyperventilate first (2 or 3 deep breaths is enough, you don't have to get dizzy or anything like that). 69.228.170.24 (talk) 02:56, 7 May 2010 (UTC)
- That's exactly what I did before my attempt. I think I could go longer if I was laying down and not having to support my torso as I'm doing now sitting upright on my couch. Dismas|(talk) 03:03, 7 May 2010 (UTC)
- According to Static Apnea, the world record for breath-holding is eleven and a half minutes [11]. I had thought that it was around six! Man, sometimes I have a hard time going eleven and a half minutes between ginger snaps. (Note that the record was just eight minutes a decade ago, which suggests that being able to get anywhere close to that is highly unusual.) Paul (Stansifer) 03:23, 7 May 2010 (UTC)
- It depends on if the breath hold is under water or not. Due to the mammalian diving reflex humans can hold their breath much longer if their face is in cold water. Ariel. (talk) 05:11, 7 May 2010 (UTC)
Not a scientific response, but personal experience: I sometimes find it easier if I slowly exhale as I reach the point where it starts to burn. Riffraffselbow (talk) 06:52, 7 May 2010 (UTC)
- I know that with practice humans can hold their breath for several minutes, but I am very surprised by the above comments. If I'm anything like Mr. Average I struggle to hold my breath for longer than one minute, unless I practice ... a lot. Astronaut (talk) 13:06, 7 May 2010 (UTC)
- With hyperventilation I can hold it for 1 1/2 minutes. I am 15. --Chemicalinterest (talk) 13:26, 7 May 2010 (UTC)
- The weird thing I find is that I can't hold my breath for even a minute just sitting down doing it (I'm 54 years old - so I don't last as you young-un's) - but I can easily swim between two and three lengths of my back-yard pool underwater - and hold my breath for well over a minute to a minute and a half, despite the obvious extra exertion due to the swimming burning up oxygen faster. I don't understand that. But I suspect it's because the "rules of the game" are a bit different. When I sit here holding my breath, it somehow seems to be cheating to release air from my lungs while I'm "holding" by breath - resulting in an explosive exhalation when I finally have to give up. When swimming underwater, it seems OK to gradually release air as I swim - so long as I don't surface and take another breath. Does this somehow explain the difference in my times? I think so...but I don't understand why. When I release air underwater, it's not like I'm breathing out just the CO2 that I don't want and keeping the left-over oxygen in my lungs! What I'm breathing out has the same percentage of oxygen as what's left behind. So are you folks who can manage an impressive 2 minutes allowing yourself to exhale while doing it? SteveBaker (talk) 14:06, 7 May 2010 (UTC)
- Steve, I posted about this a little higher up, the reason is the mammalian diving reflex. Even though you are exerting your mussels, they are probably working non-aerobically, at least at first, and the reflex prevents them from using up your oxygen. Ariel. (talk) 21:07, 7 May 2010 (UTC)
- In my youth I once held it for over 3.5 minutes, hyperventilating first. Claims of 11.5 minutes just do not seem plausible. Most people seem to give up at the point where a "burn" or oxygen need is first sensed, which seems like a pretty healthy response in general, but if you were under water it would not end well. Edison (talk) 15:47, 7 May 2010 (UTC)
Hyperventilating before swimming under water is dangerous, because the most important stimulus for breathing is the CO2 content of the blood, and not hypoxia. By hyperventilating, you artificially lower the CO2 content of the blood, thereby suppressing the urge to breath, making it possible to stay under water until you pass out because of hypoxia. See shallow water blackout. --NorwegianBlue talk 16:59, 7 May 2010 (UTC)
What's a tumor like?
I've heard about tumors that are "inoperable" because they're wrapped around organs or whatever. So what is a tumor like? From the way that people talk about them, I guess they aren't some semi-solid mass that you can just cut bits off until you get something that is small enough to just pull out. So what are they like? Dismas|(talk) 03:02, 7 May 2010 (UTC)
- Well Tumor has the basics, as well as links to what you probably want, like Cancer. Calling a tumor inoperable usually means that it's not safe to remove the cancerous growths. Often this occurs because the cancer was detected late in the game, and has already metastasized to other parts of the body (thus making it very hard to remove). Also, cancerous tumors don't have to be wrapped around something, it can be withing something. A relative of mine, for example, currently has a tumor growing inside a kidney. Tumors inside vital organs, such as the liver or spleen, can be very tricky to remove properly. ~ Amory (u • t • c) 03:21, 7 May 2010 (UTC)
- Tumors generally originate in an organ, and often invade other nearby structures. There isn't always a clear dividing line, since it infiltrates into surrounding tissue. And if vital structures are involved, it may not be possible to remove it, as Amorymeltzer points out. And yes, if a cancer has metastasized (spread), there may be no benefit to removing the primary tumor (and surgeries always carry risk). Does that help?
- I would have to respectfully disagree, Amory, regarding your specific examples of inoperable tumors. In the cases you presented, the surgeons would remove the entire spleen. Inoperable tumors exist in or around crucial organs, such as the brain, in which case an operation to remove the tumor may necessarily lead to immediate loss of life/function while allowing the tumor to remain would likely lead to loss of life/function later down the line. The parotid gland, for example, exists with the facial nerve running and branching within it -- so it's a difficult task to remove the parotid because removing it en masse' would leave the patient with a unilateral facial muscle paralysis. But there are ways around this problem, such as testing each and every bit of tissue for conduction ability prior to resection. The point is that each case is different and a particular benefit/cost analysis of each and every case would need to be carried out. DRosenbach (Talk | Contribs) 13:06, 7 May 2010 (UTC)
- Tumors generally originate in an organ, and often invade other nearby structures. There isn't always a clear dividing line, since it infiltrates into surrounding tissue. And if vital structures are involved, it may not be possible to remove it, as Amorymeltzer points out. And yes, if a cancer has metastasized (spread), there may be no benefit to removing the primary tumor (and surgeries always carry risk). Does that help?
- Tumors are the result of uncontrolled cell division. As such, tumors are relatively similar in physiology to normal cells with a few exceptions. They usually have poor blood supply, and they don't differentiate to perform special functions like their normal counterparts. Instead they just grow, and grow, and grow. Some invade surrounding tissues very easily, others do not. Some metastasize (when a few cells break off and travel through the blood or lymph system) and form new tumors in other locations; again, others don't. There are no hard and fast descriptions of a tumor, but instead several different descriptions depending upon the type. Regards, --—Cyclonenim | Chat 14:25, 7 May 2010 (UTC)
Reverse dial gauge/indicator alignment
How to carry out shaft alignment using reverse dial gauge/indicator alignment method? —Preceding unsigned comment added by 202.79.203.51 (talk) 03:31, 7 May 2010 (UTC)
- Please rephrase your question. I don't think anyone understood it. Ariel. (talk) 22:00, 7 May 2010 (UTC)
- Does this help at all? Reverse Dial Alignment--Aspro (talk) 16:01, 8 May 2010 (UTC)
archae biotech
Biotechnologically, what are the manipulations made to some archae present deep inside the earth? —Preceding unsigned comment added by 125.21.50.214 (talk) 03:43, 7 May 2010 (UTC)
How many slots on GEO are available for communication satellites?
Obviously a limited number, but how big is it? See also orbit allocation. 70.48.64.135 (talk) 05:09, 7 May 2010 (UTC)
- The problem is not room for the satellites - there's tons of room. The problem is all the satellites use more or less the same radio frequency, and if they are too close together you will pick up signals from more than one at a time. So there is no real way to answer you question - if you stagger the frequencies you can fit more in. But maybe you need a specific frequency in a specific spot and you can't. Also, some places are better than others, for example over the ocean is not a desirable spot. Ariel. (talk) 06:25, 7 May 2010 (UTC)
- It is obvious that the physical space for satellite not a problem (how could it even be? Have you have slightest idea of how big GEO?). It is also absolutely obvious that the problem restricting number of COMSATs on GEO is RFI. If you know nothing about subject, please refrain from answering the question. Reading link I provided above might also help. PS. I'm almost speechless about your remark about "spot over the ocean". Yes, while it is true that for the spot over the ocean, signal must travel longer distance, the relative overhead would be minimal, if worth of mentioning. For a country over equator signal from satellite flying directly above the country must travel ~36 000km, while from satellite that 10 000 km from the first one and "over the ocean" signal traveling distance would be 37 400km. Please also read geostationary orbit article. 70.48.64.135 (talk) 08:12, 7 May 2010 (UTC)
- QUOTE from the link I provided above, before you start talking ignorant nonsense about "no real way to answer you question": The requirement to space these satellites apart to avoid harmful radio-frequency interference during operations means that there are a limited number of orbital "slots" available, thus only a limited number of satellites can be operated in geostationary orbit. This has led to conflict between different countries wishing access to the same orbital slots (countries at the same longitude but differing latitudes) and radio frequencies. These disputes are addressed through the International Telecommunication Union's allocation mechanism. 70.48.64.135 (talk) 08:24, 7 May 2010 (UTC)
- Unless you know which frequencies are desired, and how many satellites overlap in a given area+frequency how are you supposed to know how many can fit? It all depends on what they want to do with them. And some satellites have more narrow broadcasting (spot beam), and others are wider. You could fit tons of spot beams, and just a few wide ones, there is no single number for how many will fit. The problem with the ocean is that it's over the horizon for your customers unless they have tall masts (and satellite communication is line of sight), it's not the distance to the satellite. Ariel. (talk) 09:07, 7 May 2010 (UTC)
- To help you understand, the radio waves from the satellite dish spread out and cover a particular angle, called the main lobe. For a small satellite TV dish this could be 3 degrees. So you cannot have two satellites sending to the same area closer than 3 degrees. If you have a bigger dish, such as might see for C band satellite TV, the beam will be around 1 degree, and those satellites sending to large dishes could be closer together. There could be sidelobes as well that pickup interference, so the response from the antenna to a neighbouring satellite should be very small. However most of the money will be from pay TV on Ku band, so that will determine the economic distance. Over the mid Pacific Ocean there are very few satellites, so there are plenty of free slots, but few users. Graeme Bartlett (talk) 12:33, 7 May 2010 (UTC)
Stars
Sorry for being dumb! Why are stars born and dead without living? If they live, what life do they lead? - anandh, chennai —Preceding unsigned comment added by 125.21.50.214 (talk) 05:57, 7 May 2010 (UTC)
- There are many types of stars, and they lead very different lives. Light stars would take billions or even trillions of years to run out of fuel, then slowly burn out into a cinder. Intermediate stars like the sun would take several million years to use up their fuel, and heavy stars burn out quickly then erupt in a supernova. See the article on Stellar evolution for more information. --The High Fin Sperm Whale 06:04, 7 May 2010 (UTC)
- Stars like the Sun last for billions of years, not just millions. StuRat (talk) 19:49, 7 May 2010 (UTC)
- Sperm Whale: his question was more etymological than scientific; In short, stars are born and die because humans, or at least western culture, think of stars as quasi-living beings. It's anthropomorphism, akin to Mother Nature or the concept of a living sea. Riffraffselbow (talk) 06:50, 7 May 2010 (UTC)
- In other words, they aren't alive, they aren't born, they don't die, and they don't "live different lives" as Whale said -- we just talk about them as if they do. It's a metaphor. People also speak of places and companies and lots of other non-living things as being born and dying, when we mean they start existing and stop existing. --Anonymous, 06:55 UTC, May 7, 2010.
- Yes, but... Define "life". Stars "metabolise" hydrogen into helium. They certainly react to some stimuli, and when they die, they seed the interstellar medium in a way that influences the next generation of stars. Are viruses alive? ;-) --Stephan Schulz (talk) 07:22, 7 May 2010 (UTC)
- You're asking a philosophy question on the science refdesk. Scientists hate philosophy, it makes them grumpy. Riffraffselbow (talk) 07:43, 7 May 2010 (UTC)
- Yes, but... Define "life". Stars "metabolise" hydrogen into helium. They certainly react to some stimuli, and when they die, they seed the interstellar medium in a way that influences the next generation of stars. Are viruses alive? ;-) --Stephan Schulz (talk) 07:22, 7 May 2010 (UTC)
- In other words, they aren't alive, they aren't born, they don't die, and they don't "live different lives" as Whale said -- we just talk about them as if they do. It's a metaphor. People also speak of places and companies and lots of other non-living things as being born and dying, when we mean they start existing and stop existing. --Anonymous, 06:55 UTC, May 7, 2010.
well, thanks for making me a philosopher. All i need to know is about the duty of stars during their life time. —Preceding unsigned comment added by 125.21.50.214 (talk) 10:51, 7 May 2010 (UTC)
- It's not philosophy - it's linguistics. Wiktionary has half a dozen meanings for words like "born", "life", "live", and "die" - just pick the appropriate ones and not the inappropriate ones and the problem disappears.
- Anyway, the definition of "life" is fuzzy. It is increasingly clear that there is no hard boundary between things that are clearly alive (Zebras) and things that clearly aren't (Rocks) with things like Viruses being on the borderline. I think most people would put stars rather closer to the "Rock" end of the scale than the "Zebra" end - and probably somewhere lower down the scale than Viruses. However, there are some attributes of stars (like that they do, somewhat, in a sense, 'reproduce') that puts them above rocks on that scale. But you can't ask science to answer a question that only relates to the arbitary use of a fuzzy word. Stars are whatever stars are - no matter whether we call them "alive" or "inanimate".
- SteveBaker (talk) 15:05, 7 May 2010 (UTC)
- I echo The High Fin Sperm Whale in saying the stellar evolution article is probably what you are looking for. Comet Tuttle (talk) 19:37, 7 May 2010 (UTC)
Nitrogen in tyres
I've read a couple of things on filling tyres with nitrogen, which basically boil down to: it leaks less than air, it corrodes wheel and tyre less than air, it expands/contracts less with temperature than air. And almost all of that is due to the fact that the nitrogen sold to you is dry, whereas the air from the local service station has water vapour in it, and water vapour expands and contracts strongly with temperature change and with the help of the oxygen causes the corrosion. So filling tyres with dry air gives you all of the benefits of using nitrogen, except for the leaking thing.
So, a few questions: 1. If I fill my tyres on a cold (5°C) low humidity day do I get the same benefits? 2. Presuming I fill on such a dry day, and always top up in similar conditions, will my tyres become ever closer to being filled with pure nitrogen as oxygen leaks more quickly than nitrogen? 3. I'm a brewer, I have bottled CO2, how does CO2 compare? Should I use that instead to fill my tyres? 4. Should I use this as an excuse to buy a bottle of nitrogen which will also contribute to better stouts and other British beers?
I also note that the companies selling nitrogen tell you that you must never top up your tyres with air if you're using nitrogen as it will ruin any benefit - (5) does this ruin my plan, with the tiny amount of water included in low humidity air? (6) Does this ruin the tyre companies' plans if the tyre has a reasonable amount of air in it (from being installed onto the wheel in a normal air atmosphere).
Wow, a lot more questions than I had when I started thinking about and typing this question. So, one more for luck (7) If I'm stating with a tyre full of normal, wet, air, and I wanted to refill it with dry air or whatever gas, how many times would I have to deflate it to near zero net pressure and inflate it to 200kPa before I could be reasonably assured that there were no more than trace amounts of what was in the tyre before? --Psud (talk) 07:48, 7 May 2010 (UTC)
- Remember, they want to sell you something. How often have you seen a tire "corroded" (from the inside) before its tread has worn down? So go by point 3 and leave your car alone ;-). --Stephan Schulz (talk) 08:21, 7 May 2010 (UTC)
- Using carbon dioxide will cause the tyres to get hotter. This gas is has a higher molecular mass, so the work done on it by the flexing tyre will heat it more than pure nitrogen and more the normal air. You did not say what type of vehicle you were considering this for. If you are a microbrewery, then it is not worth it, but if you have a fleet of commercial lorries, then you could save because you could have the same old tyres remoulded time, after time, after time. [16] As for nitrogenated beer -sacrilege! --Aspro (talk) 11:40, 7 May 2010 (UTC)
- That doesn't make sense. Higher heat capacity ==> temperature rises less quickly. John Riemann Soong (talk) 19:40, 7 May 2010 (UTC)
- Uhmm. Maybe then, you are thinking still in terms of the classical experiments, with those same values that were fixed (by the design) and those values that were the variables (which aided the formulation of the laws).--Aspro (talk) 21:12, 7 May 2010 (UTC)
We discussed this issue in 2007, and brought it up peripherally in a 2009 discussion about nitrogen. Nimur (talk) 06:16, 8 May 2010 (UTC)
space objects on earth
What prevents the space objects (moving randomly) from hitting the earth? Rarely does that happen. - anandh, chennai. —Preceding unsigned comment added by 125.21.50.214 (talk) 09:51, 7 May 2010 (UTC)
- Well, they're spread pretty thinly, and small objects burn up in the atmosphere before they hit the surface. Most things in the solar system are pretty comfortable in their orbit around the Sun, too. 212.219.39.146 (talk) 10:20, 7 May 2010 (UTC)
I think we have impact craters on the surface of the earth. 125.21.50.214 (talk) 10:48, 7 May 2010 (UTC)
- See meteorite. The friction of the atmosphere gases vaporizes the rocks. --Chemicalinterest (talk) 11:56, 7 May 2010 (UTC)
- Nothing stops space objects from hitting the Earth and many thousands of objects hit the Earth every day. The vast majority of these objects are moving very fast and are very small such that they quickly burn up in the Earth's atmosphere as meteors. On rare occasions an object might be large enough to survive its trip through the atmosphere and fall to Earth as a meteorite. On even rarer occasions the object is so large that it hits the Earth's surface with enough force to leave a crater, sometimes with devestating effects. Astronaut (talk) 12:48, 7 May 2010 (UTC)
- One thing that helps us here is that most of the big things that were in orbits that would bring them close enough to the earth to impact it have already done so - hence the early history of the planet (before life formed here) was exceedingly violent with large chunks of rock and ice pummeling the planet all the time. In fact, it is generally agreed that our moon was formed from debris that resulted when another entire planet (the size of Mars!) hit the earth very early on in it's history. Fortunately, that super-violent era is over now - and even though (as User:Astronaut says) we're hit by a lot of stuff, most of it is small and the odds of a big rock hitting something important is rather small. You can see this happening at particular times of year during meteor showers. Every year, if you go out late at night (midnight until maybe 2am is good) between August 9th and 14th - in an area far from city lights and other 'light pollution' you'll be treated to a most amazing light show from the Perseids - which are bits of an old broken-up comet that cross the earth's orbit on the exact same few days every year, peppering our planet with teeny-tiny rocks and other debris. Most of those burn up in the air - making for a spectacular show of "shooting stars". There is only one recorded instance of a person being hit by a falling piece of space debris - and a mere handful of cases of buildings and cars getting hit. However, there are still gigantic rocks out there and there is always a chance of one of them hitting us and wiping out an entire city - or perhaps killing all life on earth! SteveBaker (talk) 13:52, 7 May 2010 (UTC)
Space materials-luminosity
Might be basic. What is responsible for the luminous efficacy/luminosity of sun, star and other space objects that 'glow'? Are there any reactions behind hydrogen and helium, eventually resulting in the luminosity of certain space objects? - anandh, chennai —Preceding unsigned comment added by 125.21.50.214 (talk) 11:18, 7 May 2010 (UTC)
- Most people think it is nuclear fusion. See Sun and nuclear fusion. --Chemicalinterest (talk) 11:54, 7 May 2010 (UTC)
- And it is very hot! Graeme Bartlett (talk) 12:23, 7 May 2010 (UTC)
- It is indeed nuclear fusion. The sun's gravity is so strong that it crushes the hydrogen atoms together and turns them into helium. In the process of doing that, a tiny fraction of the mass of the hydrogen gets converted into energy - and because E=m.c2, that's an enormous amount of energy! That's why the sun can burn for billions of years without running out of fuel. However, the sun has already burned through about half of it's hydrogen - and in another billion or so years, it'll have to start converting the helium that it's made...and that will result in the destruction of the earth and everything that lives on it! SteveBaker (talk) 13:40, 7 May 2010 (UTC)
- That's a bit oversimplified. Stars of the size of the sun are fairly stratified, with helium "ash" accumulating in the center. When it goes red giant, a significant amount of hydrogen will still be left. The sun has gone through about half of its main sequence life, but not through half of its hydrogen. And if the Earth will be destroyed is somewhat uncertain - every few months a new study comes out claiming the opposite of the preceding one. But it will certainly become rather warm... --Stephan Schulz (talk) 14:00, 7 May 2010 (UTC)
- The fusion provides the heat. Stars then glow because of Thermal radiation. It's essentially the same reason why hot iron glows red. Everything with a temperature above absolute zero will glow in this sense, but for most temperatures that we're used to, they primarily glow predominately at wavelengths longer than what we can see. Humans, for example, glow predominately in the infrared range. When you heat something up, it will emit more light of shorter wavelengths. That's why iron that's somewhat hot will emit a dull red light (the longest wavelength we can see), while it will emit orange, then yellow light when heated further. When you heat something even more (like a light bulb filament), it will emit light at all visible wavelengths, giving white light. The sun is very hot, so not only does it emit "white light" by emitting light at nearly all wavelengths in the visible range, it also emits a more generalized "white light" by emitting across nearly the entire electromagnetic spectrum, from short wavelength gamma rays to long wavelength radio waves. Buddy431 (talk) 14:34, 7 May 2010 (UTC)
Light & escape velocity
Why is light (irradiated from the surface of the earth) still on earth when it has more speed than the escape velocity of earth? If it escapes, can that be seen from/in the space? - anandh, chennai —Preceding unsigned comment added by 125.21.50.214 (talk) 11:24, 7 May 2010 (UTC)

- The Earth reflects light that is emitted from the Sun. About 30% of the light eneregy that the Earth receives from the Sun is reflected back into space - another way of expressing this is to say that the Earth has an albedo of about 0.3. This reflected light is how astronauts see the Earth (or, at least, its day side) from space stations in orbit or from the surface of the Moon. The other 70% of the light energy is absorbed by the oceans, atmosphere, soil, plants etc. (and some of it bounces off objects and is then absorbed by your eyes, which is how you see stuff). The overall effect of this absorbed energy is to keep the Earth warm - if the Earth had a higher albedo, its average temperature would be colder. Gandalf61 (talk) 11:45, 7 May 2010 (UTC)
- Atmospheric diffraction and atmospheric refraction ensures that a lot of light gets bounced around before it escapes or gets absorbed.--Aspro (talk) 11:53, 7 May 2010 (UTC)
- Furthermore, and more importantly, light is just another form of energy. Plenty of it does escape. What you see when you, say, turn on a lightbulb, is the conversion of electrical energy into light energy and heat energy. Riffraffselbow (talk) 12:06, 7 May 2010 (UTC)
- Note that even the the classic "daytime" views of the earth (Blue Marble) are keen evidence that light escapes. Everything you can see is light that is reflecting off of Earth from the Sun. If light could not escape Earth, it would look like a Black hole. --Mr.98 (talk) 13:14, 7 May 2010 (UTC)
- Light has both speed and direction. The light that bounces off of something in just the right direction and hits your eyes before it can escape the planet is what you "see" - most of the other light hits some other thing (the ground, your head, an oxygen molecule in the air) and either gets absorbed and turned into heat or maybe bounces off again. The remainder is the light that "gets away", that you don't see - and which (as you say) has enough speed to escape the earth's puny gravity and head off into space. The average "albedo" of the earth is about 0.37, which means that (very roughly) 37% of the light that comes from the sun and the stars is reflected off into space - and the remaining 63% bounces around until it's absorbed by something and turns into heat. Someone (or something) that's out there in space looking at the earth is only able to see our planet at all because of that 37% of reflected sunlight.
- (I said it was "very roughly" 37% because there is a lot of complication about light that is outside of our visual range (infrared and ultraviolet) and electromagnetic radiation in general, and whether we're talking about 37% of the energy or 37% of the photons...that complicates the explanation about what "albedo" means). SteveBaker (talk) 13:34, 7 May 2010 (UTC)
- In terms of energy, earth emits 100% as much light as it receives. The other 6x% is blackbody radiation in the infrared, which is invisible to our eyes but not to telescopes. In terms of particle count, earth emits far more photons than it receives. I'm thinking the ratio is about 20 (= 6000 K / 300 K), but don't quote me on that. -- BenRG (talk) 19:12, 7 May 2010 (UTC)
- Surely it emits more than it receives, due to radioactive decay, as well as a general cooling of the interior? Buddy431 (talk) 19:23, 7 May 2010 (UTC)
- You're right, but I think it's a small correction. Geothermal gradient says "total heat loss from the earth is [...] about 1/10,000 of solar irradiation". -- BenRG (talk) 20:33, 7 May 2010 (UTC)
- Hmm... I'm surprised it's that small, but I guess it makes sense when I think about it, considering how much energy the sun dumps on us. Buddy431 (talk) 21:34, 7 May 2010 (UTC)
- You're right, but I think it's a small correction. Geothermal gradient says "total heat loss from the earth is [...] about 1/10,000 of solar irradiation". -- BenRG (talk) 20:33, 7 May 2010 (UTC)
- Surely it emits more than it receives, due to radioactive decay, as well as a general cooling of the interior? Buddy431 (talk) 19:23, 7 May 2010 (UTC)
- In terms of energy, earth emits 100% as much light as it receives. The other 6x% is blackbody radiation in the infrared, which is invisible to our eyes but not to telescopes. In terms of particle count, earth emits far more photons than it receives. I'm thinking the ratio is about 20 (= 6000 K / 300 K), but don't quote me on that. -- BenRG (talk) 19:12, 7 May 2010 (UTC)
- Light does continuously leave Earth, but more is continuously supplied by the Sun, Moon (reflected from the Sun), and stars. If they all went dark, then the Earth would, too (except for Las Vegas, of course :-) ). StuRat (talk) 20:58, 7 May 2010 (UTC)
minimum oxygen requirements and carbon dioxide toxicity
Is there a list of the minimum amount of oxygen required to sustain life for different species and the maximum amount of carbon dioxide that would not be toxic including "guesstimate" for extinct species like dinosaurs? 71.100.0.29 (talk) 17:40, 7 May 2010 (UTC)
- By "amount", do you mean percentage of oxygen in the air ? The larger dinosaurs may have needed the higher oxygen levels in the air at the time, so presumably their oxygen requirements were higher than ours. StuRat (talk) 20:48, 7 May 2010 (UTC)
- I recall that reptiles react to insufficient o2 while humans react to too much co2 or vice versa but yes by amount i mean percent o2 and percent co2 in the "air." Too little o2 or too much co2 will kill you and I'm looking for a list that shows what range various species can tolerate of each. Ther must be such a list somewhere. 71.100.0.29 (talk) 00:42, 8 May 2010 (UTC)
- I don't understand your first sentence. Insuffient oxygen and excessive carbon dioxide are big problems for any multicellular creature. If you are looking for specifics values of how much oxygen dinosaurs needed, or how much CO2 would be too much for a dinosaur, I think you're going to be pressed to find an accurate answer since they are, as you said, extinct. For humans, hypercapnia is defined as 45 mmHg or above CO2 in the blood. Hypoxia is any stage down to 40 mmHg of oxygen, at which point it, roughly speaking, becomes lethal. Obviously these aren't exact values for every human, just rough averages. Regards, --—Cyclonenim | Chat 00:30, 9 May 2010 (UTC)
- I recall that reptiles react to insufficient o2 while humans react to too much co2 or vice versa but yes by amount i mean percent o2 and percent co2 in the "air." Too little o2 or too much co2 will kill you and I'm looking for a list that shows what range various species can tolerate of each. Ther must be such a list somewhere. 71.100.0.29 (talk) 00:42, 8 May 2010 (UTC)
wear earplugs 24 hours?
do army wear earplugs 24 hours or shoot without them in war? —Preceding unsigned comment added by Tom12350 (talk • contribs) 17:46, 7 May 2010 (UTC)
- This article from Army Times says that earplugs have been standard issue for the US Army since 2002, but a challenge has been getting soldiers to use them. Comet Tuttle (talk) 19:35, 7 May 2010 (UTC)
- There are times when there is a much higher probability of gunshots than others. For example, someone in the "Green Zone" in Iraq is obviously safer (although not entirely safe) than someone on patrol. So, I suspect they avoid wearing them when in safer areas.
- Then there's the issue of whether decreased hearing ability while the earphones are in place makes soldiers wearing them more susceptible to ambush. Perhaps a more sophisticated option is needed, like noise canceling earphones with a pickup on the gun to only counter noises it makes, not the noises the enemy makes. StuRat (talk) 20:45, 7 May 2010 (UTC)
- Artillery gun crews of course wear better protection and more often than infantry. 75.41.110.200 (talk) 23:40, 10 May 2010 (UTC)
- I don't know how anyone can complain about not being able to get them to wear ear protection. Hearing is an extremely important sense when you're in a warzone. Better sllightly damaged hearing than death (I bet most ipod users do more damage to their ears while listening to it on high volume than a soldier does when shooting). Besides an M16 rifle isn't very loud, the loudest weapon in a squad is likely the M249 light machine gun.--92.251.166.171 (talk) 18:23, 11 May 2010 (UTC)
Hurricane gustav
did hurricane Gustav damage Louisiana? —Preceding unsigned comment added by 169.244.148.235 (talk) 19:26, 7 May 2010 (UTC)
- Yes; see our article Hurricane Gustav, which has an "Impact" section that has what you want. Comet Tuttle (talk) 19:32, 7 May 2010 (UTC)
Lung capacity vs. holding my breath
For most of my life I can hold my breath for longer than most people, but when I did what was AFAICR a lung capacity test in my doctor's office a few years ago, I did quite poorly and was recommended exercise, I think. Is holding breath a learned skill rather than a useful sign of health? Thanks. 67.243.7.245 (talk) 19:48, 7 May 2010 (UTC)
- How long you can hold your breath is dependent on more than just lung capacity. Some other factors:
- 1) Your level of activity at the time. Obviously you need less oxygen while sitting quietly than while running a marathon.
- 2) Your basal metabolic rate also affects oxygen consumption.
- 3) Your tolerance for high carbon dioxide levels, as that's what triggers a new breath.
- 4) Your mass. More mass means more oxygen is needed. StuRat (talk) 20:38, 7 May 2010 (UTC)
- We can't say anything about your breathing rate and the results of your lung capacity test or anything else about you. But I'd like to point out that even if holding your breath for extended periods can be learned, that doesn't mean that it's not also a useful sign of good health. For example Cardiorespiratory fitness says
- "Exercise improves the respiratory system by increasing the amount of oxygen that is inhaled and distributed to body tissues"
- and according to Physical exercise,
- "Physical exercise is any bodily activity that enhances or maintains physical fitness and overall health".
- Lung volumes says that athletes have larger volumes than non-athletes and Breathing says
- "Medical respiratory data ... suggest that sick people breathe about 2-3 times more air at rest than the medical norm".
- See this disclaimer. Zain Ebrahim (talk) 21:01, 7 May 2010 (UTC)
- The episode of Bang Goes the Theory shown on 3 May in the UK had a segment at the start on practising breath-holding as preparation for free diving (Series 2, Episode 7 viewable online in the UK only i think). According to that "you can almost double your breath-holding ability just by practising regularly". Your body builds gets used to lower levels of oxygen and higher levels of carbon dioxide, and your brain gets better at overriding the signals to breathe without panicking. Qwfp (talk) 09:50, 8 May 2010 (UTC)
I want to destroy a culture...
Specifically, an active yogurt culture. Can they be killed off without ruining the yogurt ? Does anyone sell yogurt without "active cultures". I imagine they could be killed without affecting the product via irradiation, but I obviously can't do that at home. StuRat (talk) 20:32, 7 May 2010 (UTC)
- Campden tablets (the brewer asking about nitrogen couold hav etold you this as well)--Aspro (talk) 21:15, 7 May 2010 (UTC)
- So, you'd crush the tablets and stir them in ? Would it diffuse through the yogurt that way, or would it be too thick for that ? StuRat (talk) 21:56, 7 May 2010 (UTC)
- Crush, stir in, then ten or twenty minutes later stir again. I think it was done because we would then add pulped raw fruit (which also so treated). During the post-war austerity period, few people had refrigeration and we did not want it to start a different type fermentation that may have given it a less than pleasant tang. However, some asthmatics are sensitive to metabisulphites and so it might be easier in the long run just to heat it in a Bain-marie or something. According to page 2 of [17] heating in a hot water bath to 65 C and holding for 3 min should do the trick. --Aspro (talk) 11:15, 8 May 2010 (UTC)
- At least in my experience (UK) any yoghurt which is not in a chiller cabinet at the store will not have active cultures. I don't know if there is a way you can positively confirm this though other than writing to the manufacturer. 131.111.185.69 (talk) 21:51, 7 May 2010 (UTC)
- I think it's always refrigerated here in the US. StuRat (talk) 21:56, 7 May 2010 (UTC)
- In the UK, yoghurt with an active culture will say something like "live yoghurt" (or, these days "probiotic") somewhere on the tub: other yoghurts are available which do not contain active cultures. Often the yoghurt marketed to children, for example. 86.180.48.37 (talk) 20:04, 9 May 2010 (UTC)
Invasive surgery on small animals
Does anyone know what's the smallest animal that a veterinarian has been able to successfully perform complex brain/thoracic/abdominal surgery on? Is it humanly possible, to perform open heart surgery (just for example) on a mouse, or a zebra finch? --95.148.107.169 (talk) 21:19, 7 May 2010 (UTC)
- I suspect that larger tumors would be more easily found and removed from smaller animals, whereas delicate surgery, like neurosurgery, would definitely be out. StuRat (talk) 22:03, 7 May 2010 (UTC)
- Actually not "out", if you count surgery for experimental research reasons instead of therapeutic reasons. Lots of surgical procedures get done on mice and rats, including implantation of brain stimulators in specific nuclei. alteripse (talk) 02:42, 8 May 2010 (UTC)
- Heh. Complex surgery has been performed on fruit flies (for example see PMID 20053645.) With powerful microscopes, micromanipulators, and lasters, pretty much nothing is impossible. Delicate surgery on mice is everyday stuff nowadays. Looie496 (talk) 03:14, 8 May 2010 (UTC)
- Undergraduate college students have routinely done stereotaxic brain surgery on rats, mice and even frogs, doing some removal of portions of the cortex, or inserting microelectrodes to record brain potentials. Edison (talk) 03:32, 8 May 2010 (UTC)
It goes even smaller than fruit flies, in fact. Microsurgery is regularly done on Caenorhabditis elegans, which are only 1mm long as adults. Researchers can laser ablate individual cells, and surgically remove individual sensory neurons for research purposes. Someguy1221 (talk) 09:36, 8 May 2010 (UTC)
- Researchers can cut open a single-celled organism, surgically remove the nucleus of the cell, and put a new nucleus in! (Nuclear transfer) It doesn't get much smaller than that! APL (talk) 22:42, 8 May 2010 (UTC)
- OP here (probably with a different IP) - thanks for the replies. I didn't know that surgery on organisms that small was possible but when I asked my question, I was thinking more about a veterinarian performing surgery on small domestic/pet animals. Say if I had a zebra finch that needed open heart surgery (I don't, I'm not asking for medical advice here), would a typical veterinarian be able to perform the op these days, if he/she decided that a rough long term suvival vs. risk of death on the table vs. desire of the owner for his pet to live calculation suggested that it was worth performing? --95.148.104.246 (talk) 20:28, 9 May 2010 (UTC)
Shell Oil Company
In Fortune Magazine i believe the last issue in October of 2008 I read that Shell Oil Company has under it's control in the United States enough shale rock from which they could make oil that would supply our needs for as much as 50 years and Shell could make money doing so at $30 a barrel. Could you revue this and update these estimates. Thanks —Preceding unsigned comment added by Norwood jones (talk • contribs) 22:33, 7 May 2010 (UTC)
- There is a lot of Oil shale in the US and Canada, so the time estimate is probably right, but $30 a barrel sounds wrong. The article says it's more like 70-95 (at least at first). See the article I linked, it's really good and has a ton of details. Make sure to read the sub-articles it points to as well. Ariel. (talk) 22:55, 7 May 2010 (UTC)
- Note that if oil prices stay up in that area, however, then oil demand will drop, and alternative sources of energy will be used, instead. So, that "50 year supply" could last a lot longer at that reduced level of usage. StuRat (talk) 03:48, 8 May 2010 (UTC)
Reaction of metal hydrides from nickel-metal hydride cell
When the metal hydride anode of a nickel-metal hydride cell is reacted with acid, it forms an almost black solution, then changes dark green solution, fizzing all the while. I added the ammonia and the hydrogen peroxide as described in the article for cerium to test for cerium ions. I came up with a green precipitate, a little lighter than ferrous hydroxide. When it is just neutralized by ammonia, it forms a blue precipitate. What is the green precipitate? --Chemicalinterest (talk) 23:20, 7 May 2010 (UTC) A help would be that the filtrate from the neutralization with ammonia of the anode solution is blue. When it reacts with hydrogen peroxide, it turns green and fizzles. --Chemicalinterest (talk) 00:45, 8 May 2010 (UTC)
May 8
Reaction of chromates with acid
I am still analyzing chromates in my home lab. When hydrochloric acid is added to chromates, it turns black instantly. About 2 seconds later, it fizzes Cl2 furiously and turns green (the color of CrCl3) again. When sodium hypochlorite is added, it turns yellow again, showing the formation of chromates. My question is: What is the intermediate unstable (seems like strong oxidizer, produces Cl2) black substance? It looks similar to CrO5, chromium peroxide, but I didn't add any peroxide to the solution, just HCl. Thanks.--Chemicalinterest (talk) 00:41, 8 May 2010 (UTC)
- Could you please give me the formula for the chromate you react HCl with? --The High Fin Sperm Whale 02:12, 8 May 2010 (UTC)
- Na2CrO4 --Chemicalinterest (talk) 11:22, 8 May 2010 (UTC)
- Could it be CrO2Cl2? Just an idea. 67.170.215.166 (talk) 05:10, 8 May 2010 (UTC)

Manganese dioxideGraeme Bartlett (talk) 08:10, 8 May 2010 (UTC)- And where, may I ask, did the manganese come from? 67.170.215.166 (talk) 08:13, 8 May 2010 (UTC)
- No Mn in the solution, just Na2CrO4 and NaCl with a very little H2O2, not enough to make the dark black color. --Chemicalinterest (talk) 11:25, 8 May 2010 (UTC)
- In the chromyl chloride article it talks about reaction of potassium chromate with hydrochloric acid, followed by sulfuric acid as a desiccant. What happens if the sulfuric acid is not added, which is similar to what I did? --Chemicalinterest (talk) 11:52, 8 May 2010 (UTC)
- A possible reaction to form CrO2Cl2 is: Na2CrO4 + 2 HCl → CrO2Cl2 + 2 NaOH --Chemicalinterest (talk) 11:55, 8 May 2010 (UTC)
- Then: 2 CrO2Cl2 + 8 HCl → 2 CrCl3 + 4 H2O + 3 Cl2 --Chemicalinterest (talk) 18:02, 8 May 2010 (UTC)
- The big issue I have with that mechanism is that you seem to be proposing that HCl forms chromic acid. Then apparently Cl- is strong enough of a nucleophile to attack the Cr(VI) centre and push out OH- as a leaving group. OH- is a horrible leaving group. Now, perhaps what happens is that the oxygens get "doubly protonated" and leave as water. Is Cr(VI) that weak of a Lewis acid that Cl- can displace HOH from it? John Riemann Soong (talk) 03:26, 10 May 2010 (UTC)
- Btw, in water the reverse reaction is strongly favoured. Chromyl chloride is basically a Cr(VI) version of an acyl chloride or a phosphoryl chloride -- it hydrolyses to give HCl. John Riemann Soong (talk) 03:34, 10 May 2010 (UTC)
- No Mn in the solution, just Na2CrO4 and NaCl with a very little H2O2, not enough to make the dark black color. --Chemicalinterest (talk) 11:25, 8 May 2010 (UTC)
- And where, may I ask, did the manganese come from? 67.170.215.166 (talk) 08:13, 8 May 2010 (UTC)
I've been trying to work out a possible mechanism on paper... and here's what I think happens.
- Chromate gets protonated in water to form various protonated species of chromic acid.
- Chloride doesn't attack the Cr(VI) centre directly to form chromyl chloride. Cl- is too soft of a nucleophile to do that. Instead it donates into an oxygen (does the Cr=O bond involve pi bonds, or no?), reducing Cr(VI) to Cr(IV), and forming a "chromium (IV) hypochlorite".
- This Cr(IV) formation indirectly allows for the displacement of a water molecule as a leaving group.
- Another Cl- ion comes along and reduces the hypochlorite into an oxide, forming chlorine gas.
- Anhydrous Cr(IV) is chromium dioxide ... an insoluble black precipitate. Industrially it is formed at high temperatures at 30000 psi but it would form a plausible reactive intermediate in acidic conditions.
- However, Cr(IV) dioxide exists in equilibrium with its protonated, soluble form, but dissolution is not complete.
- The oxidation of Cl- via the reduction Cr(IV) to Cr(III) is slow. I say this because it's hard to find a plausible mechanism for it. Cr(IV) is less electronegative. Two options: Cr(IV) to Cr(II), which is instantly oxidised to Cr(III), or Cr(IV) to Cr(III) via single electron transfer. John Riemann Soong (talk) 04:42, 10 May 2010 (UTC)
- I might not understand all of your terms since I just finished 11th grade, but I guess what you're saying in the first part is that HCl does not react with Na2CrO4 to form H2CrO4 and NaCl.
- Why is the chromate protonated in water? I added HCl, which would protonate it easier than water. Normally neutral solutions, such as Na2CrO4 and NaCl, do not hydrolyze to form chromic acid and sodium hydroxide, or hydrochloric acid and sodium hydroxide respectively. It would probably be protonated by the HCl.
- Hypochlorites aren't too easy to form. I don't think Cr(VI) is strong enough.
- But why does it fizzle so rapidly when HCl is added? Maybe the reaction mechanism is impure. If you want to try it, you can. Dissolve nichrome heating element in HCl (dark green solution). React it with alkali and filter the precipitate(dark green precipitate, turns brown). React the precipitate with household bleach (some fizzing, yellow solution). Add small amounts of hydrogen peroxide to get rid of the remainder of bleach so the bleach doesn't react with the acid directly(some fizzing, if too much H2O2 then it turns dark purple). Filter the solution and collect the filtrate. Add about an equal amount of acid to the filtrate. It should turn black instantly, then start fizzing furiously, clearing up to a green solution again. Add more bleach and it will turn yellow again, with the possible release of Cl2. --Chemicalinterest (talk) 12:16, 10 May 2010 (UTC)
- Some reactions I figured out, John Riemann Soong:
- 2 Na2CrO4 + 4 HCl → 2 CrO2 + 2 NaOCl + 2 NaCl + 2 H2O [black precipitate]
- 2 CrO2 + 8 HCl → 2 CrCl3 + Cl2 + 4 H2O [fizzing, clearing to green solution]
- These reactions are not proven. --Chemicalinterest (talk) 14:45, 10 May 2010 (UTC)
- Well, CrO4- is a very weak base. I meant it gets protonated by HCl, of course. Chromic acid has a pKa of 0.74. H3O+ has a pKa of approximately -1.7 -- H3O+ will protonate chromate outright. No indeed, it might form some equivalent of NaCl and chromic acid.
- The thing to notice is the reduction potential of Cl2 (1.36) versus Cr(VI) (+1.33 for dichromate). Calculate the net redox potential for the entire reaction. In fact the potentials are so close that I think you could very well have an equilibrium reaction. An excess of hypochlorite will lead to formation of Cr(VI) from Cr(III). An excess of Cr(VI) (or chloride!) will lead to the formation of hypochlorite and the reduction of Cr(VI) to Cr(III). Also hypochlorite + acid <== (eq) ==> chlorine + water.
- Try varying the excesses. Try lots of chromate and a modest amount of acid, or a modest amount of chromate and a generous amount of acid. Just how much hypochlorite did you add? John Riemann Soong (talk) 19:31, 10 May 2010 (UTC)
- What would be really telling, if after you reoxidised Cr(III) into Cr(VI) with hypochlorite, is if you added some plain ole NaCl. If it is indeed an equilibrium reaction, an excess of Cl- will reduce some of the Cr(VI) into Cr(IV) or Cr(III).John Riemann Soong (talk) 19:39, 10 May 2010 (UTC)
Is it possible to shoot a bear in the head?
Hello all, I'm a writer and I've recently completed a novel in which a weatherman gets attacked by a bear and the Civil Air Patrol has to drop a surgeon to him by parachute to save his life. Well anyway, in the opening scene the bear attacks the weatherman, whose own shotgun backfires in his face (I believe some of you might remember me asking about it under a different IP address), and the weatherman (having been partially, but not completely, blinded but still fully conscious) tries to fight off the bear with his bare hands and by hitting it with the gun butt until his partner arrives and shoots the bear in the head. So far, most of the folks who have read the novel seem to like the action, but many of them have also pointed out some bad details to me (mostly in the dialogues). One reviewer, however, objected to something entirely different: he told me that a bullet would not pierce a bear's skull because it's too thick and also sloped like the armor on a tank, so the bullet would just bounce off. Is it true, what he said? And if so, does it depend on the aspect angle of the hunter with respect to the bear (e.g. possible to shoot from the side but not from the front)? I'm not a hunter, so I'd really like to know. Thanks in advance! 67.170.215.166 (talk) 05:08, 8 May 2010 (UTC)
- Well, if it's a brown bear (which I presume it is, since black bears don't attack people that way), the really implausible thing is that a person could put up meaningful resistance using bare hands and a rifle butt -- your weatherman better be one hell of an athlete. But that being said, whether a bullet would penetrate the skull depends a lot on the gun. Is is a pistol or a high-powered rifle? If it's a pistol, not only would the bullet probably not penetrate the skull, but the brain is so small compared to the head that you'd be likely to miss it in any case. Shooting for the heart is a much more viable proposition. Looie496 (talk) 05:50, 8 May 2010 (UTC)
- The preferred way to take a bear is to shoot it in the shoulder, with a high-powered, fairly small-bore rifle (say, a .30-06 caliber). These rounds are small and fast, and can penetrate the thick skin and fur (and bone), and shatter the shoulder. This incapacitates the bear; it can then be approached and finished with a larger-caliber weapon, e.g. a pistol. Even a high-powered rifle round will (supposedly) bounce off the skull; at the very least, it is not considered fatal and is considered unnecessarily cruel and harmful to the bear (induces slow death). (I am not a hunter and don't really approve of taking bears for sport, but I know a bit about the subject. During radio-science field-work in the great Alaskan interior and Kodiak Island, I regularly stayed in hunting lodges, a few of which were operated by commercial bear hunt guides - there aren't always motels where you want to study radio signals!) Nimur (talk) 06:00, 8 May 2010 (UTC)
- You probably have underestimated the power a gun can generate. It is nearly one mile per second! Yes, small bores like .22 or .25 or may be even a 9mm can not do much damage, but bores greater than that can really incapacitate a bear etc. in no time. A buckshot from a short-barrled 12 gauge will tear bear's skull apart. Anything larger, a 10-gauge if you can manage will blow the skull literally off. But the recoil may hurt even you (the shooter)People have killed elephants using big guns.Besides there are special revolvers made by Smith and Wesson to kill bears [[18]] Jon Ascton (talk) 07:50, 8 May 2010 (UTC)
- For this discussion, assume Colt 45 automatic to the side of the head at point-blank range. And FYI, the bear is a polar bear. 67.170.215.166 (talk) 08:01, 8 May 2010 (UTC)
- Oh, and did I say that the bullets are of the hollow-point type? 67.170.215.166 (talk) 08:09, 8 May 2010 (UTC)
- Hilaire Belloc recommended platinum bullets. Gandalf61 (talk) 10:07, 8 May 2010 (UTC)
- Even if the bullet didn't penetrate the bear's skull, it would have caused it a lot of pain. The sound produced by the gun would scare it. Both effects would hamper the bear's attack and may even cause it to run away122.169.221.144 (talk) 12:51, 8 May 2010 (UTC)
- Break the details down into believable chunks. 1) Most guns carried into the wilderness -even if there are bears about- would I think - be light and practical ( I'm using 'light' in the relative sense). Therefore, his partner could have an ordinary and popular type firearm, which the reader may have well fired himself down a the local range. This takes the emphases away from the hardware and focuses it more on the action. I.e., instead of making the gun do all the hard-work, it makes his partner the brave hero instead. 2) Gun magazines for decades have been encouraging people to buy bigger guns by pointing out that rounds can be deflected by a bears scull. So don't go against popularly held beliefs -and in this case it does seem true in some cases. 3) Its bad form for a writer to let the any of the good guys kill with the first shot (unless the hero is down to his very last BB). So let the first round ricochet off into the blue yonder. 4) The suggestions posted above, give a good idea where to put the other slugs. If you look at the these polar bear and grizzly bear skulls one can easily believe the partner in a final act of desperation (perhaps the bear swipes his rifle from is hands) pulling out his grandfather's old Colt 36 and severing the spinal cord with a lucky shot in through the mouth. If the partner gets this close, don't forget to comment on weather this bear also has Halitosis . If the shot is at point blank, don't forget to consider the hot flash going out sideways and the smell of a bit of singed fur. Films and CSI aside, who really carries around a heavy Colt 45 ? Finally: Writer Cory Doctorow has discovered, he sells more books if he also releases them under a Creative Commons licence. HowTo Negotiate a Creative Commons License. --Aspro (talk) 12:55, 8 May 2010 (UTC)
- Purely on your last point, Aspro, while that works well for Cory Doctorow who already has a doubly-established reputation, as a fiction writer, and as a champion of Creative Commons, it might not work so well for the (presumably as-yet unknown) OP. 87.81.230.195 (talk) 17:10, 8 May 2010 (UTC)
- Break the details down into believable chunks. 1) Most guns carried into the wilderness -even if there are bears about- would I think - be light and practical ( I'm using 'light' in the relative sense). Therefore, his partner could have an ordinary and popular type firearm, which the reader may have well fired himself down a the local range. This takes the emphases away from the hardware and focuses it more on the action. I.e., instead of making the gun do all the hard-work, it makes his partner the brave hero instead. 2) Gun magazines for decades have been encouraging people to buy bigger guns by pointing out that rounds can be deflected by a bears scull. So don't go against popularly held beliefs -and in this case it does seem true in some cases. 3) Its bad form for a writer to let the any of the good guys kill with the first shot (unless the hero is down to his very last BB). So let the first round ricochet off into the blue yonder. 4) The suggestions posted above, give a good idea where to put the other slugs. If you look at the these polar bear and grizzly bear skulls one can easily believe the partner in a final act of desperation (perhaps the bear swipes his rifle from is hands) pulling out his grandfather's old Colt 36 and severing the spinal cord with a lucky shot in through the mouth. If the partner gets this close, don't forget to comment on weather this bear also has Halitosis . If the shot is at point blank, don't forget to consider the hot flash going out sideways and the smell of a bit of singed fur. Films and CSI aside, who really carries around a heavy Colt 45 ? Finally: Writer Cory Doctorow has discovered, he sells more books if he also releases them under a Creative Commons licence. HowTo Negotiate a Creative Commons License. --Aspro (talk) 12:55, 8 May 2010 (UTC)
- A writer's biggest problem is obscurity. Who is going to buy a book that they don't know exists, or a book from an author who is unknown to them? By distributing some work under a CC licence, they attract a wider audience. The same has happened, to people who thought of themselves as non writers but have been surprised with offers of book deals based on what the publisher has read on their blogs. Pop-stars understand this. You have to be 'seen' if you want to be noticed. So, this exposer could be even more valuable to any of the little know story tellers out there. --Aspro (talk) 18:54, 8 May 2010 (UTC)
- Fair points, Aspro, but people like Cory Doctorow and blogger/author John Scalzi spent many years slowly building on-line reputations, and had the abilities necessary to do so; random unpublished authors like the OP may not possess the latter or want to spend the time necessary for the former, so the traditional publishing route via direct (or agent-mediated) submissions to a Publishing House who will exercise its long-honed professional skills and do much of the marketing for them from the off (and who may or may not incorporate the strategies discussed), is still in my view a better bet. My view however is shaped by a former career in traditional-model bookselling and publishing, so may be increasingly irrelevant. 87.81.230.195 (talk) 01:12, 10 May 2010 (UTC)
- A writer's biggest problem is obscurity. Who is going to buy a book that they don't know exists, or a book from an author who is unknown to them? By distributing some work under a CC licence, they attract a wider audience. The same has happened, to people who thought of themselves as non writers but have been surprised with offers of book deals based on what the publisher has read on their blogs. Pop-stars understand this. You have to be 'seen' if you want to be noticed. So, this exposer could be even more valuable to any of the little know story tellers out there. --Aspro (talk) 18:54, 8 May 2010 (UTC)
- A polar bear attacking an adult man is comparable to an adult man attacking a four year old child. In your case it would be a blind child waving a small stick. To make the story even halfway plausible you need something to slow down the bear. Why not give your guy some pepper spray? That wouldn't necessarily drive off the bear, but might slow it down enough to make the encounter last longer than 5 seconds. Youtube has a bunch of videos of bears attacking various things -- you might be able to write a more realistic account if you watched a few of them. Looie496 (talk) 22:07, 8 May 2010 (UTC)
- Hey everyone, I see a lot of good ideas here on how to make this scene more dramatic (it's pretty dramatic as it is, but from reading your replies, I see that I could make it even more dramatic) as well as more plausible. Now, I have to remind y'all that I'm not a hunter, and for this reason sorting out all this plausible but contradictory info will require some qualified advice. (I think I'll talk to the guy at the local gun store, he should know this kind of stuff in some detail.) Thank y'all for your ideas, and clear skies to you! 67.170.215.166 (talk) 07:14, 10 May 2010 (UTC)
Conventional Explosive v/s Plastic explosives
Conventional explosive like gunpowder cannot do damage if not held air-tightly. If we open a 12-gauge shot cartidge (NEVER, NEVER TRY IT AT HOME. I AM JUST GIVING EXAMPLE), and put all the smokless "powder", which is of course not plastic explosive, and set it light it will burn away with a SWOOSH and a very brillant flame, but there will not be any explosion. Even a fire-cracker has to be contained in something from which gases cannot escape without destroying it. But as far as plastic explosives are concerned, if I am not very much wrong, they do not need to be air-tightened. A piece of C-4 or semtex can explode in open when properly detonated (can't do it with fire, methinks). But is that also true about nitroglycine. Is it not necessery to hold nitroglycrine in a tight container to cause explosion ? Jon Ascton (talk) 08:51, 8 May 2010 (UTC)
- I believe the difference is that gunpowder is a "low explosive" which, technically, deflagrates rather than detonates. This makes it suitable as a propellant in munitions and fireworks, but not damaging unless in a confined space. On the other hand, C-4, Semtex and nitroglycerin are all "high explosives", which undergo true detonation. Incidentally, you don't need always need fire to initiate detonation - some explosives are very sensitive and can be detonated by pressure, friction, electric shock, sound or even light. However, such sensitive explosives are usually used in only small amounts in detonators to trigger a larger quantity of more stable "seondary" explosive. See our article on explosive material for more details. Gandalf61 (talk) 10:01, 8 May 2010 (UTC)
- Yeah, that is what I wanna know : Will a small quantity of nitroglycrine, let's say 1/10 litre - which is an oily liquid - when not in airtight position, that is lying in open, detonates cause a real explosion practically (like a bomb), or it will just burn with a SWOOSH sound....? Jon Ascton (talk) 10:43, 8 May 2010 (UTC)
- It depends on the speed of the shock wave caused by the suddenly expanding gases released by the explosive. --Chemicalinterest (talk) 11:26, 8 May 2010 (UTC)
- Read the links. Nitroglycerin#Detonation says "... a self-sustained shock wave ... propagates through the explosive medium at some 30 times the speed of sound as a near-instantaneous pressure-induced decomposition of the fuel into a white hot gas. Detonation of nitroglycerin generates gases that would occupy more than 1,200 times the original volume at ordinary room temperature and pressure; moreover, the heat liberated raises the temperature to about 5,000 °C (9,030 °F)". That sounds like a "real explosion" to me. Gandalf61 (talk) 12:07, 8 May 2010 (UTC)
- And 1/10 litre of Nitroglycerin is not "a small quantity". When we made it in school, we detonated microscopic amounts for a quite noticeable bang.--Stephan Schulz (talk) 15:03, 8 May 2010 (UTC)
- For example, consider the iconic image of a stick of dynamite. It's essentially nitroglycerine and sand, wrapped in a cardboard (or paper) tube. The sand reduces the shock-sensitivity, but the paper tube isn't there to confine the blast - only to wrap the material conveniently. That stick of dynamite is still dangerous and can explode. Now, if you're performing road construction and want to remove rock from the highway, you might want to confine the blast to maximize the transfer of energy towards productive, useful purposes - so you might drill a borehole into a rock and use that confined space to harness the blast energy. But the explosive will detonate, confined or unconfined. As far a "plastic", the entire purpose of plasticizer is to reduce shock sensitivity - it has plays very little chemistry role, other than diluting the active ingredients. Nimur (talk) 16:11, 8 May 2010 (UTC)
- Gosh, that's mixed up. It's not sand but diatomaceous earth that is the phlegmatizing agent in nitroglycerine. Second, plasticizer are added to make it 'plastic ' hence plastic explosive!--Aspro (talk) 16:31, 8 May 2010 (UTC)
- It can be anything - sand, silt, mud, sawdust, ground up pieces of paper - it doesn't matter, as long as it's absorbent... The plasticizer replaces the role of this material, and as an added bonus allows the material to be molded. Nimur (talk) 17:02, 8 May 2010 (UTC)
- Gosh, that's mixed up. It's not sand but diatomaceous earth that is the phlegmatizing agent in nitroglycerine. Second, plasticizer are added to make it 'plastic ' hence plastic explosive!--Aspro (talk) 16:31, 8 May 2010 (UTC)
- Think you may be getting mixed up with Gelignite and even that article is inaccurate, as it does sweat – that's what old jelly is renowned for. Although the Dynamite article mentions sawdust, and Nobel used it for his early blasting sticks, Nobel's patent for Dynamite was for the absorption by dichotomous earth (which just happen to be mined close to him in Sweden). The reason why Dynamite was such an improvement on safety was that blasting sticks that used sawdust also 'sweated' - Dynamite didn't. By definition, a stick using anything else is not Dynamite. Just don't believe everything you read on Wikipedia.--Aspro (talk) 17:51, 8 May 2010 (UTC)
- Like Kleenex, dynamite has become a genericized name; but you are absolutely correct. Nimur (talk) 17:54, 8 May 2010 (UTC)
- Think you may be getting mixed up with Gelignite and even that article is inaccurate, as it does sweat – that's what old jelly is renowned for. Although the Dynamite article mentions sawdust, and Nobel used it for his early blasting sticks, Nobel's patent for Dynamite was for the absorption by dichotomous earth (which just happen to be mined close to him in Sweden). The reason why Dynamite was such an improvement on safety was that blasting sticks that used sawdust also 'sweated' - Dynamite didn't. By definition, a stick using anything else is not Dynamite. Just don't believe everything you read on Wikipedia.--Aspro (talk) 17:51, 8 May 2010 (UTC)
- Actually, dynamite is perfectly safe unless detonated. It can be thrown in a fire without exploding. --The High Fin Sperm Whale 22:09, 8 May 2010 (UTC)
- Same with C-4, our guys in 'Nam actually used it as campfire fuel! Nitro, on the other hand, is so sensitive that even a static spark from your pants/skirt could set it off! We didn't do any experiments with nitro in school, and for good reason. 67.170.215.166 (talk) 07:10, 9 May 2010 (UTC)
- But one manual about making nitroglycrine I read says that it burns with a clear blue flame. In fact this is the test for real nitro... Jon Ascton (talk) 13:41, 9 May 2010 (UTC)
- Yeah, that's what it does, 'less it blows up in your face. Care to try it some time? 67.170.215.166 (talk) 07:16, 10 May 2010 (UTC)
irradiating an egg
What happens to an egg when it is irradiated? 71.100.0.29 (talk) 12:45, 8 May 2010 (UTC)
- The bacteria are killed in it, but I don't think the egg itself is harmed. --Chemicalinterest (talk) 12:47, 8 May 2010 (UTC)
- It depends mainly on how much irradiation they are subjected to In-shell irradiation of eggs . Here in Europe, health grounds tend to be considered before profit, therefore, this process is not permitted for most food stuffs -including eggs. I think this link covers most of the objections The Irradiation of Eggs: The Details.--Aspro (talk) 13:22, 8 May 2010 (UTC)
- Let's be clear -- if there is a living embryo in the egg, it will be killed by irradiation. Most scientists think that the edibility of the egg would not be impaired. Looie496 (talk) 18:24, 8 May 2010 (UTC)
- FWIW I've found a document that give the European position, which also gives examples of which food stuffs are sometimes irradiated over here. Commission adopts first EU report on irradiated food--Aspro (talk) 19:21, 8 May 2010 (UTC)
- If those EU blockheads really considered health grounds rather than all that ultra-left-wing neo-Luddite green hype, they would've allowed most foods to be radiated -- it's one of the most effective ways to kill any critters living in the food, as well as being a lot better for the consumer's health than salting it or boiling it or putting in a bunch of antiseptic additives that could affect human metabolism. Too bad that they fall so easily for all those lies from the green movement who want us all to start living like the Apache Indians or something... 67.170.215.166 (talk) 07:18, 9 May 2010 (UTC)
- Isn't it better to simply eat fresh or frozen food unprocessed food? I don't know where you live but as Europe adopts more and more American style food processing techniques, so it discovers that it becomes ever more burdened with American style health problems. Currently we are getting very alarmed with childhood obesity and childhood diabetes, which is racing up to USA levels fast. These days you don't have to eat salt beef and things -so those arguments are something of a non sequitur. As I'm not against irradiation per se you can't call me a Green either. Remember, it took years of big fines and bad publicity to reach the current level compliance in the food industry. Letting the fox guard the chick shed, (i.e., letting lobbyist tell US politician what to allow in the food industry) is I would say - being the blockhead. --Aspro (talk) 12:55, 9 May 2010 (UTC)
- Aspro, I wasn't talking about lobbyist influence on the FDA (which I do not condone in any way -- the FDA should be free to do the right thing about food safety without political pressure), but specifically about the Green blockheads opposing irradiation, which is a perfectly safe food-preservation procedure that has no known effects on the food's nutritional value as opposed to salting or canning, which could in some cases introduce unhealthy substances into the food (e.g. excessive sodium in the case of salting). My beef (no pun intended) is with the statement that [because of health concerns], irradiation is not permitted in the EU, and the blockheads I refer to are members of that (mostly Green) community which objects to irradiation because of some imaginary "health concerns" (even though there are none) and thus by their influence consign many tons of fresh food to the landfill through spoilage that irradiation could have easily prevented. In short, this is not a debate about food safety in general, but specifically about irradiation and the raving lunatics and pandering politicians who oppose it despite its merits. In other words, as bad as it is to let the food industry set food safety standards, it's just as bad to leave this task to the madmen in the Green movement who have a vested interest in taking our way of life back to pre-industrial levels. 67.170.215.166 (talk) 07:39, 10 May 2010 (UTC)
- Isn't it better to simply eat fresh or frozen food unprocessed food? I don't know where you live but as Europe adopts more and more American style food processing techniques, so it discovers that it becomes ever more burdened with American style health problems. Currently we are getting very alarmed with childhood obesity and childhood diabetes, which is racing up to USA levels fast. These days you don't have to eat salt beef and things -so those arguments are something of a non sequitur. As I'm not against irradiation per se you can't call me a Green either. Remember, it took years of big fines and bad publicity to reach the current level compliance in the food industry. Letting the fox guard the chick shed, (i.e., letting lobbyist tell US politician what to allow in the food industry) is I would say - being the blockhead. --Aspro (talk) 12:55, 9 May 2010 (UTC)
- Food irradiation is generally used on fresh foods. As 67.170 alludes to, processed foods usually have other means of being kept bacteria free (i.e. cooking, drying, salting, canning, or using other preservatives), and so irradiation is not needed or used. For what it's worth, I always assume that my eggs are contaminated, and make sure to cook them thoroughly. Buddy431 (talk) 16:08, 9 May 2010 (UTC)
Insoluble chromate
Is there any insoluble hexavalent chromium compound? I reacted sodium hypochlorite with the product of the reaction of Nichrome with hydrochloric acid. It formed a dark orange-brown precipitate. What is that precipitate? It lightens when hydrogen peroxide is added to it, and dissolves in hydrochloric acid to turn yellow. --Chemicalinterest (talk) 14:43, 8 May 2010 (UTC)
- Remember the nickel? Sodium hypochlorite is alkaline and will neutralise the acid precipitating hydroxides. Take a look at nickel chromate and nickel hydroxide. Are either of these possible? Graeme Bartlett (talk) 03:36, 9 May 2010 (UTC)
- It probably is nickel chromate, but I don't know why it is insoluble. --Chemicalinterest (talk) 19:15, 9 May 2010 (UTC)
- Whoops, it is insoluble. --Chemicalinterest (talk) 19:25, 9 May 2010 (UTC)
- 2 NiCl2 + 2 CrCl3 + 10 NaOH + 3 NaClO → 2 NiCrO4 + 10 NaCl + 5 H2O This only happens when there is no residual acid in the reaction between Nichrome and hydrochloric acid. If there is, it forms Cl2 and yellow solution. --Chemicalinterest (talk) 19:40, 9 May 2010 (UTC)
- The yellow solution will probably be the result of the dissolution of NiCrO4. --Chemicalinterest (talk) 19:44, 9 May 2010 (UTC)
- What does NiCrO4 produce when it dissolves in acid? (Question in lower section) --Chemicalinterest (talk) 11:07, 10 May 2010 (UTC)
- The yellow solution will probably be the result of the dissolution of NiCrO4. --Chemicalinterest (talk) 19:44, 9 May 2010 (UTC)
- 2 NiCl2 + 2 CrCl3 + 10 NaOH + 3 NaClO → 2 NiCrO4 + 10 NaCl + 5 H2O This only happens when there is no residual acid in the reaction between Nichrome and hydrochloric acid. If there is, it forms Cl2 and yellow solution. --Chemicalinterest (talk) 19:40, 9 May 2010 (UTC)
- Whoops, it is insoluble. --Chemicalinterest (talk) 19:25, 9 May 2010 (UTC)
Chromium and acid
Does chromium react with acids to form chromium(II) chloride or chromium(III) chloride? I have some information that may support the former. --Chemicalinterest (talk) 18:44, 8 May 2010 (UTC)
- I think CrCl2, because 2CrCl3 + H2 → 2CrCl2 + 2HCl. However, I think in high concentrations of acids, CrCl3 will from. --The High Fin Sperm Whale 20:45, 8 May 2010 (UTC)
Because the resulting solution reacts with ammonia to form an easily oxidized green precipitate. --Chemicalinterest (talk) 22:16, 8 May 2010 (UTC)
- In that case, I think that your getting CrCl3. I think the reaction goes like this:
3HCl + Cr → CrCl3 + 1½H2
then:
CrCl3 + NH3 → CrN + 3HCl
--The High Fin Sperm Whale 04:44, 9 May 2010 (UTC)
- My first question is how you know that the reaction product with ammonia is easily oxidized green precipitate. Chromium(II) compounds are instable and difficult to make, so you and always with chromium(III). Chromium(III) chloride with ammonium you get a complex which will change colour to dark blue or violet on heating and go back to green after some time. The problem you encounter could also be that you add to much base and you precipitate chromium(III) hydroxides which at the end will be transformed to chromium(III) oxide. But if you like that kind of old chemistry get a Gmelin Handbook of chemistry from early 19th century, there most of your questions will be answered. --Stone (talk) 08:36, 9 May 2010 (UTC)
- Why would it be CrN? CrCl2 + 2 NH4OH → Cr(OH)2 + 2 NH4Cl
- Also, why are the links to chromium(II) chloride when the formula is CrCl3?
- After the ammonia is reacted with the chromium chloride, it forms the hydroxide precipitate. The hydroxide precipitate is green, then after about 5 minutes of exposure to air, it turns brown, whether wet or dry. It is the precipitate of the hydroxide when I react with ammonia. Note: I only use ammonia because I don't have much of any other base. I only have about 1 g of KOH, and no NaOH. Is the chromium(II) hydroxide more green than the chromium(III) hydroxide, which is why the brown color comes out. --Chemicalinterest (talk) 19:24, 9 May 2010 (UTC)
- To Stone: I think that the reaction of chromium with hydrochloric acid forms chromium(II) chloride; it becomes oxidized over time to form chromium(III) oxide. --Chemicalinterest (talk) 19:30, 9 May 2010 (UTC)
- The chromium(II) is very reactive and without proper Schlenk-methods you will not be able to obtain it. Even with them the light blue Chromium(II) reduces the water and with production of hydrogen you get the Chromium(III). --Stone (talk) 21:21, 9 May 2010 (UTC)
- With ammonia you get the very dark blue green chromium(III) hydroxide.
- The colour change in chromium(III) salt solutions from green to violett is no oxidation or reduction, but a simple change in the ligands of the chromium. --Stone (talk) 21:21, 9 May 2010 (UTC)
My first question is how you know that the reaction product with ammonia is a easily oxidized?--Stone (talk) 21:22, 9 May 2010 (UTC)
- Because of the color change; that's why I think it is oxidized. It did not change from green to violet, which would be dehydration, but from green to brown, like the oxidation of ferrous hydroxide to ferrous oxide(which turns from green to brown). The chromium(II) wouldn't reduce the water: at least not at room temperature. The reduction potential for Cr3+ (or the oxidation of Cr2+) is -0.42. Only an oxidizing agent which has a number above (less negative) it can oxidize it.The potential for the oxidation of water (2 H2O + 2 e- → H2 + 2 OH-) is -0.8277. But if the water is a little acidic, the potential for the reduction of acid is (2 H+ + 2 e- → H2) +0.00, which it can easily oxidize. If it is alkaline, it can precipitate the chromium(II). That is why it is only stable in very pure solution.
- So chromium(II) cannot form in acid then. The reactions would be: Cr + 2 HCl → CrCl2 + H2 2 CrCl2 + 2 HCl → 2 CrCl3 + H2 --Chemicalinterest (talk) 11:05, 10 May 2010 (UTC)
Idea! The easily oxidized green precipitate is just chromium and nickel oxides. They are easily oxidized when alkaline, such as in the reaction with ammonia. They react to form nickel chromate. 4 NiO + 2 Cr2O3 + 3 O2 → 4 NiCrO4 Then NiCrO4 + 2 NaClO → Na2CrO4 + NiCl2 + O2 If it is green, i.e. still nickel and chromium oxides, when it is reacted with NaClO it turns brown, (nickel chromate) then forms sodium chromate. --Chemicalinterest (talk) 12:57, 10 May 2010 (UTC)
is it possible to have a defective sense of rhythm?
is it possible to have a defective sense of rhythm or no sense of rhythm? Thank youl. 84.153.199.22 (talk) 18:51, 8 May 2010 (UTC)
- Yeah. I know quite a few drummers like that! —Preceding unsigned comment added by RampantFairy (talk • contribs) 19:04, 8 May 2010 (UTC)
- See Amusia#Temporal relations. Qwfp (talk) 19:51, 8 May 2010 (UTC)
- Comment from Roger Taylor on a BBC programme about guitar players last night. "How do you know if there's a drummer at the door?" "Because they knock three times then come in late." An alternative. "Who hangs around with musicians?" "Drummers". --Phil Holmes (talk) 09:50, 9 May 2010 (UTC)
- Sorry I can't resist my fav drummer joke: What's the difference between a drummer and a drum machine? You only have to punch the song into a drum machine once. :D Vespine (talk) 23:11, 10 May 2010 (UTC)
gas vent
can some1 explain how the monoxide vent to gas central heating works? its been cold in my basment and i hear wind gushing thru my ducts. i suspects the cap on the monoxide vent is missing or something. —Preceding unsigned comment added by Tom12350 (talk • contribs) 19:41, 8 May 2010 (UTC)
- Get a technician. You'll want to find the carbon monoxide leak if there is one, it's very toxic and very unnoticeable by smell, sight or taste. Regards, --—Cyclonenim | Chat 00:34, 9 May 2010 (UTC)
- Get a qualified engineer to look at it. But at the earliest opportunity, I suggest you try to find the outlet yourself and check that it is not obstructed.--Shantavira|feed me 08:20, 9 May 2010 (UTC)
- You can also buy carbon-monoxide detectors - if you have that kind of a system, I'd strongly recommend getting one. SteveBaker (talk) 16:22, 9 May 2010 (UTC)
omg thers no monoxide leaking. thats not my question i was asking how they work. can some1 give me a diagram. —Preceding unsigned comment added by Tom12350 (talk • contribs) 18:28, 9 May 2010 (UTC)
- I've never heard of a monoxide vent. Where did you hear that term? There most definitely is not carbon monoxide flowing through your heating ducts unless you have a very very severe failure of your system. Ariel. (talk) 22:04, 9 May 2010 (UTC)
- Googling suggests that some people refer to the ordinary exhaust vent as a "monoxide vent", but you're right that its primary purpose is to vent ordinary exhaust gases & vapours, of which carbon monoxide should be a very small component, though there will always be some present: my own gas water heater typically tests out at an acceptable 12 parts per million, for example.
- I suggest to Tom12350 that you contact the manufacturer to ask for a handbook for your particular appliance/system, as there is doubtless a great deal of variation between makes and models. I'd also endorse Shantavira's suggestion to get a professional gas appliance engineer to look at it: this stuff is too potentially dangerous for anyone unqualified to mess with, and in the UK it's illegal - see Gas safe register. 87.81.230.195 (talk) 00:43, 10 May 2010 (UTC)
Yellowjackets
Hoe many body lengths do they fly per minute? This just a curiosity question. —Preceding unsigned comment added by Unambiguous13 (talk • contribs) 21:01, 8 May 2010 (UTC) Find out how many miles per hour they fly. Divide it by sixty to get miles per minute. Multiply that by 5280 to get how many feet per minute. Find out how long a wasp's body is. Divide 1 foot into the length of the body in feet. Multiply that by the number of feet per minute it flies. That will give you the body lengths. PS: Sorry if you don't live in the US. --Chemicalinterest (talk) 23:18, 8 May 2010 (UTC)
- (Edit Conflict) That would depend very much on whether they (generally called 'wasps' outside North America, by the way) were foraging amongst food sources, covering distance purposefully, escaping from danger, or attacking. Our article Yellow jacket gives a typical (worker) body length of ½"/12mm. This site quotes a (non-foraging) wasp flight speed of 0.094mph (in contrast to a foraging flight speed of 0.008mph), while this one gives a wasp flight speed of 2.5m/s (= 5.59mph). (Both sites found in the first half dozen Google hits from searching 'wasp flight speed'.) If my maths are correct, these two figures give body length per minute figures of about 198 and 11,806 (or more realistically 200 and 12,000) respectively. 87.81.230.195 (talk) 23:38, 8 May 2010 (UTC)
- "Generally called wasps outside North America"? What are you talking about? They're called wasps in North America, too, because they are wasps. But not all wasps are yellowjackets. It's a specific kind of wasp. --Trovatore (talk) 06:54, 9 May 2010 (UTC)
- The word "yellowjacket" isn't used outside North America, though. They are just called "wasps". If you like you can talk about specific species of wasps, but most people never would. --Tango (talk) 15:39, 9 May 2010 (UTC)
- "Generally called wasps outside North America"? What are you talking about? They're called wasps in North America, too, because they are wasps. But not all wasps are yellowjackets. It's a specific kind of wasp. --Trovatore (talk) 06:54, 9 May 2010 (UTC)
- That second site is linked to as the source for the first site. The first site is just a random idiot on the internet and should be ignored. --Tango (talk) 02:49, 9 May 2010 (UTC)
- Good spot, Tango. It's not entirely clear if the first site got those specific figures from the second, but in any case different sites cite widely varying figures for speeds of various types of flight, so a definitive single answer is uncalculable. 87.81.230.195 (talk) 00:26, 10 May 2010 (UTC)
did people really have to stay quiet on submarines?
did people really have to stay quiet on submarines or is that just a movie thing? 85.181.146.182 (talk) 22:06, 8 May 2010 (UTC)
also: can you literally hear a high pitch "ping" if another ship or sub is acti ely sonarinf your position?
also, why in films do subs try to go very deep to become impervious cant ships see them there too or drop charges? 85.181.146.182 (talk) 22:49, 8 May 2010 (UTC)
- Well if you did not want to be detected on the submarine it is a good idea to be quiet. The ship could have a microphone in the water to listen to submarine engines or other sources of noise. A submarine near the surface may be visible through the water and mat disturb the surface with a wake, ALso deep down it will be harder to hit, and harder to detect on a sonar, due to increased range, and particularly hard if it was close to the sea floor as the reflection of the sea bed will mask the vessel. Graeme Bartlett (talk) 03:24, 9 May 2010 (UTC)
- Plus, in deep water it's possible for the submarine to go below a thermocline layer, which would tend to reflect sound and make it harder for a surface ship to detect the sub. Fair winds to you 67.170.215.166 (talk) 07:23, 9 May 2010 (UTC)
- I have a work colleague who spent many years on submarines during the 'cold war' period and he relates that absolute silence was paramount to avoid detection. Crew used slippers not shoes to avoid detectable footfall. Remember that water is a hugely more efficient transmitter of sound than air, ask the whales (no, not that Whales!) Caesar's Daddy (talk) 07:56, 9 May 2010 (UTC)
- Plus, in deep water it's possible for the submarine to go below a thermocline layer, which would tend to reflect sound and make it harder for a surface ship to detect the sub. Fair winds to you 67.170.215.166 (talk) 07:23, 9 May 2010 (UTC)
- We have an article on sonar. The ping or chirp given out by the other vessel would not be audible to the human ear because the frequency is just a bit too high for most people to hear, and it wont re-radiated into the sub through the pressure hull anyway. However, the sonar operator can hear it, because the frequency is lowered into the audible range via a heterodyne circuit. See also Counter Sonar measures.--Aspro (talk) 11:18, 9 May 2010 (UTC)
- Modern SONAR is far outside of the range of frequencies that humans can hear - but since many submarine movies are set in World War II, we're not really talking about modern SONAR. I think the technology of the time would have made using audible frequencies quite attractive since you wouldn't need any fancy electronics to decode it - just a guy with sharp hearing. If the guy on the anti-submarine ship could hear the return echo (albeit with sensitive directional microphones) then the ping that was emitted would have had to be pretty loud. The guys inside the submarine would be hearing the sound after it had travelled only half the distance that the sonar guy would be hearing it at - so for them, it would be at least four times as loud - more than that since some energy would be lost in the reflection off of the submarine's hull. Since they are all trying to be super-quiet, I think it's possible that they could hear the 'pings'. SteveBaker (talk) 16:20, 9 May 2010 (UTC)

- If they could have done that without the ambient noise swamping the signal they may have adopted (or stayed with) audible frequencies. Also, higher frequencies are more directional and it is important to not only obtain the range but the direction of the returning echo too. Therefore, they used a range of about 20kHz to 25 kHz. The article on Hearing_range reads: “Specifically in humans, we have a maximum aural range of 12 Hz under ideal laboratory conditions[1] to 20,000 Hz in some individuals, but the range shrinks during our lifetime, usually beginning at around the age of 8 with the higher frequencies fading. If you look at the equal-loudness contours diagram on the right you will see that sensitivity falls off dramatical with increasing pitch. The background noises in the ocean would swamp the echo out preventing (any children they would have to employ) from detecting the sub. Also, heterodynes are hardly fancy electronics – the common domestic radios of the era used one. --Aspro (talk) 18:16, 9 May 2010 (UTC)
- A modern nuclear submarine in operation underwater makes about as much noise as a motorcycle or a brass band, due to propeller noise, fluid flow through pipes, and pumps.. Slippers versus normal shoes would seem to be the least of their worries. In the old WW2 movies, when a diesel electric sub was operating on batteries and the sonar was crude, shutting down pumps and fans and generally making no sound would have made more of a difference. Copied from my earlier posting on the topic: The Russian Sierra class had a reported 120 decibels and the U.S. Los Angeles class produced 110 decibels. By comparison, a loud rock concert is reported to be about 115 decibels, a power saw at 3 feet 110 db, and a motorcycle 100 db. There is a lot of heavy equipment operating on a submarine while it is in motion. Another source on the noise levels of various nations' subs, both diesel, battery and nuke, is at [19]. AWW2 diesel/electric sub submerged did not have to operate pumps to cool a reactor core, and could basically shut down all motors and drift. Edison (talk) 00:06, 10 May 2010 (UTC)
- Wouldn't the important factor be how much louder the sub is than the oceans' background noise at the particular frequencies (no doubt a wide range) the sub makes? (added) Perhaps Ambient noise level is closer to what I was thinking of. --220.101.28.25 (talk) 05:14, 10 May 2010 (UTC)
- A modern nuclear submarine in operation underwater, Edison, can make use of natural convection in the reactor core while operating at slow speeds (which is what they do most of the time, precisely to reduce noise), thus allowing the reactor pumps to be shut down and cutting the noise down to a whisper. The figures you gave are only valid when the sub is racing at full speed, and this is because of propeller cavitation (which is a factor on all subs at high speed, regardless of whether they're diesel or nuclear); the powerplant noise (except on some kinds of subs like the Alfa) is generally a much smaller component of total noise (though still significant at high speeds). Also, modern subs have rubber tiles on the hull to absorb engine noise. The Sierra class submarines were indeed noisy, because they were designed mostly for high speed without regard for noise -- just like the earlier Alfas, they were mainly designed for inshore defense and for chasing down carrier groups. High-frequency sonar is absorbed much more by the water, so there's a tradeoff between better resolution and maximum range. The Los Angeles sub used 2 different sonars, an audible sonar for long-range detection and an ultrasonic sonar for more precise location at shorter ranges (don't know about the new Virginia class submarines, though). FWiW 67.170.215.166 (talk) 08:03, 10 May 2010 (UTC)
Bird/crocodile grouping
Is there a taxonomic group that includes birds and crocodiles but excludes mammals? I remember reading that birds and crocodiles have similar lung structures, which suggests that they are more closely related than ether are to mammals, but the last common ancestors of the two I can find are Amniote and Tetrapod, which each include mammals. —Arctic Gnome (talk • contribs) 22:28, 8 May 2010 (UTC)
- You are thinking of the clade Sauropsida (Goodrich, 1916), if I am not mistaken. Intelligentsium 22:45, 8 May 2010 (UTC)
- The minimal group that includes birds and crocodiles is archosauria, I believe. The split between mammals and the others goes back to the very early split between diapsids (birds, crocodiles, reptiles) and synapsids (mammals and many extinct groups), which diverged well over 300 million years ago. Looie496 (talk) 23:56, 8 May 2010 (UTC)
- Thanks. So is Archosaur a subgroup of Sauropsida? The relationship between the two groups isn't fully explained in either article. Also, the article on crocodilia seems to have merged the terms into Archosauromorpha. —Arctic Gnome (talk • contribs) 01:33, 9 May 2010 (UTC)
- The tree diagrams in Sauropsida and archosauromorpha show the relationships -- archosauria is a subset of archosauromorpha; everything outside that subset is extinct, though. Looie496 (talk) 01:50, 9 May 2010 (UTC)
- Thanks. So is Archosaur a subgroup of Sauropsida? The relationship between the two groups isn't fully explained in either article. Also, the article on crocodilia seems to have merged the terms into Archosauromorpha. —Arctic Gnome (talk • contribs) 01:33, 9 May 2010 (UTC)
- The minimal group that includes birds and crocodiles is archosauria, I believe. The split between mammals and the others goes back to the very early split between diapsids (birds, crocodiles, reptiles) and synapsids (mammals and many extinct groups), which diverged well over 300 million years ago. Looie496 (talk) 23:56, 8 May 2010 (UTC)
May 9
Neanderthal genes in modern humans
I read here that I may be partially Neanderthal. What I don't understand is how it is possible that you could only have a small nonzero Neanderthal contribution to the human genome. I mean, the scientists are surprised that it seems to be as large as 1% to 4% instead of a much lower number or zero. But how can any nonzero Neanderthal contribution to someone's DNA be less than an entire chromosome? Count Iblis (talk) 00:23, 9 May 2010 (UTC)
- Chromosomes come in pairs, and the two members of a pair can swap parts via the process of chromosomal crossover. After a sufficient number of generations you get a very high level of mixing, even between parts of a single chromosome. Looie496 (talk) 00:35, 9 May 2010 (UTC)
- (edit conflict)I think, emphasis on that, that they are referring to the original DNA that is left in your DNA. Clearly when the Neanderthal mated with a Homo sapien, they're contributing chromosomes, but over time this DNA is going be diluted with new DNA from other Homo sapiens. I think I explained that badly. In short, the 1% is the remaining original Neanderthal DNA. Regards, --—Cyclonenim | Chat 00:37, 9 May 2010 (UTC)
- Yeah, Looie explains it better. Regards, --—Cyclonenim | Chat 00:37, 9 May 2010 (UTC)
- What confused me (speaking as someone who almost failed high school biology), is that since we have 46 chromosomes, I would have thought that the smallest (non-zero) amount of neanderthal DNA we could posses is 1/46th. Does this "chromosomal crossover" that Cyclonenim mentioned mean that two parents can have more than 2^46 different possible offspring? 24.68.41.132 (talk) 02:55, 9 May 2010 (UTC)
- Yes. Nil Einne (talk) 08:41, 9 May 2010 (UTC)
- The first-generation offspring will have 23 chromosomes that are "all-neanderthal", and 23 chromosomes that are "all-modern-human". The number of possible neanderthal chromosome combinations that the neanderthal parent could transmit to the child is a lot greater than 223, due to recombination between the two variants of neanderthal chromosomes that the neanderthal parent has inherited from the neanderthal grandparents. Likewise of course with the human parent. When the hybrid child grows up, and starts producing reproductive cells, there will again be recombination between the pairs of chromosomes, but now the recombination will occur between a neanderthal-derived chromosome and a modern-human-derived chromosome, resulting in chromosomes that are partly derived from neanderthals and partly derived from modern humans. If the hybrid's children continue breeding with modern humans, the neanderthal contribution to the genomes of their offspring will be diluted. However, if some of the neanderthal genes turn out to be advantageous, natural selection will ensure that these are not lost. --NorwegianBlue talk 09:57, 9 May 2010 (UTC)
Thanks for the answer everyone! (I should have kept biology on my high school curriculum, I guess). So, how fragmented will the Neanderthal genetic material be in the modern population? I roughly estimated this as follows. Suppose that I have a Neanderthal gene on one of my chromosomes, orginating from a hybrid that lived 60,000 years ago. Then, if I understand this article correctly, on average two genes separated by a million base pairs will have a P = 1% probability of being separated in the next generation. So, the probability of a gene that is L*10^6 basepairs away not being separated after N generations is (1-P*L)^N, which is valid for L not much larger than 1. If I take N = 3000 and equate the probability to 1/2, I find L = 0.023. So already at a distance of 23,000 basepairs there is a 50% chance of separation. Count Iblis (talk) 14:56, 9 May 2010 (UTC)
- Natural selection is not random. 67.243.7.245 (talk) 15:16, 9 May 2010 (UTC)
- Yes it is! SteveBaker (talk) 16:09, 9 May 2010 (UTC)
- This is actually a misconception about natural selection. The process wherein DNA mutations occur is random, as is the combination of genetic information from one's parents. However, as DNA encodes physiology and behavior, these two random process have consequences in the environment, which means genetic information is subject to nonrandom processes of selection. Over time, the genes that encode for physiology and behavior that work best in a given environment persist at a greater frequency than those that don't; this is natural selection.
- Bringing it to the subject at hand, this means that if any Neanderthal genetic information has environmental consequences (including those from the social environment), then there would be non-random influence on populations descending from such intermixing. For example, if the Modern-Humans who came to Europe after Neanderthals had very dark skin, then they would have difficulty producing vitamin D; relevent genetic information from the native Neanderthals, who surely would have already acquired lighter skin, would thus be favored over generations. — Ƶ§œš¹ [aɪm ˈfɹ̠ˤʷɛ̃ɾ̃ˡi] 19:17, 9 May 2010 (UTC)
- So a random frog mutates and gets the gene for eternal life and for being irresistable to frogs of the other sex - but is eaten by your pet cat just one hour before it reached maturity and passed this remarkable gene onto the next generation. OF COURSE IT'S RANDOM!!! SteveBaker (talk) 23:51, 9 May 2010 (UTC)
- Does eternal life mean that the frog is more likely to survive and pass on its genes in this particular environment? I don't think so, but even if it did, it's not a guarantee. Advantagious changes usually only give a slight advantage over others. If natural selection were random, then there would be nothing pushing species to adapt to their environments.
- Keep in mind that when there are no selective pressures on a given set of DNA, it is subject to genetic drift, which is also random. — Ƶ§œš¹ [aɪm ˈfɹ̠ˤʷɛ̃ɾ̃ˡi] 01:22, 10 May 2010 (UTC)
- (Edit Conflicts) But in that example, Steve, natural selection hasn't occurred, because it never had a chance to. The selection happens over generations, after one or more frogs have (semi-randomly) mutated that gene and successfully introduced it into the frog gene pool, when nature/the environment (including pet cats) operates on it (or more accurately its phenotypic expression(s)) and selects for or against it. Individual carrier frogs may well be subject to random accidents (ones not related to the genes' effects), and others to random 'good fortune', but the effects of these will average out in the gene pool as a whole, so the selection pressure on that gene is the opposite of random. Compare it to Monopoly where, although individual moves are affected by the limited randomness of dice scores (e.g. you can't score 1 or 13), a skilful player will still usually beat less skilful ones in the long run, though he/she might still be wiped out by unusual events. 87.81.230.195 (talk) 01:49, 10 May 2010 (UTC)
- So a random frog mutates and gets the gene for eternal life and for being irresistable to frogs of the other sex - but is eaten by your pet cat just one hour before it reached maturity and passed this remarkable gene onto the next generation. OF COURSE IT'S RANDOM!!! SteveBaker (talk) 23:51, 9 May 2010 (UTC)
- Yes it is! SteveBaker (talk) 16:09, 9 May 2010 (UTC)
- Steve, your frog example is not random. it is stochastic. These are two different things. Natural selection is not random. Dauto (talk) 05:38, 10 May 2010 (UTC)
- I suggest you look up the word "stochastic" in the dictionary. Wiktionary provides just one meaning - it says "stochastic: Random, randomly-determined.". Randomness that tends to average out in the long run is still random. There is still always the possibility that a thousand species of genetically perfect dinosaurs get wiped out by a random meteor strike. SteveBaker (talk) 16:20, 10 May 2010 (UTC)
- If, over thousands of generations, a species changes to adapt to its environment, then that change itself can't be random. At the individual level, the advantage of beneficial genes is much smaller than it is at the group level over generations. Your examples seem to assume that environment is irrelevent when it comes to fitness. In your frog example, the most advantagious genes are the ones that make frogs cat resistant. In your dinosaur example, the meteor strike has altered the environment so drastically that the genes that didn't fit the new environment didn't survive. When the environment changes drastically and none of the individuals in a species have genes that favor that environment, extinction is a lot more likely. — Ƶ§œš¹ [aɪm ˈfɹ̠ˤʷɛ̃ɾ̃ˡi] 18:05, 10 May 2010 (UTC)
- I suggest you look up the word "stochastic" in the dictionary. Wiktionary provides just one meaning - it says "stochastic: Random, randomly-determined.". Randomness that tends to average out in the long run is still random. There is still always the possibility that a thousand species of genetically perfect dinosaurs get wiped out by a random meteor strike. SteveBaker (talk) 16:20, 10 May 2010 (UTC)
- Steve, your frog example is not random. it is stochastic. These are two different things. Natural selection is not random. Dauto (talk) 05:38, 10 May 2010 (UTC)
- One thing that I think we're missing here is that Neanderthal and Modern-Human DNA would be remarkably similar to start with. Heck Chimpanzee DNA and Human DNA is about 94% identical - we'd imagine that Neanderthals and humans would be more similar than Chimps and humans. So even if we had one complete Neanderthal chromosome - we'd still only have a tiny, tiny percentage of DNA that looked different from a Neanderthal-free modern human. SteveBaker (talk) 16:09, 9 May 2010 (UTC)
- To quote Wikipedia, "While unable to definitively conclude that interbreeding between [humans and Neanderthals] did not occur, analysis of the nuclear DNA from the Neanderthal suggests the low likelihood of it having occurred at any appreciable level". Source: Hayes, Jacqui (15 November 2006). "DNA find deepens Neanderthal mystery". Cosmos. http://www.cosmosmagazine.com/news/853/dna-find-deepens-neanderthal-mystery. Retrieved 18 May 2009.
- That 2006 quote is pretty irrelevant now. It relates to the same group, and the same project. The data they had aquired in 2006 did not give evidence for admixture. Today, when more data is available, it turns out that some interbreeding probably occured. --NorwegianBlue talk 20:21, 9 May 2010 (UTC)
- To quote Wikipedia, "While unable to definitively conclude that interbreeding between [humans and Neanderthals] did not occur, analysis of the nuclear DNA from the Neanderthal suggests the low likelihood of it having occurred at any appreciable level". Source: Hayes, Jacqui (15 November 2006). "DNA find deepens Neanderthal mystery". Cosmos. http://www.cosmosmagazine.com/news/853/dna-find-deepens-neanderthal-mystery. Retrieved 18 May 2009.
Paintballing science
Where I went paintballing they sold pressurized CO2 chambers, as well as more expensive pressurized N2 chambers (used for firing the balls). Why would nitrogen be better than carbon dioxide? Isn't it just dependent on the pressure, which is independent of the gas used? Also, my facemask kept fogging up and I was wondering what could have been done to reduce that. But before that, I'm not too sure why it would fog up...my guess is that it fogs up because the moist air from our lungs (moist because it was raining?) hits the cold visor, causing the air to cool down which makes the water in the air condense. I'm not completely satisfied by this because the air cools down to the same temp as the surrounding air, which can hold its water without too much trouble. I was hopeing someone could help me out with this. But anyways, about preventing the fogging...I heard spitting on the visor helps, but why? Any other useful tips? Thanks. 173.179.59.66 (talk) 01:49, 9 May 2010 (UTC)
- After looking at some paintball forums, it seems that the nitrogen is stored as a compressed gas, but the CO2 is stored as a liquid. As a gas (nitrogen) expands, it gets slightly cooler, but as liquid CO2 evaporates it gets very cold. Apparently this makes the gun cold and leads to less consistent shots. See [20].24.150.18.30 (talk) 02:27, 9 May 2010 (UTC)
- As for the fogging mask, smearing a drop of saliva may work, as I know (from experience) that this is useful in SCUBA diving (I'm sure that a fogged up mask is no fun in paintball, but it can be an absolute nightmare underwater!). The fogging is caused by water vapour in your breath hitting a cold surface (the mask) and condensing into tiny droplets. My theory is that if there is already a thin layer of moisture (saliva) on the mask that is smooth enough to see through, then any drops that form from you exhaling will just become part of that layer and won't affect the light passing through. Dive shops and maybe ski stores may sell antifog lens cleaners which could help.24.150.18.30 (talk) 02:37, 9 May 2010 (UTC)
- The other option is to get a better quality mask with vents to allow the moisture out (not an option for SCUBA divers, but it works well for masks used in air - I've used one for skiing and you could really tell the difference if you blocked the vents, not that I can work out why goggles that only covers your eyes, not your nose or mouth, would fog up so much...). --Tango (talk) 02:54, 9 May 2010 (UTC)
- On preventing your mask from fogging up, I often see people at paintball fields putting shaving cream on their facemask lenses in order to prevent fogging. No idea what the theory behind this is or why it works, but I guess people wouldn't do it if it didn't have some degree of effectiveness. And yes, if you're serious about paintball, definitely invest in a high-quality mask with vents and anti-fog coating on the lenses. --Cerebellum (talk) 03:31, 9 May 2010 (UTC)
- The other option is to get a better quality mask with vents to allow the moisture out (not an option for SCUBA divers, but it works well for masks used in air - I've used one for skiing and you could really tell the difference if you blocked the vents, not that I can work out why goggles that only covers your eyes, not your nose or mouth, would fog up so much...). --Tango (talk) 02:54, 9 May 2010 (UTC)
- Smearing the visor with spit can reduce fogging because of the spit's small tendency to act as a surfactant - it prevents the condensate from forming small drops and instead it forms a uniform layer, which you can see through. Other liquids, such as shaving foam, potato juice and detergent do the same thing. The best way of reducing condensation is to use a double-layered mask, which is very common in skiing. The inner one warms up and so does not suffer from condensation. The outer one gets cold, but the warm damp air does not reach it, and so it doesn't get fogged either. Many motor-cycle helmets do the same sort of thing, often with a system called a pin lock. --Phil Holmes (talk) 09:45, 9 May 2010 (UTC)
- And least anybody forgets to mention it. Wikipedia -as always- has an article. Paintball equipment.--Aspro (talk) 12:26, 9 May 2010 (UTC)
- Also see Anti-fog... Cacycle (talk) 22:11, 9 May 2010 (UTC)
Thanks a bunch! 173.179.59.66 (talk) 02:05, 10 May 2010 (UTC)
Angular momentum transform
If the angular momentum of a particle about the point (x0,y0) is known, how do I calculate the angular momentum with respect to another position, say (x1,y1)? --142.151.129.67 (talk) 02:54, 9 May 2010 (UTC)
- You can't. They are independent. Imagine you have a particle with mass 1kg moving in a circle of radius 1m centred at (0,0) at 1 m/s - it has a constant angular momentum around (0,0) of 1 Nms. The same particle's angular momentum around (0,1) will depend on its position in the circle, eg. when it is at (0,1) its angular momentum will be zero, when it is at (0,-1) its angular momentum will be 2 Nms. If there was a transformation from one centre to another then a constant angular momentum would have to transform into a constant angular momentum, so clearly there is no such transformation. --Tango (talk) 03:03, 9 May 2010 (UTC)
- A good discussion of the mathematics of angular momentum under various coordinate transforms is given in Chapter 9.4 of Classical Dynamics of Particles and Systems ($150 at Amazon). You can probably find this book at a university library. The derivation is too complex to write here, but can be summarized: "The total angular momentum about an origin is the sum of the angular momentum of the center of mass about that origin and the angular momentum of the system about the position of the center of mass." This is closely related to the parallel axis theorem, which describes the transform of moment of inertia to other coordinate systems. I am not sure if Tango's statement above is correct - I think what he means is that the angular momentum can not be easily represented. In the case of a non-inertial coordinate system, the angular momentum will appear to not be conserved, and will be time-varying. This is one way to describe Coriolis force, for example - a "mysterious" generation of angular momentum - but all that is really happening is that we are observing conservation of momentum in a system from the viewpoint of a non-inertial frame. Nimur (talk) 02:05, 10 May 2010 (UTC)
- It's not difficult to represent, it is impossible. The Coriolis force has nothing to do with it; we haven't changed reference frame. I've shown that given an angular momentum around one point I can find two situations both with that angular momentum around that point but with different angular momenta around a different point. That means you cannot determine the angular momentum around the second point without more information. --Tango (talk) 02:33, 10 May 2010 (UTC)
- Tango, I think you are incorrect; what you have shown failed to take into account the Parallel Axis Theorem; in other words when you gave your circle example, you did not account for the angular momentum of the center of mass (in the new coordinate system - you moved the origin!). This is where the mysterious 2 Nms has disappeared / appeared - it should have been the angular momentum relative to the new position of the center of mass. Your derivation was incomplete, and that's why there's the inconsistency - you have performed half of the transform but forgot to transform the system's angular momentum about the center. If you decompose the vector to the center, r, into (ra + R), where R is the vector to the new origin, and ra is the vector to the true center of mass of the object, and then proceed with your derivation, you will find a new term in R (which describes, in your case, the orbit of the object around an arbitrary circle, and varies with time). The sum of the angular momenta from the system (M R x dR/dt) and the object's rotation (Iara) will be conserved - that is the total angular momentum of the entire system. Think of Earth revolving around the sun, and also rotating on its own axis. The total angular momentum of the system is constant, but if you observe from the point of view of Earth, (which is a moving reference frame), you "forgot" to account for the angular momentum of the whole orbit. This must be accounted for by computing the center of mass for the entire system, and taking a coordinate transform to your new origin of choice. In an n-body problem, where the choice of origin is arbitrary, how else would we be able to analyze angular momentum? It is definitely possible to represent angular momentum as observed from any arbitrary origin; this is done by decomposing the angular momentum into a rotation about the new origin and a rotation about the object's center of mass. For each arbitrary origin, the numeric values of this decomposition vary. In the most general coordinate transforms (rotations and translations), we need one or more inertia tensors to describe the system, and can not drop any terms. Nimur (talk) 15:16, 10 May 2010 (UTC)
- I'm talking about single particle. The centre of mass is the location of the particle and the angular momentum of the system around the centre of mass is zero. There is only one term. I don't need any theorems since I'm calculating the angular momentum for a specific system using the definition of angular momentum. --Tango (talk) 21:22, 10 May 2010 (UTC)
- Unless I'm very much mistaken, isn't angular momentum defined within a plane, and not "about" anything? In which case, the question isn't very meaningful.--Leon (talk) 21:41, 10 May 2010 (UTC)
- The definition of angular momentum, as given in our article, is so given an arbitrary coordinate system, r and v are going to be different. Because we know that angular momentum is a conserved quantity, something has to account for these changes in r and v. That would be the parallel axis theorem, which is a direct consequence of the definition of angular momentum in generalized coordinates. Nimur (talk) 07:10, 11 May 2010 (UTC)
- Correct, and the cross product yields a bivector, or oriented plane segment.
- The parallel axis theorem allows one to determine the moment of inertia of a body about a given axis if the moment of inertia about its centre of mass is known. From this, if one knows the angular velocity about this new axis, one can furthermore calculate the angular momentum. That is not the same as being able to ascertain the angular momentum about some new point if all that is known is the angular momentum about some other.--Leon (talk) 07:44, 11 May 2010 (UTC)
- That is the definition of angular momentum around the origin. The numbers I've quoted above are the magnitude of that vector (my system was planar, so there was no reason to state the direction - it is clearly perpendicular to that plane). Angular momentum is conserved - that means it stays the same if you change the time. It doesn't stay the same if you change the origin. There is clearly a relationship between the angular momentum around one point and the angular momentum around another, but that relationship depends on the details of the system in question so you can't do anything if you are just given the angular momentum. You need to be given the details of the system as well. --Tango (talk) 10:56, 11 May 2010 (UTC)
- The definition of angular momentum, as given in our article, is so given an arbitrary coordinate system, r and v are going to be different. Because we know that angular momentum is a conserved quantity, something has to account for these changes in r and v. That would be the parallel axis theorem, which is a direct consequence of the definition of angular momentum in generalized coordinates. Nimur (talk) 07:10, 11 May 2010 (UTC)
- Unless I'm very much mistaken, isn't angular momentum defined within a plane, and not "about" anything? In which case, the question isn't very meaningful.--Leon (talk) 21:41, 10 May 2010 (UTC)
- I'm talking about single particle. The centre of mass is the location of the particle and the angular momentum of the system around the centre of mass is zero. There is only one term. I don't need any theorems since I'm calculating the angular momentum for a specific system using the definition of angular momentum. --Tango (talk) 21:22, 10 May 2010 (UTC)
- Tango, I think you are incorrect; what you have shown failed to take into account the Parallel Axis Theorem; in other words when you gave your circle example, you did not account for the angular momentum of the center of mass (in the new coordinate system - you moved the origin!). This is where the mysterious 2 Nms has disappeared / appeared - it should have been the angular momentum relative to the new position of the center of mass. Your derivation was incomplete, and that's why there's the inconsistency - you have performed half of the transform but forgot to transform the system's angular momentum about the center. If you decompose the vector to the center, r, into (ra + R), where R is the vector to the new origin, and ra is the vector to the true center of mass of the object, and then proceed with your derivation, you will find a new term in R (which describes, in your case, the orbit of the object around an arbitrary circle, and varies with time). The sum of the angular momenta from the system (M R x dR/dt) and the object's rotation (Iara) will be conserved - that is the total angular momentum of the entire system. Think of Earth revolving around the sun, and also rotating on its own axis. The total angular momentum of the system is constant, but if you observe from the point of view of Earth, (which is a moving reference frame), you "forgot" to account for the angular momentum of the whole orbit. This must be accounted for by computing the center of mass for the entire system, and taking a coordinate transform to your new origin of choice. In an n-body problem, where the choice of origin is arbitrary, how else would we be able to analyze angular momentum? It is definitely possible to represent angular momentum as observed from any arbitrary origin; this is done by decomposing the angular momentum into a rotation about the new origin and a rotation about the object's center of mass. For each arbitrary origin, the numeric values of this decomposition vary. In the most general coordinate transforms (rotations and translations), we need one or more inertia tensors to describe the system, and can not drop any terms. Nimur (talk) 15:16, 10 May 2010 (UTC)
- It's not difficult to represent, it is impossible. The Coriolis force has nothing to do with it; we haven't changed reference frame. I've shown that given an angular momentum around one point I can find two situations both with that angular momentum around that point but with different angular momenta around a different point. That means you cannot determine the angular momentum around the second point without more information. --Tango (talk) 02:33, 10 May 2010 (UTC)
- A good discussion of the mathematics of angular momentum under various coordinate transforms is given in Chapter 9.4 of Classical Dynamics of Particles and Systems ($150 at Amazon). You can probably find this book at a university library. The derivation is too complex to write here, but can be summarized: "The total angular momentum about an origin is the sum of the angular momentum of the center of mass about that origin and the angular momentum of the system about the position of the center of mass." This is closely related to the parallel axis theorem, which describes the transform of moment of inertia to other coordinate systems. I am not sure if Tango's statement above is correct - I think what he means is that the angular momentum can not be easily represented. In the case of a non-inertial coordinate system, the angular momentum will appear to not be conserved, and will be time-varying. This is one way to describe Coriolis force, for example - a "mysterious" generation of angular momentum - but all that is really happening is that we are observing conservation of momentum in a system from the viewpoint of a non-inertial frame. Nimur (talk) 02:05, 10 May 2010 (UTC)
Boiling and vapor
From my understanding, boiling a solution evacuates solely the water as vapor and causes the solutes to remain in solution. But what happens when all the solvent has been vaporized; is the solute in crystal form along the walls of the pot? My particular question relates to boiling milk. Does the water content boil off and the fats, sugars, etc. remain in solution, albeit more concentrated? What exactly is included in the steam that comes off of a pot of boiling milk? DRosenbach (Talk | Contribs) 03:19, 9 May 2010 (UTC)
- Yes the steam will be mostly water. The scum left behind will be largely protein and lactose, with liquid butter fat making up the oily component. The lactose in water will form a syrup, which will boil at a higher temperature, and this will eventually caramelize. Graeme Bartlett (talk) 03:30, 9 May 2010 (UTC)
- Mostly...what other than water would boil off? And when you say that the syrup will boil at a higher temp, so that would be carbohydrate in gas form? DRosenbach (Talk | Contribs) 03:32, 9 May 2010 (UTC)
- The vapour consists of the compounds of the liquid in the relation to their partial pressure at the given temperature and that is depending on the vapour pressure of the compound. So at 100°C a little of the fat will be also evaporate, not enough to see it but not zero molecules. With milk I would guess that the vapour is more than 99.999% water. --Stone (talk) 08:42, 9 May 2010 (UTC)
- Mostly...what other than water would boil off? And when you say that the syrup will boil at a higher temp, so that would be carbohydrate in gas form? DRosenbach (Talk | Contribs) 03:32, 9 May 2010 (UTC)
- Strictly, your original statement is not true. If we dissolve some ethanol in water and then raise the temperature of the solution, the initial vapour will be composed predominantly of ethanol with some water also. As stone says, the proportion of ethanol to water would depend on their partial pressures at the temperature the solution had been raised to. So "boiling a solution" does not evacuate "solely the water as vapour". --Phil Holmes (talk) 09:39, 9 May 2010 (UTC)
- Well to start with the most volatile substances will boil out first. These would be nitrogen oxygen carbon dioxide. You would also have noticed a smell, this is also due to volatile substances that have evaporated along with the water. Graeme Bartlett (talk) 12:31, 9 May 2010 (UTC)
- When you boil milk, it is almost entirely water that evaporates. If you boil it down until almost no water is left, the result is fudge -- an almost solid mass made a bit squishy by the fat content. (The fudge you buy in stores has a lot of sugar added, and usually other flavorings such as chocolate, but basic fudge is what you get by boiling the water out of milk.) Looie496 (talk) 16:07, 9 May 2010 (UTC)
Plastic&Electric kettles
from what kind of plastic are electric kettle made? —Preceding unsigned comment added by Ha-y Gavra (talk • contribs) 11:04, 9 May 2010 (UTC)
- PEEK comes to mind but it is expensive.[21] More common high temp. plastics are polypropylene and POM.--Aspro (talk) 12:40, 9 May 2010 (UTC)
- My kettle says >PP< which I'm pretty sure means polypropylene: the article says this is a common material to make kettles out of. 86.180.48.37 (talk) 19:45, 9 May 2010 (UTC)
Moon distance
how far is moon from the earth —Preceding unsigned comment added by 41.184.96.83 (talk) 12:54, 9 May 2010 (UTC)
- 384,405 km (from center to center), according to a diagram in the moon article. DRosenbach (Talk | Contribs) 13:04, 9 May 2010 (UTC)
- Which makes for about 376,296 km (233,819 miles) subtracting out both sphere's radii. DRosenbach (Talk | Contribs) 12:08, 11 May 2010 (UTC)
- Well, as the moon's orbit is best described by an ellipse there are a few key distances which can all be found in the first few lines of the infobox here. The apogee is the furthest distance it ever gets from Earth and the perigee the closest. Martlet1215 (talk) 16:55, 9 May 2010 (UTC)
river discharge
What is the relationship between the average discharge of a river and maximum discharge? —Preceding unsigned comment added by Amrahs (talk • contribs) 13:32, 9 May 2010 (UTC)
- There isn't a relationship between those two figures. Rivers vary: maximum discharge is the largest amount of water that flows through the river in a certain amount of time, that usually happens in winter time. Average discharge is the average (usually mean) amount of water that flows through in a certain amount of time.--92.251.131.97 (talk) 14:24, 9 May 2010 (UTC)
- Have you read Discharge (hydrology).--Aspro (talk) 14:30, 9 May 2010 (UTC)
which one according to u are better; SAS of British army and NSG of India?
thank you —Preceding unsigned comment added by 117.197.245.199 (talk) 13:37, 9 May 2010 (UTC)
- Well if we compare the performance of the SAS during the Iranian embassy seige and both Iraq wars to the performance of the NSG during hte Mumbai terror attacks I think SAS is better.--92.251.131.97 (talk) 14:21, 9 May 2010 (UTC)
- The Wikipedia (science) reference desk is not the place to ask for opinions. The best one can possibly do to answer this question is to attempt to list records of each service's performances in similar situations. As the activities of these organisations are largely kept secret, it is quite difficult to attempt to gather empirical evidence of any relevance. 88.90.16.140 (talk) 15:43, 9 May 2010 (UTC)
- Why isn't a place to ask for opinions? Sure this isn't science it should be in humanities or misc, but we can give opinions.--92.251.131.97 (talk) 16:43, 9 May 2010 (UTC)
- The reference desks were originally set up to assist people with writing articles. Our opinions don't go in the articles and so the ref desks are about facts not opinions. Theresa Knott | token threats 16:46, 9 May 2010 (UTC)
- Have you read the bit at the top of the page where it says "The reference desk does not answer requests for opinions"? --Phil Holmes (talk) 17:27, 9 May 2010 (UTC)
- Why isn't a place to ask for opinions? Sure this isn't science it should be in humanities or misc, but we can give opinions.--92.251.131.97 (talk) 16:43, 9 May 2010 (UTC)
Is there much Greek blood in southern Italians?
Approx what % of people from southern Italy have some Greek ancestors?
Approx what % of people from the Po valley have some Celtic ancestors?
thanks--92.251.131.97 (talk) 14:19, 9 May 2010 (UTC)
- Happy to help, but a little google searching goes a long way! Greeks in Italy...and still looking around for Po valley --rocketrye12 talk/contribs 15:11, 9 May 2010 (UTC)
- Sorry I'm already aware of the history of greek people in Italy so that article doesn't help much, I'm asking how many actual Italian people have greek blood. —Preceding unsigned comment added by 92.251.131.97 (talk) 16:25, 9 May 2010 (UTC)
- You are going to have to give a better definition of Greek blood. If you just mean a person with an ancestor who has lived in Greece in the past, the number would be almost all of them. Graeme Bartlett (talk) 21:54, 9 May 2010 (UTC)
- Sorry I'm already aware of the history of greek people in Italy so that article doesn't help much, I'm asking how many actual Italian people have greek blood. —Preceding unsigned comment added by 92.251.131.97 (talk) 16:25, 9 May 2010 (UTC)
I'm going to suggest that you **** blood. DNA sequencing is where it's at. You want to look for the allele/haplotype frequencies (or their combinations) for molecular phenotypes that tend to be particular to "Greeks" (though you're going to have to define what type of Greek -- Mycaenean, Hellenistic, etc.?) John Riemann Soong (talk) 07:28, 10 May 2010 (UTC)
- I think it's rather likely that the OP is simply using 'blood' in the colloquial fashion as used in Kinship terminology to mean related by a common ancestor (i.e. genetically related) rather then literally having anything to do with blood. DNA sequencing is of course simply a method of attempting to measure this relationship. Of course you can use a blood sample for DNA sequencing although it's rare given the difficulty of taking blood compared ot other easier methods of obtaining a DNA sample like a cheek swab. Nil Einne (talk) 08:58, 10 May 2010 (UTC)
- I agree. And by that definition, I'd bet on 99+% for both "celtic blood" and "greek blood" unless one defines a non-negligible threshold. --Stephan Schulz (talk) 09:16, 10 May 2010 (UTC)
travel speed in space
how fast a spaceship travel in space? —Preceding unsigned comment added by Pedfp (talk • contribs) 14:21, 9 May 2010 (UTC)
- It would depend on where it's going. It would have to be going fast enough to achieve the appropriate Escape velocity.--Aspro (talk) 14:33, 9 May 2010 (UTC)
- It's not necessary to reach escape velocity to escape a planet (at least not the escape velocity at the surface), as mentioned at Escape velocity#Misconception. -- BenRG (talk) 04:03, 10 May 2010 (UTC)
- Are you asking, how fast can spaceships currently existing travel, or how fast could they travel, or how fast is theoretically feasible with different methods? Because it will be different for each of them. --Mr.98 (talk) 15:03, 9 May 2010 (UTC)
- The fastest manned spacecraft was Apollo 10 on it's return journey from the moon when it hit 39,900 kilometers per hour (24,791 mph) in 1969. That's also the fastest that any human has travelled.
- The fastest any human built unmanned spaceship has ever gone (and actually, is continuing to go right now) is the New Horizons probe that NASA has sent to Pluto. It is travelling at 16.3 kilometers every second which is 58,500 kilometers per hour, 10.1 miles per second or 36,400 miles per hour. It will gradually slow down as it gets closer to Pluto. The long-term fastest spacecraft are the Voyager probes - which are going fast enough to escape the sun's gravity completely - around 34,000 miles per hour.
- The fastest practical (just!) spacecraft we've ever designed would be the Project Orion nuclear bomb-powered spacecraft that in some versions would be theoretically capable of reaching about 10% of the speed of light and getting us to the nearest star in about 1000 years. An Orion-class spacecraft would (by necessity) be simply gigantic - with a massive 'pusher plate' about 20 kilometers across! You could house an entire city full of people inside - but it would be ruinously expensive to build - and if you launched it, it woudl make the area for hundreds of miles around its launchpad uninhabitable due to radioactive fallout - possibly causing a 'nuclear winter' scenario and ending life on earth in the process! However, if mankind had to leave the Earth in a hurry for some reason of impending catastrophy (a major earth-killing asteroid impact, for example) then it would perhaps be feasible to build such a thing in order to save a half million people and save some vestige of humanity for the future!
- There have been highly theoretical concepts for anti-matter powered spacecraft that would permit speeds up to 80% of light speed...but we have no idea how we could build such things in practice.
- The fastest that any spaceship (or anything else for that matter) could theoretically go is just a hair short of the speed of light...no matter how it's built or propelled, that's a "final" limit because no physical object can travel at the speed of light.
- SteveBaker (talk) 15:52, 9 May 2010 (UTC)
- An Orion drive's pusher plate doesn't have to be that big. According to the designs, the plate wouldn't be wider than the ship itself, and the ship's diameter can be less than 50 meters. ScienceApe (talk) 20:25, 9 May 2010 (UTC)
- And it could be built in orbit or (if small enough) launched into orbit using a conventional rocket, thus avoiding radioactive fallout at ground level. 67.170.215.166 (talk) 08:22, 10 May 2010 (UTC)
- The problem is that Orion only works if it is exceedingly heavy. It has to survive being pounded by nuclear weapons! Not just once - but hundreds or even thousands of times! That makes building it in orbit impractical. I suppose you could possibly build it on the moon using locally mined materials though. SteveBaker (talk) 11:49, 10 May 2010 (UTC)
- And it could be built in orbit or (if small enough) launched into orbit using a conventional rocket, thus avoiding radioactive fallout at ground level. 67.170.215.166 (talk) 08:22, 10 May 2010 (UTC)
- An Orion drive's pusher plate doesn't have to be that big. According to the designs, the plate wouldn't be wider than the ship itself, and the ship's diameter can be less than 50 meters. ScienceApe (talk) 20:25, 9 May 2010 (UTC)
- Well at least an equation tells us that we'd need infinite energy to go the speed of light, but I'm not so sure. I mean I bet all the "empty space" inside atoms will turn out not to be empty at all (maybe that will solve the dark matter speculation). Science is pretty primitive even now considering what it may be in even 100 years, never mind 1,000 or if/when humans have colonized other planets.--92.251.131.97 (talk) 16:29, 9 May 2010 (UTC)
- Matter not reaching the speed of light is more of a structural requirement of our current understanding of physics. It's not just an issue of taking too much energy. In order for the laws and the speed of light to be the same in all inertial frames, things just can't work such a way that matter can move like that. Rckrone (talk) 17:04, 9 May 2010 (UTC)
- And dismissing what is pretty solid science on the basis that there might be a magical workaround is, well, magical thinking. It's possible that Magiconium exists and somehow allows us to break the basic rules of special relativity, but there's absolutely no good reason to think so. --Mr.98 (talk) 18:45, 9 May 2010 (UTC)
- The equations of special relativity have been exceptionally well tested. When you sling material around in something like the Large Hadron Collider, you can see how it takes increasingly large amounts of energy to get asymptotically closer to lightspeed - precisely in accordance with that equation. There is no realistic possibility that the equation is wrong. Sad and annoying though it is - the speed of light is an exceptionally well researched "hard limit" that's not going to be exceeded by conventional means. We're down to "wormholes" and "warp drives" and other highly unlikely technologies that 'get around' the lightspeed limit...but those are all very dubious science and stink of wishful thinking rather than scientifically derived possibilities. SteveBaker (talk) 11:49, 10 May 2010 (UTC)
- Not saying you are wrong here Steve, but the same things were said about aircraft and the speed of sound through the 20s and early 40s. Aren't all scientific principals in theory only one experiment (ok, not counting verification experiments) away from being discarded? Googlemeister (talk) 13:34, 10 May 2010 (UTC)
- That was an entirely different matter. They already knew that (for example) a rifle bullet or the tip of a bull-whip could go faster than sound - the issue wasn't a fundamental one. The doubt was that an airplane could be controlled above the speed of sound. Also, they hadn't done any experiments to try this on a small scale. We've done precisely that. We've found that the amount of energy it takes to accelerate a bunch of electrons or protons from (say) 90% of the speed of light to 99% of the speed of light is less than it takes to get from 99% to 99.9% - which in turn is less than it takes to get from 99.9% to 99.99% and so on. The rate of increase exactly fits the equation. Also, the idea that an aircraft couldn't fly faster than sound was based on gut feel and untested ideas. The speed of light limit emerged from simple mathematics and solid experimental evidence - and every single subsequent experiment (and there have been an awful lot of them) has backed it up to the hilt. Nobody is saying that it's absolutely, utterly impossible that we're wrong about this - that would be unscientific - but what we can say is just how improbable it is that we're wrong given the vast pile of evidence we've amassed since the theory was put forward that precisely match the predictions it makes. It's hard to express a measure of 'believability' here...um...I would say (for example) that it is more likely that the Simulation hypothesis is true than that Special relativity is wrong. SteveBaker (talk) 22:54, 10 May 2010 (UTC)
- Not saying you are wrong here Steve, but the same things were said about aircraft and the speed of sound through the 20s and early 40s. Aren't all scientific principals in theory only one experiment (ok, not counting verification experiments) away from being discarded? Googlemeister (talk) 13:34, 10 May 2010 (UTC)
- The equations of special relativity have been exceptionally well tested. When you sling material around in something like the Large Hadron Collider, you can see how it takes increasingly large amounts of energy to get asymptotically closer to lightspeed - precisely in accordance with that equation. There is no realistic possibility that the equation is wrong. Sad and annoying though it is - the speed of light is an exceptionally well researched "hard limit" that's not going to be exceeded by conventional means. We're down to "wormholes" and "warp drives" and other highly unlikely technologies that 'get around' the lightspeed limit...but those are all very dubious science and stink of wishful thinking rather than scientifically derived possibilities. SteveBaker (talk) 11:49, 10 May 2010 (UTC)
- And dismissing what is pretty solid science on the basis that there might be a magical workaround is, well, magical thinking. It's possible that Magiconium exists and somehow allows us to break the basic rules of special relativity, but there's absolutely no good reason to think so. --Mr.98 (talk) 18:45, 9 May 2010 (UTC)
- Matter not reaching the speed of light is more of a structural requirement of our current understanding of physics. It's not just an issue of taking too much energy. In order for the laws and the speed of light to be the same in all inertial frames, things just can't work such a way that matter can move like that. Rckrone (talk) 17:04, 9 May 2010 (UTC)
- Well at least an equation tells us that we'd need infinite energy to go the speed of light, but I'm not so sure. I mean I bet all the "empty space" inside atoms will turn out not to be empty at all (maybe that will solve the dark matter speculation). Science is pretty primitive even now considering what it may be in even 100 years, never mind 1,000 or if/when humans have colonized other planets.--92.251.131.97 (talk) 16:29, 9 May 2010 (UTC)
- That's a great answer, Steve, but how would traveling at .1c get you to Proxima Centauri in 1000 years? Or did you mean nearest stars? — Ƶ§œš¹ [aɪm ˈfɹ̠ˤʷɛ̃ɾ̃ˡi] 19:02, 9 May 2010 (UTC)
- You don't just take off from earth, turn on the hyperdrives and instantly start going at 0.1c. The Orion would slowly gain speed up to 0.1c halfway through the trip - then have to turn around and slow down to zero by the time they get to their destination. Anyway, that number came from our Project Orion article...I suppose it might be wrong. SteveBaker (talk) 23:30, 9 May 2010 (UTC)
- Assuming uniform acceleration, the average speed is half the maximum speed. 4ly/0.05c=80 years. I'll go and check that article... --Tango (talk) 01:48, 10 May 2010 (UTC)
- Ah, I see what happened. You took the time from the original paper and the speed from the later studies - they were different versions of the design and had different speeds. --Tango (talk) 01:51, 10 May 2010 (UTC)
- I don't follow the logic behind Project Orion. The relativistic rocket equation is m ∂α/∂t = −β ∂m/∂t, where α is the ship's rapidity, m is its proper mass, β is the exhaust velocity, and t is proper time. Integrating gives mi / mf = eΔα / β. Plugging in Δα = 0.1 (10% c) and β = 30 km/s gives mi / mf ~ 10434, i.e., you need 10434 times as much fuel as payload to get to that speed, or 10868 if you want to slow down again at the other end. That's assuming a perfect cylindrical explosion and perfect elastic reflection off the reaction plate. The article's source for the claim of 0.1 c is "Cosmos" by Carl Sagan, which does say "Orion and Daedalus might travel at 10 percent the speed of light"—with no source or justification, of course. In context it's easy to believe that Sagan just made the number up. The other problem with traveling at 10% c is the particles of the interstellar medium striking you at a speed of 30,000 km/sec and a rate of maybe 10,000 per square meter per second. The whole thing seems to me quite ludicrous. -- BenRG (talk) 04:03, 10 May 2010 (UTC)
- Our article gives an exhaust velocity of up to 30,000 km/s, not 30 km/s. --Tango (talk) 18:10, 10 May 2010 (UTC)
- I don't follow the logic behind Project Orion. The relativistic rocket equation is m ∂α/∂t = −β ∂m/∂t, where α is the ship's rapidity, m is its proper mass, β is the exhaust velocity, and t is proper time. Integrating gives mi / mf = eΔα / β. Plugging in Δα = 0.1 (10% c) and β = 30 km/s gives mi / mf ~ 10434, i.e., you need 10434 times as much fuel as payload to get to that speed, or 10868 if you want to slow down again at the other end. That's assuming a perfect cylindrical explosion and perfect elastic reflection off the reaction plate. The article's source for the claim of 0.1 c is "Cosmos" by Carl Sagan, which does say "Orion and Daedalus might travel at 10 percent the speed of light"—with no source or justification, of course. In context it's easy to believe that Sagan just made the number up. The other problem with traveling at 10% c is the particles of the interstellar medium striking you at a speed of 30,000 km/sec and a rate of maybe 10,000 per square meter per second. The whole thing seems to me quite ludicrous. -- BenRG (talk) 04:03, 10 May 2010 (UTC)
- Ah, I see what happened. You took the time from the original paper and the speed from the later studies - they were different versions of the design and had different speeds. --Tango (talk) 01:51, 10 May 2010 (UTC)
- Assuming uniform acceleration, the average speed is half the maximum speed. 4ly/0.05c=80 years. I'll go and check that article... --Tango (talk) 01:48, 10 May 2010 (UTC)
- You don't just take off from earth, turn on the hyperdrives and instantly start going at 0.1c. The Orion would slowly gain speed up to 0.1c halfway through the trip - then have to turn around and slow down to zero by the time they get to their destination. Anyway, that number came from our Project Orion article...I suppose it might be wrong. SteveBaker (talk) 23:30, 9 May 2010 (UTC)
- That's a great answer, Steve, but how would traveling at .1c get you to Proxima Centauri in 1000 years? Or did you mean nearest stars? — Ƶ§œš¹ [aɪm ˈfɹ̠ˤʷɛ̃ɾ̃ˡi] 19:02, 9 May 2010 (UTC)
- You need to be careful with speeds in space - you need to specify what they are relative to. I think your Apollo 10 speed is relative to the Earth and your New Horizon's speed is relative to the Sun, so they aren't directly comparable. --Tango (talk) 21:31, 9 May 2010 (UTC)
- Again, I read the numbers from their respective articles. You can check them for yourself. SteveBaker (talk) 23:30, 9 May 2010 (UTC)
- I'm not saying the numbers are wrong, just meaningless without specifying the reference frame. --Tango (talk) 01:48, 10 May 2010 (UTC)
- I understand perfectly what you're saying - and I agree with you 100% - I'm just asking that you don't shoot the messenger! I'm merely repeating what the respective articles say. Unless the Guinness Book of Records sets a standard for what speeds have to be relative to...all bets are off. The Earth orbits around the sun at 67,000 mph - which is more than the numbers specified for any of those spacecraft - so it's hardly an insignificant matter! FWIW, I'm pretty sure the Apollo 10 numbers are relative to the Earth where the New Horizons and Voyager numbers are relative to the Sun - but I can't tell for sure. SteveBaker (talk) 11:49, 10 May 2010 (UTC)
- I'm not saying the numbers are wrong, just meaningless without specifying the reference frame. --Tango (talk) 01:48, 10 May 2010 (UTC)
- Again, I read the numbers from their respective articles. You can check them for yourself. SteveBaker (talk) 23:30, 9 May 2010 (UTC)
- You need to be careful with speeds in space - you need to specify what they are relative to. I think your Apollo 10 speed is relative to the Earth and your New Horizon's speed is relative to the Sun, so they aren't directly comparable. --Tango (talk) 21:31, 9 May 2010 (UTC)
I think that new Stephen Hawking Into The Universe with Stephen Hawking show, where he goes on about how it may be possible for us to travel forwards in time, mentions the fastest spacecraft created. You can watch it on Youtube if you haven't seen it already, or are interested. There are three episodes. I think he mentions how fast the Voyagers are speeding out of the solar system. An awesome show, I think.--Brianann MacAmhlaidh (talk) 08:38, 10 May 2010 (UTC)
- I know - I watched it on TV a few nights ago and winced when they said that. The Voyager probes have been the fastest man made spacecraft for almost 30 years and are very famous for that fact - so it's written up that way in an awful lot of books and web sites. The New Horizons spacecraft only beat their record fairly recently after some slingshot manouver or other and it will soon slow down again, leaving the two Voyagers as the 'current' fastest once more. Both Voyagers are also slowing down of course - they are still influenced by the sun's gravity, albeit feebly. It's perfectly possible that Hawking's show is out of date (when was it written?) - or simple inadequately fact-checked at the time. SteveBaker (talk) 11:36, 10 May 2010 (UTC)
What does crab use to dig a hole ?
I'd like to know what crab uses to dig a hole on a beach or muddy area. Thanks. —Preceding unsigned comment added by Srkim793 (talk • contribs) 18:29, 9 May 2010 (UTC)
- I would imagine they all use the same method as this, even if their digging into river banks etc.Ghost Crab digging a hole Fort Desoto Beach, FL--Aspro (talk) 19:09, 9 May 2010 (UTC)
Invention of high blood pressure medication
I am trying to find when the first high blood pressure or hypertension medication was first developed, who developed it, was it meant to be used for this disease or was it found to be better for another. I found who invented the blood pressure test, but for life of me can't find anything on the medication. Thank you so much —Preceding unsigned comment added by 166.183.201.189 (talk) 18:43, 9 May 2010 (UTC)
- Do you mean modern synthetic pharmaceutical drugs? Herbalists have used plants that achived the same effect long before the start of 'recorded' history; so that date is not available.--Aspro (talk) 19:17, 9 May 2010 (UTC)
- Hypertension has only been recognized as a disease since the 19th century, so I find Aspro's assertion that it's been treated intentionally since before recorded history to be unlikely. Drugs used to treat high blood pressure are known as Antihypertensive drugs, of which there are many types. There's no history on that page, which is unfortunate. The hypertension#history article only briefly mentions a history, giving a couple of the people who first recognized that it existed. Buddy431 (talk) 19:36, 9 May 2010 (UTC)
- Where did I state that they knew they where treating high blood pressure? Were the Ancient Egyptians able to explain the metallurgical science behind adding tin to copper, which enabled them to defend their empire? Or was it just obvious it was harder? Can bumble bees fly, although they are aerodynamically ill equiped to do so? --Aspro (talk) 20:08, 9 May 2010 (UTC)
- Back up your assertion then. Find a natural substance used for a long time that lowers high blood pressure. Buddy431 (talk) 20:18, 9 May 2010 (UTC)
- Reading both articles further, a major type of High Blood Pressure medication is ACE inhibitors. There is some History both on that page, as well as the more technical article ACE inhibitors drug design. The first article indicates that the first ones were discovered in the 1950s and 1960s, but it wasn't until 1981 that the U.S. Food and Drug Administration approved captopril for use. Buddy431 (talk) 19:42, 9 May 2010 (UTC)
- According to this article: "Until the late 1940s, treatment for hypertension had been largely limited to sedatives, nitrates, or the complete surgical ligation of the sympathetic nerves running alongside the spinal cord. Over the course of the ensuing decade, four entirely new classes of antihypertensive drugs emerged in a dizzying array of branded combination preparations. Though high levels of adverse effects at initial dosages limited their widespread use, by 1958 these antihypertensives had more or less displaced surgical treatment for acute hypertensive crises." The four classes Greene identifies are ganglionic blockers (the "earliest class of specific antihypertensives"), hydralazine ("thought to neutralize an unknown “pressor substance,” is still in use today at lower dosages"), rauwolfia compounds, and veratrum alkaloids. That's all pretty much Greek to me, but the article itself is about Diuril, which was initially a diuretic, but later found to be a good anti-hypertension drug, and is credited as radically changing the way hypertension is treated (e.g. treating chronic and symptomless cases rather than just acute ones). Greene also has a book out that talks a lot about this. --Mr.98 (talk) 19:47, 9 May 2010 (UTC)
- Here's another good, easily read (though unsourced) article about the history of Hypertension treatment: [22]. The site is all about high blood pressure, and looks reasonable (though I'm no medical professional). They say that Veratrum alkaloids were the first types of drugs, used as early as the 1930's, but that their effectiveness was limited (they were also quite toxic). Buddy431 (talk) 19:54, 9 May 2010 (UTC)
- Another thought. Just as different herbs would be given to treat different causes of high blood pressure, so different synthetic drugs would been developed to treat those different causes too. So, perhaps you need to defocus from the major symptom, to each of the underlying causes. Let me know of what's not clear and I'll rephrase it (we don't give medical advice so I'm playing-safe here) --Aspro (talk) 19:55, 9 May 2010 (UTC)
- Please back up your assertions. Hypertension's only been recognized recently, and people generally couldn't have treated a risk factor that they didn't know existed. While it's true that some high blood pressure drugs were developed from natural products (like ACE inhibitors from the venom of Bothrops jararaca), I can find no mention that these substances were used medicinally before the heavy research done starting in the 1950s. It appears that different substances were tested more or less at random in a guinea pig to find one that worked. Treating high blood pressure with straight up snake venom would have been very bad, and unless you can find a citation asserting so, I won't believe it. Buddy431 (talk) 20:16, 9 May 2010 (UTC)
- “unless you can find a citation asserting so, I won't believe it.” I'm not posting here to prove to you, or anyone else. OP 166.183.201.189 has asked a question, and a good one; and I am doing what I can, to guide them to the answer that their looking for. I am 'aware' that some countries have adopted teaching methods, where students end up demanding that they get spoon fed, like some, cuckoo chick in a nest. However, if they expect regurgitated factoids from me, without context, and without doing any of the slog themselves - then can take a running jump.--Aspro (talk) 21:16, 9 May 2010 (UTC)
- As soon as Google 'cardiaca' I get Motherwort, the next thing that comes to mind is kidneys, so what comes up - dandelions. Oh silly me, of course! The real reason why they recorded these things in these old books is that Dr Who left a copy of the BNF and some modern herbalists books!--Aspro (talk) 20:18, 9 May 2010 (UTC)
- I'll grant you motherwort: it appears to contain a mild vasodilator that can reduce blood pressure, and it was (and is) used for heart problems (among other things). You also make a good point about diuretics (what dandelion's were used for), in that they can be used to treat hypertension. However, most modern diuretics used to treat hypertension (like Thiazide) are actually effective in lowering blood pressure even at levels below which they increase urine production. I'm sorry I was so dismissive of you, but I'd really appreciate it if you could give examples, rather than make bold assertions without backing them up. Buddy431 (talk) 20:50, 9 May 2010 (UTC)
- As soon as Google 'cardiaca' I get Motherwort, the next thing that comes to mind is kidneys, so what comes up - dandelions. Oh silly me, of course! The real reason why they recorded these things in these old books is that Dr Who left a copy of the BNF and some modern herbalists books!--Aspro (talk) 20:18, 9 May 2010 (UTC)
- For those that find this discussion difficult to fathom (and this should not be a discussion page but a ....). A manufacture can only get pattern protection for something that has been invented. A patted treatment can be sold for thousands of times its cost to manufacture. Even after spending millions on drug development, it still makes more money than it would if it manufactured a generic drug. So what is a manufacturing company going to do ?
- As an aside: Charles Dickens died from an apoplectic fit from which he never recovered, (I only say this because I have read an original copy of the Times newspaper giving the details) . In those days, surgeons where the top rank of the medical profession but herbs were an anathema to them. --Aspro (talk) 20:43, 9 May 2010 (UTC)
- Actually, physicians, not surgeons, were still "the top rank" of the medical profession in 1870. Surgical training had only become integrated into medical school a few decades earlier. The first appendectomy in England had not yet been performed and Joseph Lister was early in his career. alteripse (talk) 00:27, 10 May 2010 (UTC)
- As an aside: Charles Dickens died from an apoplectic fit from which he never recovered, (I only say this because I have read an original copy of the Times newspaper giving the details) . In those days, surgeons where the top rank of the medical profession but herbs were an anathema to them. --Aspro (talk) 20:43, 9 May 2010 (UTC)
Using oil from an oil spill
Is it possible to use oil from an oil spill? I know it's mixed up with seawater and other gunk, but can't they use some chemical process to extract the oil and then refine that into petroleum products like gasoline? ScienceApe (talk) 20:19, 9 May 2010 (UTC)
- Yes. They refine it and sell it like normal. Ariel. (talk) 21:40, 9 May 2010 (UTC)
- When crude oil comes out the ground it is mixed up with salty water, grit and all sorts of stuff. It just costs so much more to recover and process it when it floats about on the ocean. The ancient Greeks however, found it very profitable to collect.--Aspro (talk) 21:41, 9 May 2010 (UTC)
- Oil-water emulsions are routinely collected and re-refined, mostly from the water used to wash the tanks on oil tankers (which is illegal to dump into the ocean, obviously, because of toxic hazards). The oil-water mixture first has to be desalted and dewatered to prevent corrosion problems downstream -- where I work, we use electrostatic desalters to get rid of the salt, and multi-plate oil separators to separate out the water (these would also get rid of the grit). Generally, oil collected from an oilslick in the ocean (or from washing tankers) has to be desalted/dewatered in a separate unit from the oil that comes from the well, because the former contains a lot more salt and water; once desalted and dewatered, though, it can be fed to the same atmospheric distillation unit as the oil from the well. So yeah, it's very much possible to collect and refine oil from an oil spill. 67.170.215.166 (talk) 08:37, 10 May 2010 (UTC)
Why don't our severed limbs/appendages just grow back?
Say if I was to lose a finger, or a hand, or part of my arm or something in an accident. Why doesn't the missing part just grow back eventually, considering that the flesh and bones of our bodies have the ability to repair themselves? --95.148.104.246 (talk) 20:33, 9 May 2010 (UTC)
- The body is limited in its ability to regenerate since that same ability is what leads to tumours. Finger tips often do grow back, but that's about it for humans. Some reptiles have more impressive regenerative abilities (I don't know if they have more cancer - I think they have shorter lifespans, which probably reduces the extent of the problem). There is some information on this topic here: Regeneration (biology). --Tango (talk) 21:38, 9 May 2010 (UTC)
- Evolutionarily that's not strictly true. While this is one factor, a more prominent one is that it would be so unlikely for you to lose a major body part in your everyday life, so if you had genes for regeneration, it wouldn't give you enough of an advantage over people who don't so that they would be perpetuated. If you do lose a limb, you would probably die before it could regenerate (without the miracles of modern medicine, that is), so you couldn't reproduce. So evolutionarily, the major genetic changes that would allow this have no incentive to occur. 22:49, 9 May 2010 (UTC) —Preceding unsigned comment added by 68.248.227.1 (talk)
- That's only true if such a trait were a "major genetic change." I think the question may be "since our cells were able to grow themselves into arms and legs once before, why can't they do so again?" I assume that there's some epigenetic explanation for why this ability is switched off after development is complete, but I don't know if that's true. — Sam 76.118.181.97 (talk) 23:05, 9 May 2010 (UTC)
- Yes, it's to do with stem cells differentiating into different tissue types. In mammals, that is a one-way process. --Tango (talk) 23:15, 9 May 2010 (UTC)
- I do not agree that fingertips grow back in humans. I have seen individuals who lost a fingertip and 50 years later it had not grown back even a little bit. Some non-mammals, like amphibians, can regenerate limbs. Edison (talk) 23:49, 9 May 2010 (UTC)
- I said often, not always. See the article I linked to above, it has a section on finger tips. --Tango (talk) 01:39, 10 May 2010 (UTC)
- I do not agree that fingertips grow back in humans. I have seen individuals who lost a fingertip and 50 years later it had not grown back even a little bit. Some non-mammals, like amphibians, can regenerate limbs. Edison (talk) 23:49, 9 May 2010 (UTC)
- Yes, it's to do with stem cells differentiating into different tissue types. In mammals, that is a one-way process. --Tango (talk) 23:15, 9 May 2010 (UTC)
- Humans notably can regenerate their livers from only a part of it. Buddy431 (talk) 00:21, 10 May 2010 (UTC)
- It's my understanding that humans actually have the ability to naturally regenerate limbs but this ability is lost as we age. A fetus that loses a limb can regrow it. Children under five years who lose fingertips can regrow them without trouble. After this, tissue regeneration only takes place over small distances (about 1 cm). There are ways being developed that use this natural ability to regenerate tissue to grow new organs. this video and this video talk about applying this in medicine. — Ƶ§œš¹ [aɪm ˈfɹ̠ˤʷɛ̃ɾ̃ˡi] 01:10, 10 May 2010 (UTC)
- Conversely however, I scratched my cheek just after I was born and it never healed even slightly! 86.7.19.159 (talk) 21:57, 10 May 2010 (UTC)
- It's my understanding that humans actually have the ability to naturally regenerate limbs but this ability is lost as we age. A fetus that loses a limb can regrow it. Children under five years who lose fingertips can regrow them without trouble. After this, tissue regeneration only takes place over small distances (about 1 cm). There are ways being developed that use this natural ability to regenerate tissue to grow new organs. this video and this video talk about applying this in medicine. — Ƶ§œš¹ [aɪm ˈfɹ̠ˤʷɛ̃ɾ̃ˡi] 01:10, 10 May 2010 (UTC)
- That's only true if such a trait were a "major genetic change." I think the question may be "since our cells were able to grow themselves into arms and legs once before, why can't they do so again?" I assume that there's some epigenetic explanation for why this ability is switched off after development is complete, but I don't know if that's true. — Sam 76.118.181.97 (talk) 23:05, 9 May 2010 (UTC)
- Evolutionarily that's not strictly true. While this is one factor, a more prominent one is that it would be so unlikely for you to lose a major body part in your everyday life, so if you had genes for regeneration, it wouldn't give you enough of an advantage over people who don't so that they would be perpetuated. If you do lose a limb, you would probably die before it could regenerate (without the miracles of modern medicine, that is), so you couldn't reproduce. So evolutionarily, the major genetic changes that would allow this have no incentive to occur. 22:49, 9 May 2010 (UTC) —Preceding unsigned comment added by 68.248.227.1 (talk)
We can regenerate organs. We just have to find the correct combination of cellular signals to the turn the system back "on". It's quite about evolutionary incentives. Our liver cells are under constant daily attack from oxidants and all sorts of toxins we eat. So naturally they have been selected to regenerate quite often. John Riemann Soong (talk) 03:22, 10 May 2010 (UTC)
- The primary error is in your premise -- you ask why we cannot regenerate if we can repair. They are too entirely separate things, although obviously somewhat related. It's sort of like asking "I don't understand why we can't fly if we can walk!" DRosenbach (Talk | Contribs) 14:27, 10 May 2010 (UTC)
Are vacuums in Europe more powerful than in North America?
In North America the practical limit to a household motor is 12-13 amps, i.e. 1300-1500 watts. But in Europe the voltage is higher, plus they have high amp ring circuits. So do they make the vacuums with more powerful motors? Ariel. (talk) 22:12, 9 May 2010 (UTC)
My vacuum is a Henry. A very popular brand in the UK. It has a 1200W motor and plenty of suction. I don't see why anyone would need any more. Theresa Knott | token threats 22:15, 9 May 2010 (UTC)
- Things like kettles are faster boiling in Europe. My kettle's 3,100 watts. With vacuums there is little point in higher power. --Aspro (talk) 22:29, 9 May 2010 (UTC)
- Wouldn't a higher power vaccum be harder to push? 68.248.227.1 (talk) 22:39, 9 May 2010 (UTC)
- I don't live in Europe but of course a substanial portion of the world uses 220-240V outside of Japan, Taiwan, most parts of the Americas (some of which do use 220-240V as well) and a few other various places File:Weltkarte der Netzspannungen und Netzfrequenzen.svg and NZ isn't one of those places.
- Anyway my vacuum which is this (I think) [23] is rated 2,300W. However it has a powerhead and at a guess 100-200W at least would be for that when in use. It also has adjustable power. It got good reviews from the NZ Consumer's Institute at the time I think (I didn't read them but the person who purchased it did I believe) which was about 4 years ago, I think the powerhead is good for pet hairs and the like, as well as especially dirty carpets (probably good since I don't vacuum as much as I should). See also [24].
- Note that that also says "Vacuum cleaners are often promoted on the basis that more watts equals better performance. This is not true. Our tests show no relationship between performance and rated watts." [25].
- I can easily find 2000W vacuums [26] [27] [28] [29] [30] [31] [32] [33] [34] [35].
- Two of those are in the UK i.e. Europe, so the answer to this question is apparently some are. For the NZ ones, you can see the price of those with higher power ranges from cheap to expensive so although a primarily a gimmick, it appears many do it. And the answer to the wider but unasked question about places outside Europe with 220-240V is also some are.
- Nil Einne (talk) 08:19, 10 May 2010 (UTC)
Soot
Where does the soot come from in my gas fire? —Preceding unsigned comment added by 79.76.139.1 (talk) 22:24, 9 May 2010 (UTC)
- Impurities in the gas (or impurities that are picked up in the pipes or the mechanisms of the fire itself). If there is a significant amount of soot then something is wrong and you should call a gas engineer out. --Tango (talk) 22:34, 9 May 2010 (UTC)
- (Edit conflict)Soot is made of small particles of carbon that cool off before they have time to burn. Soot is the same as smoke, only smoke is in the air. When you burn anything with carbon in it, whatever is left that is black is carbon that has not burned. Hope this helps, --The High Fin Sperm Whale 22:37, 9 May 2010 (UTC)
- Presuming it is mostly Methane and so, it will have carbon atoms as part of its composition. The soot come from incomplete combustion of the gas.--Aspro (talk) 22:40, 9 May 2010 (UTC)
Specifically - if the gas is burning smokily and leaving soot - then there isn't enough oxygen present for complete combustion. Carbon burns in oxygen - if there is carbon left, then it couldn't find oxygen to combine with to make carbon dioxide. That's bad because it also means that there could easily be more carbon monoxide than there should be - and that's a fairly serious health risk. SteveBaker (talk) 23:22, 9 May 2010 (UTC)
- "Fairly serious health risk" means you could die in a matter of hours. Jc3s5h (talk) 23:27, 9 May 2010 (UTC)
- Depending on the amount of ventilation - yes, that's perfectly possible. But (for example) candle flames burn very smokily - and produce a lot of carbon monoxide too - but you don't generally have enough of them for that to be a problem. However, with the amount of fuel that a gas heater gets through - there is ample scope for problems. I think our OP should get his fire looked at by an expert - there is plenty of scope here for personal danger! SteveBaker (talk) 11:23, 10 May 2010 (UTC)
How do wheels on a cannon affect the force on the cannon ball?
Hi all,
I always thought that wheels on cannons were just the prevent the cannon from, say, ripping out ship floorboards, and that having them "robbed" the cannon ball of some of its energy, but my friend says that the cannon ball actually travels further when the cannon has wheels on it.
I have two arguments. The first is slightly less persuasive even to me: The cannon rolling back requires energy, which is taking energy away that could have gone into the ball. The answer to this is probably: if the cannon is bolted down, this energy probably just goes into changing the spin of the Earth in some minute way, or something.
My second argument is: the ball is propelled forward by expansion of the air between the ball and the back of the cannon, much like a spring pushing two things apart. If you put two marbles on either side of a compressed spring and let go, both would travel a little way. If you fixed one end down and just had one marble, the one marble would travel further when you let go. Right?
So... what's the real science? Thanks! Sam — 76.24.222.22 (talk) 22:55, 9 May 2010 (UTC)
- You're right that the ball would move faster if the cannon weren't allowed to move, assuming you didn't just destroy everything. Conservation of momentum requires that when the cannon ball moves forward, something else has to be moving back with the same amount of momentum, but if that object is the Earth rather than only the cannon it will get much less energy out of the interaction (since energy is proportional to velocity squared, while momentum is linear in velocity) and the ball will receive more of it. That's really the same thing that happens with the marbles, since the fixed end of the spring is also pushing against the Earth. Rckrone (talk) 23:14, 9 May 2010 (UTC)
- The primary reason for wheels on cannons is to enable the artillerymen to move them around; it's far simpler to limber a cannon than to take it all apart and carry the pieces around on waggons. Nyttend (talk) 01:40, 10 May 2010 (UTC)
- The tackle on a shipboard gun carriage from the age of sail is a simple form of recoil mechanism that prevents the firing shock from tearing the ship apart. It softens the opposite reaction by spreading the force in time - otherwise, the effect on the firing ship would be similar to the cannonball hitting the opposing ship. It also brings the muzzle of a gun inboard, allowing for reloading without having to haul the gun backwards to get access to the front end of a muzzle-loading cannon. Acroterion (talk) 01:48, 10 May 2010 (UTC)
Ok, so let's see if I get this right. Say the the cannon weighs 100 kg and the cannon ball weighs 1 kg.
- From conservation of momentum, we know that the cannon will travel at V backwards and the cannon ball will travel at 100V forward, right?
If the total energy of the explosion was 100 Joules, then
- cannon KE + cannon ball KE = 100
- {(1/2)100 * 1V^2} + {(1/2) * 100V^2} = 100
- 100 V^2 = 100
- V = 10
So the cannon ball travels forward at 10 * 100 = 1000 m/s? Is that right? And the units?
Now we bolt the cannon to the ship, so the whole thing weighs 1000kg.
- From conservation of momentum, we know that the cannon will travel at V' backwards and the cannon ball will travel at 1000V' forward, right?
If the total energy of the explosion was 100 Joules, then
- cannon KE + cannon ball KE = 100
- {(1/2)1000 * 1V'^2} + {(1/2) * 1000V'^2} = 100
- 1000 V'^2 = 100
- V' = 0.31
So the cannon ball travels forward at 0.31 * 1000 = 310 m/s?
Blah, that doesn't work. I just proved that the cannon ball goes slower when the cannon is attached the the ship. Where did I go wrong? — Sam 76.24.222.22 (talk) 03:40, 10 May 2010 (UTC)
- I didn't read the whole thing, but as a general rule, you don't get accurate results in ballistics by working with energy, mainly because it's too difficult to model how much of the energy gets dissipated. --Trovatore (talk) 03:44, 10 May 2010 (UTC)
- Perhaps your friend is getting it mixed up with a trebuchet, which does work better with wheels. This is because the counterweight moves back when the ball moves forward, so it results in the trebuchet moving forward more, and because the kinetic energy of the weight is more down and less backwards, causing it to throw the ball up more too. — DanielLC 05:21, 10 May 2010 (UTC)
- Without commenting on the math, a gun firing a 32-lb ball weighed about 6000 lb (with another 1000 lb or so for the carriage) with a muzzle velocity of something a little less than 2000 ft/sec (sorry about the units - no metric in those days). Acroterion (talk) 11:51, 10 May 2010 (UTC)
Pitot tube pressure/height
I was reading the article on pitot tubes and I saw that the difference between static and dynamic pressures is used to calculate the airspeed on an aircraft. What I wanted to know (because it's not explained in the article) is how exactly one finds the static and dynamic pressures are measured within the tube. Some sort of spring apparatus? --130.216.46.2 (talk) 23:48, 9 May 2010 (UTC)
- Pressure sensor. Ariel. (talk) 00:03, 10 May 2010 (UTC)
- The static pressure is measured by the static sensor. The whole process is better explained at Pitot-static system. Dismas|(talk) 00:41, 10 May 2010 (UTC)
- It is the dynamic pressure (or impact pressure) that is relevant to the indicated airspeed of an aircraft, not the difference between static and dynamic. For a low-speed aircraft, the difference between the stagnation pressure and static pressure is called dynamic pressure. At speeds faster than about 0.3 Mach the difference between stagnation and static pressures is called impact pressure. Static pressure is conveyed by a conduit to one side of a diaphragm (ie an aneroid capsule or bourdon tube) and stagnation pressure is conveyed (from the pitot tube) to the other side. The deflection of the aneroid or bourdon is proportional to the dynamic pressure. The deflection drives the airspeed pointer, and the face of the airspeed indicator is calibrated so that it displays indicated airspeed in units of knots, km/hr, miles per hour etc. See Airspeed indicator#Operation. Dolphin (t) 03:22, 10 May 2010 (UTC)
May 10
rate for humans
What is the maximum number of calories a human could burn an hour without setting themselves on fire? (by burning I mean through exercise and by setting themselves on fire I mean with a match like some crazy protester.) 71.100.0.29 (talk) 02:15, 10 May 2010 (UTC)
- I seem to recall that the highest scientifically measured metabolic rate was during tests of the Gossamer Condor, a human-powered aircraft. (Gossamer Condor and Albatross: A Case Study in Aircraft Design, AIAA, 1980). I vaguely recall numbers on the order of feeding the cyclist a 10,000-calorie-per-day diet, and working all those calories out of him by having him pedal at top efficiency for many hours each day. [36]. This was about the maximum; feeding the cyclist more sugar, or training him harder each day, did not effectively get any more energy out of him. This thesis, Human Powered Helicopter (1991, Naval Postgraduate School), has a nice plot on page 12, indicating maximum power output plotted vs. time that power can be sustained. It is feasible to sustain approximately 1.5 horsepower (or about 1000 calories-per-hour) for about one minute. It is feasible to sustain half that rate for a much longer period of time. After a few hours at ~500 calories-per-hour, though, a human's entire daily food intake would be used up; hence the research into intense super-nutrition, sugar-syrups, and so forth, for the human-powered flight. Per the OP's question, these metabolic rates are well below the autoignition temperature of a human, so through exercise alone, it is impossible for a human to ignite. Nimur (talk) 02:31, 10 May 2010 (UTC)
- I've personally burned 1000 calories in an hour running on a treadmill, if the numbers were accurate, and I was nowhere close to a top-level athlete. Note however that the calories the body burns do not equal the power output of the person -- well over half of it goes into waste heat. My understanding is that somebody like Lance Armstrong during a race will burn over 1500 calories per hour for at least a couple of hours. Looie496 (talk) 03:29, 10 May 2010 (UTC)
- I would not believe what the treadmill says unless you have an oxygen mask on. There is a factor of ten in unknowns which they are guessing. Ranulph Fiennes has been quoted as saying that racing in the artic you lose weight on 8000 calories a day but you cannot metabolise more than this. He quotes 15000 calories output but beware trying to match the performance of someone who cut off his own finger-tips... --BozMo talk 11:41, 10 May 2010 (UTC)
- What do you mean by 'There is a factor of ten in unknowns which they are guessing.'? What unknowns, and why a factor of ten? --22:07, 10 May 2010 (UTC)87.114.95.229 (talk) signed too late—Preceding unsigned comment added by 87.114.95.229 (talk) 22:06, 10 May 2010 (UTC)
- I would not believe what the treadmill says unless you have an oxygen mask on. There is a factor of ten in unknowns which they are guessing. Ranulph Fiennes has been quoted as saying that racing in the artic you lose weight on 8000 calories a day but you cannot metabolise more than this. He quotes 15000 calories output but beware trying to match the performance of someone who cut off his own finger-tips... --BozMo talk 11:41, 10 May 2010 (UTC)
Mystery Plant
Hello! I am hoping there are some botanists here who can shed some light on this plant for me. (pun not intended.) This plant has recently come into my possession, and the prior owner was just as clueless as I am. I am hoping to find out what species this plant is, or at least it's family, so I can best take care of it. Any help is much appreciated. (A few more images are available if needed.) Avicennasis @ 04:25, 10 May 2010 (UTC)
- It is definately a type of succulent plant, likely a member of the Crassulaceae family. My best guess is this is a Crassula, likely a Jade plant or some similar. You might want to search through various genera and species of the Crassulaceae family to find a good match, if the Jade plant isn't it... --Jayron32 04:41, 10 May 2010 (UTC)
- You might, in the absence of an answer here, like to try Flikr. Accounts are free and they have a group especially for unidentified plants (craftily called "What plant is that")[37]which I can confirm provides an excellent and quick service. Whatever it is it needs much more light than it is (or has been) receiving. It definitely looks etiolated. Caesar's Daddy (talk) 06:17, 10 May 2010 (UTC)
- This is some variety of Echeveria, possibly E. elegans. Here [38]is a photo of several varieties of which the one at front centre look like yours but is in a normal form and not drawn up from lack of light. Richard Avery (talk) 19:59, 10 May 2010 (UTC)
russia
|
This question inspired an article to be created or enhanced: |
why are many (not all) russia drunks? —Preceding unsigned comment added by Tom12350 (talk • contribs) 04:36, 10 May 2010 (UTC)
- [citation needed]. Do you have any evidence that Russians are more prone to alcholism than people of other cultures? There are stereotypes of Russians as such, but there's lots of bullshit stereotypes out there, many of which have zero basis in fact. Russians drink, but so don't people from other countries. --Jayron32 04:46, 10 May 2010 (UTC)
- Alcoholism in Russia happens to be a redirect to the "Russia" section of the Binge drinking article, and that section states that Russian binge drinking is basically on par with most other European countries. It doesn't specifically discuss alcoholism in Russia. Comet Tuttle (talk) 05:27, 10 May 2010 (UTC)
- The Russians seem to think alcoholism is a "national calamity" (Pravda, 2006) and a "national disaster" (RIA Novosti, 2010). This article says "Consuming on average 32 pints of pure alcohol per person per year, Russians are among the heaviest drinkers in the industrialized world." According to this academic commentary, "Alcohol, as a central component of life in Russia, has been commented on, by Russians and by travellers from other countries, since at least the tenth century AD." Fee fi fo fum, I smell the blood of a new article, but I don't have time today. Clarityfiend (talk) 06:04, 10 May 2010 (UTC)
- Aren't the Irish the biggest drunks of all? :-) 67.170.215.166 (talk) 08:43, 10 May 2010 (UTC)
- I discussed this in a conversation with one Irish and one Russian friend of mine. I think the conclusion was that Irish drink more, but Russians drink harder. Vimescarrot (talk) 09:45, 10 May 2010 (UTC)
- I'll drink to that. Cuddlyable3 (talk) 18:29, 10 May 2010 (UTC)
- I discussed this in a conversation with one Irish and one Russian friend of mine. I think the conclusion was that Irish drink more, but Russians drink harder. Vimescarrot (talk) 09:45, 10 May 2010 (UTC)
- Aren't the Irish the biggest drunks of all? :-) 67.170.215.166 (talk) 08:43, 10 May 2010 (UTC)
but why —Preceding unsigned comment added by Tom12350 (talk • contribs) 19:15, 10 May 2010 (UTC)
- My unsourced, inexpert take on it: Drinking has been deeply ingrained in Russian culture for centuries and therefore more or less accepted. In modern times, Russia, and the Soviet Union before it, were dreary places for the masses, so they drank as an escape. Clarityfiend (talk) 19:57, 10 May 2010 (UTC)
Where can I find paid gigs to be a guinea pig for a medical experiment within 150 miles of Manhattan, KS? (With room & board included?)
Now you see, I've heard about willing members of society becoming the subjects of medical experiments where a medical school tests prototype drugs and/or experimentally new medical procedures on any willing participant.
I think nowadays would be a good time to start becoming a medical test subject. However, it must be within reasonable distance (~150 miles) of Manhattan, KS, with local lodging also paid for by the medical school or whatever medical research lab is doing these experiments.
So could someone help me find these gigs? In whatever links you give, be sure it'll also give the amounts each test subject will be paid for each experiment (or time-period of these sets of experiments).
For those of you who are about to ask me to just Google it, I already did, and the results were not at all helpful.
--Let Us Update Wikipedia: Dusty Articles 06:39, 10 May 2010 (UTC)
- It seems highly unlikely you could support yourself with such a "career", most medical test subjects receive a very small honorarium for participating in the test; usually enough to cover travel expenses and maybe give you enough cash for a night on the town (say, $50-$100). Many will also pay for medical expenses should any complications arise. I can't think of a single lab that would cover room and board, nor would it likely be possible to participate in enough tests to live off of. Many tests may be mutually exclusive, and most require you to have a medical condition first, and already be under the treatment of a doctor. For example, if they were testing a radical new cancer treatment, it would do you no good if you don't have cancer. Also, some tests you participate in may preclude you from participating in other tests. There are some paid testing where they do take "anyone off the street" who volunteers for the test, but these are so unreliable, rare, and poorly paid that I can't imagine you supporting yourself on such a gig. --Jayron32 06:48, 10 May 2010 (UTC)
- There is the NASA bed rest study, where they do provide room and board, except you have to stay in a bed without getting up. If you are interested: https://bedreststudy.jsc.nasa.gov/ Ariel. (talk) 07:24, 10 May 2010 (UTC)
- I'm a unit assistant for a clinical trials unit at my University hospital. We board test subjects for a week, and offer taxis/rides into town. We pay them 300 dollars / wk and they get 15/dollars a day for meals via hospital vouchers. But yeah, there are conditions or predispositions we target. John Riemann Soong (talk) 07:25, 10 May 2010 (UTC)
- If you can stretch your travel radius to Wales and don't mind getting the sniffles, consider signing up at the Common Cold Research Centre. Cuddlyable3 (talk) 10:02, 10 May 2010 (UTC)
Flexing axe heads
I split a fair bit of wood (not commercial, just for home), often with a standard axe and a sledge hammer. The complete book of self sufficiency says one thing you should not do is hit the back of a stuck axehead with a sledge hammer, using it as a splitting wedge. The book says doing this will break the axe handle but I find the tempation to do so irresistable when the axe sticks in a tough log. And the book is right, it breaks the axe handle (not at once but over a few hours misuse), AFAICT not because the sledge hammer ever hits the wood but apparently because the heavy steel axe head deforms enough under the strike of an eight pound sledge hammer to damage the wooden handle in the middle of it (??is that possible). The bit of the handle inside the axe head pulverises, leaving the rest of the handle intact (so I can plane off and put the axe head back on a couple of inches lower. So what's the deflection mode of the axe head and can I do anything to stop the damage to the handle? This particular bad habit is a great time saver. What if a cut the handle so it had a top and bottom gap and was held by the sides? Or put in a piece of rubber or something? Any ideas? --BozMo talk 10:26, 10 May 2010 (UTC)
- As an aside, you can get splitting wedges (basically a wide axe-head without a shaft). If the axe gets stuck in the log-end, then tap the wedge into the wood in the crack you've made, free the axe, tap the wedge in a bit more to secure it, then whack it with the sledge hammer. CS Miller (talk) 10:45, 10 May 2010 (UTC)
- Thanks I have a splitting wedge but I need to get through logs in a matter of a few seconds and collecting a wedge from where it landed, putting it into a log, tapping with a smaller hammer, wriggling out the axe and then moving the wedge centrally and then hitting it with a big hammer takes ages compared to 50% one strike 50% two strike with an axe and hammer --BozMo talk 10:49, 10 May 2010 (UTC)
- Based on what I know about axes and hammers (which is not that much), the sledge puts the back side of the ax blade under compression, causing the hollow channel in the blade that holds the handle in place to flex ever so slightly in the direction of the blow, and to put a bending stress on the handle, which eventually cracks from material fatigue. But hey, I could be wrong about this, no? As far as what you can do about it, cutting the handle so that it's only held by the sides is a very bad idea -- it will just let the ax-head wobble and maybe lead to a major accident. One possible solution would be to get an ax with a handle made of some ductile material (not rubber, but some kind of hard plastic could work), or machining a broad groove in the handle where it passes through the ax-head and putting a sleeve of hard rubber on it, then putting the ax-head back on so that it rests on that sleeve. Another alternative would be an all-metal ax, like a tomahawk or something. FWiW 67.170.215.166 (talk) 10:57, 10 May 2010 (UTC)
- (ec) I don't think it's because the axe-head is deforming and breaking the handle - I think it's because of the inertia of the handle. When you hit the head, it moves very abruptly - the unyielding metal of the hammer and the head mean that when the fast-moving hammer hits the stationary head, the axehead has to accelerate from zero to something like half the speed of the hammer head in the very short amount of time over which the two can deform. The acceleration is likely to be hundreds of g's - which is then (more gradually) reversed as the axe is slowed down again by its passage through the wood that you're chopping and is again stationary. Meanwhile - that LONG axe-handle has to follow along. The end that's attached to the axe-head has to accelerate just as the head does - but the center of mass of the handle is roughly halfway along it - so the inertia of the handle acts a long way away - so it has lots of leverage - and the result will be all sorts of really nasty forces propagating along the handle...enough (evidently) to weaken, and eventually break it.
- What surprises me is that some sledge hammers also have long handles - how come the sledge-hammer's handle doesn't break for the same reasons?
- I understand that using a splitting wedge slows you down - but not as much as a broken axe handle. This job is simply going to take longer than you thought.
- As far as I know, the primary reason for not striking the back of an axe with the hammer is avoiding dangerous splinters. Never hit steel on steel. I think you might do well to invest into a heavy rubber mallet - that should be easier on your tools and easier on your eyes, and it can be used with your current work flow (although you may need a few more blows). --Stephan Schulz (talk) 11:50, 10 May 2010 (UTC)
The OP needs a Log splitter. Cuddlyable3 (talk) 18:27, 10 May 2010 (UTC)
- Curiously I did look into it but actually the better ones used by farmers are screw head ones which our article does not even mention, run off a tractor engine and they cost a couple of thousand dollars. For my 6 or 7 metre tonnes of logs a year thats an overkill. The cheaper hydraulic ones like in the WP article seem to be okay for dealing with small diameter plantation wood or a coppice but hopeless when you have big hardwood logs which comes from falling trees in an old garden, and the very big hydraulic ones are hopelessly expensive for light use. So axe it is and it looks like I will go with Steve's advice. All aerobic anyway. --BozMo talk 19:38, 10 May 2010 (UTC)
Another way to split wide logs is to aim your axe so that the near end of axe's blade lands at the near end of log. Then on your second swing, aim the axe so that the near end of the blade hits just after the crack you've made. Hopefully the two cracks will join and the axe will go all the way through. CS Miller (talk) 20:07, 10 May 2010 (UTC)
- You can get axes with toughened heads suitable for being whacked with a hammer. I'll have a sniff about and if I can find a link to such will post it here. DuncanHill (talk) 22:17, 10 May 2010 (UTC)
- OK, what you need is a splitting maul. The blade is shaped for efficient splitting (a felling axe has a different profile, and will be less effective for splitting). The poll is toughened, so it can be used as a sledge or should be OK to use a sledge on it. Try a good manufacturer such as Gränsfors Bruks [39]. DuncanHill (talk) 22:23, 10 May 2010 (UTC)
Calculating the velocity of a cannon ball and cannon
Hi all,
I was trying to work out the velocity that a cannon ball and cannon would have after the cannon is fired in my question above (How do wheels on a cannon affect the force on the cannon ball?), but I think I failed. Can someone help?
Suppose we have a 1kg cannon ball and a 100 kg cannon. Suppose the explosion of the gunpowder provided 100 Joules, and that this was transformed perfectly into the KE of the cannon and cannon ball. How fast would the cannon ball move?
Thanks a lot, — Sam 76.24.222.22 (talk) 11:39, 10 May 2010 (UTC)
Conservation of energy: 1/2*(1kg)*(vb)^2+1/2*(100kg)*(vc)^2=100J
Conservation of momentum: 1*vb+100*vc=0
vc=-vb/100
1/2*(1kg)*(vb)^2+1/2*(100kg)*(-vb/100)^2=100J
1/2*(1kg)*(vb)^2+1/2*(100kg)*(-vb)^2/100^2=100J
(1/2*(1kg)+1/2*(100kg)/100^2)*(vb)^2=100J
vb=sqrt(100J/(1/2*(1kg)+1/2*(100kg)/100^2))=14.0719509m/s
vc=-0.140719509m/s 157.193.175.207 (talk) 13:46, 10 May 2010 (UTC)
- edited to correct sign error 157.193.175.207 (talk) 13:48, 10 May 2010 (UTC)
- (ec) I won't do your homework for you, but the key to this problem is conservation of energy and momentum. Since you can probably assume that both quantities are initially zero, you know the final energy is 100 J, and the final momentum is zero. You can work from there to find the velocities. anonymous6494 13:51, 10 May 2010 (UTC)
Interesting. So it seems from my calculations that, while it certainly makes a difference if the cannon is big and heavy, it doesn't make a huge difference
Using a cannon weight of 10kg, we get vb=sqrt(100J/(1/2*(1kg)+1/2*(10kg)/10^2)) = 13.48 m/s
Using a cannon weight of 100,000kg, we get vb=sqrt(100J/(1/2*(1kg)+1/2*(100000kg)/100000^2))=14.14m/s
Is that right? So in answer to my earlier question, bolting the cannon directly to the ship, so that the weight of the cannon would be equal to the entire weight of the ship, would make the cannon balls travel slightly faster, but not a lot. So putting wheels on the cannons (for which there are a bunch of other reasons) doesn't rob very much energy from the cannon balls. Is that right? — Sam 63.138.152.189 (talk) 14:31, 10 May 2010 (UTC)
- You could just put blocks behind the wheels and move them when you want to reload and have the best of both worlds. Googlemeister (talk) 15:41, 10 May 2010 (UTC)
Solubility of nickel chromate
Does nickel chromate react with hydrochloric acid when it dissolves, or does it just dissolve? Another related question is: Is chromic or hydrochloric acid stronger? You only have to answer one of these. --Chemicalinterest (talk) 11:40, 10 May 2010 (UTC)
- Question 1) Depends on how you define "react" and "dissolve". If nickle and chromium ions cannot coexist in solution together, then it seems unlikely that the presence of excess hydronium or chloride would effect the situation. If you are trying to make an insoluble salt dissolve, then something sort of complexation is probably needed, much like how at VERY high pH's, soluble copper hydroxide complexes form which can cause Cu(OH)2 to redissolve as the Cu(OH)42- ion. The only lewis base in your proposed system would be the chloride ion, so I would suspect that, if it DOES dissolve, you are creating NiCl42-, which is a complex ion attested to in the Nickel(II) chloride article. For Question 2) Chromic acid is not a compound which readily exists either isolated or in water. Stable "chromic acid" generally only works as a mixture of chromate (or dichromate) salts and a strong acid such as hydrochloric or sulfuric; and the strength of these chromic acid solutions probably depends on the strength of the co-acid present. From the point of view of Arrhenius theory, there is no acid which can be "stronger" than HCl anyways; it dissociates extensively in water; since acid strength is a measure of % dissociation, no acid may be stronger than HCl, though several "tie" it in terms of acid strength. --Jayron32 13:48, 10 May 2010 (UTC)
- There is: No acid dissociates 100% in water. But does it react with HCl, i.e. to form Cl2, or does it just dissociate and dissolve? According to my anionic activity theory: Ni2+ is higher up on a standard reduction potential chart than H+. If chromic acid is stronger, than the Ni2+ will stay with the chromate, causing no reaction. If hydrochloric acid is stronger, the H+ ion will take the chromate, forming chromic acid. I know some people don't like my idea of ions in solution "sticking" together, but that is what my opinion of anions is. --Chemicalinterest (talk) 14:29, 10 May 2010 (UTC)
- (edit conflict with below) My Table of standard reduction potentials shows that Cl2 is a (slightly) better oxidizer than chromate (or dichromate, whatever), which, if we believe that, means that the Cl- will not be oxidized to Cl2. As to your first question specifically, look at Nickel Chromate, where it says it dissolves in an HCl solution, forming a "yellow solution". This is, as Jayron says, likely to be Ni(Cl)42-: see here. Finally, Chromic acid is weird (as Jayron points out), so it doesn't really make sense to talk about it in the sense of a normal acid. Usually it's used as a strong oxidizing agent, rather than as an acid. The article makes is unclear as to what species is actually in solution, (Chromium trioxide vs. H2CrO4), but it really doesn't matter. Buddy431 (talk) 15:21, 10 May 2010 (UTC)
- There is: No acid dissociates 100% in water. But does it react with HCl, i.e. to form Cl2, or does it just dissociate and dissolve? According to my anionic activity theory: Ni2+ is higher up on a standard reduction potential chart than H+. If chromic acid is stronger, than the Ni2+ will stay with the chromate, causing no reaction. If hydrochloric acid is stronger, the H+ ion will take the chromate, forming chromic acid. I know some people don't like my idea of ions in solution "sticking" together, but that is what my opinion of anions is. --Chemicalinterest (talk) 14:29, 10 May 2010 (UTC)
- Look, dude, you can't just invent your own acid-base theory. Science doesn't work like that. What inadequate hole in the existing theory are you trying to plug? Not the least of which is that your theory doesn't work, as it presupposes the existance of things which do not exist. You keep insisting that substances which exist in water simply do not; like a week ago when you kept trying to find discrete NaCl particles floating in water. Lets see if we can dispense with some of the multitude of misconceptions in the above proposal you have. Regarding HCl's dissociation in water: Insofar as there is no perfection in the universe, yes, nothing is 100%. However, insofar as we must use measuring devices to detect things, there is no way to measure the amount of discrete HCl particles in water, so it is as functionally close to 100% dissociated as we need it to be. One can make comparitive gas-phase measurements of acid strength between acids, but any acid which has a higher aqueous-phase pKa than hydronium is effectively 100% dissociated in water, so it is completely moot to decide on strength between, say, HCl and H2SO4. Compare the pKa of water (14) with the pKa of Hydrochloric Acid (-7). That's a difference of 21 pKa units, or a difference in equilibrium constant of 1021. That means that at a 1 molar concentration of HCl, we have something on the order of SQRT (6 x 1023/1021) or about 25 molecules of HCl per liter. There is absolutely no way that this is not as close to 100% dissociated as you need it to be. Secondly, the reduction potential chart has NOTHING TO DO WITH ACID-BASE STRENGTH. There's just no way that the oxidation/reduction relationship between hydrogen and nickel and chromate and chloride has anything to do with how they will react in an acid-base sense. You are completely ignoring things like coordination chemistry, complex ion formation, lewis theory, stuff like that, all well established and functioning parts of chemistry. You can't just have an opinion about anions "sticking" together, with no experimental proof, and then act like this is somehow a "valid" theory. You're just making shit up, and that isn't really what science is all about!!! --Jayron32 15:06, 10 May 2010 (UTC)
- (ec) Make sure you don't base any further predictions on a model that is your opinion which contradicts reality. Even in college, we make all sorts of approximations and "known to be not quite correct" explanations, but we know when we are "not correct". Things might work well in a few limited cases, so we are very careful not to apply those things outside of their correct realm. In normal (not many-molar concentration) solutions, strong electrolytes like HCl are so dissociated there's no reason to consider the few molecules that are bound. In a water solution of two strong acids, neither takes the H+...the H+ is solvated by the water (as H3O+) because water is a stronger base than the anion from any strong acid and there is like 55 molar concentration of water in glass of water (vs only a few molar at most concentration of the counter-anions). DMacks (talk) 15:08, 10 May 2010 (UTC)
- With react I mean a redox reaction. I can demonstrate the anionic activity series by analyzing the reaction of barium chloride and copper sulfate. Since sulfuric acid is a stronger acid than hydrochloric acid, the cation of a more reactive metal will take the stronger acid, forming barium sulfate. Sodium acetate is reacted with hydrochloric acid. Since sodium is the cation of a more reactive "metal" according to the standard electrode potential page, the hydrogen will bond with the acetate, forming acetic acid and sodium chloride. Calcium hydroxide and sodium carbonate react to form sodium hydroxide and calcium carbonate. Because carbonic acid is stronger acid than water, then calcium must be expected to bond with carbonate because it is higher up on the standard electrode potential chart (not the activity series). This anionic theory is just a helpful way to predict whether certain salts will react or not. It really concerns precipitates though, not reactions in which all four of the salts are soluble, the reactants and the products. --Chemicalinterest (talk) 19:37, 10 May 2010 (UTC)
- What I mean by sticking together is that, like in the reaction of barium chloride and copper sulfate, the barium kicks the chloride out and takes sulfate, leaving copper chloride. Essentially, the copper chloride exists since the Ba2+ and SO42- ions are out of solution. --Chemicalinterest (talk) 19:40, 10 May 2010 (UTC)
- The copper chloride doesn't exist. What exists is discrete copper ions and discrete chloride ions. You are confusing a notational convenience (like writing CuCl2 (aq)) with actual reality. Let's take your theory, and show where it doesn't work: If I mix Lead(II) acetate and sodium perchlorate, I would get absolutely no precipitate. This is despite the fact that a) acetic acid is weaker than perchloric acid and b) lead is higher up on the standard reduction potential table. Using either your "which acid is stronger" theory OR your "standard reduction potential" theory, that one doesn't fit. I can find any random number of mixtures which will not work; that you have found some random ones that coincidentally do means nothing. --Jayron32 20:16, 10 May 2010 (UTC)
- What I mean by sticking together is that, like in the reaction of barium chloride and copper sulfate, the barium kicks the chloride out and takes sulfate, leaving copper chloride. Essentially, the copper chloride exists since the Ba2+ and SO42- ions are out of solution. --Chemicalinterest (talk) 19:40, 10 May 2010 (UTC)
- With react I mean a redox reaction. I can demonstrate the anionic activity series by analyzing the reaction of barium chloride and copper sulfate. Since sulfuric acid is a stronger acid than hydrochloric acid, the cation of a more reactive metal will take the stronger acid, forming barium sulfate. Sodium acetate is reacted with hydrochloric acid. Since sodium is the cation of a more reactive "metal" according to the standard electrode potential page, the hydrogen will bond with the acetate, forming acetic acid and sodium chloride. Calcium hydroxide and sodium carbonate react to form sodium hydroxide and calcium carbonate. Because carbonic acid is stronger acid than water, then calcium must be expected to bond with carbonate because it is higher up on the standard electrode potential chart (not the activity series). This anionic theory is just a helpful way to predict whether certain salts will react or not. It really concerns precipitates though, not reactions in which all four of the salts are soluble, the reactants and the products. --Chemicalinterest (talk) 19:37, 10 May 2010 (UTC)
- OK, your example had several fallacies: None of these (lead(II) acetate, sodium perchlorate, sodium acetate, and lead(II) perchlorate) is insoluble. Second, sodium is higher up on the standard reduction potential than lead. So sodium would keep perchlorate. It doesn't really apply when all of the products are soluble though. As you said, compounds don't exist as discrete ions. --Chemicalinterest (talk) 00:29, 11 May 2010 (UTC)
- "I can demonstrate the anionic activity series by analyzing the reaction of barium chloride and copper sulfate. Since sulfuric acid is a stronger acid than hydrochloric acid". You're not going to get very far in validating your hypothesis if aren't even using correct data for your fundamental example: hydrochloric acid pKa=−8 vs sulfuric acid pKa=−3. DMacks (talk) 20:47, 10 May 2010 (UTC)
Here is a question: Do stronger acids displace weaker acids from weaker acid salts? Does hydrochloric acid displace acetic acid from sodium acetate? Does hydrochloric acid displace boric acid from sodium borate? Does sulfuric acid displace hydrofluoric acid from calcium fluoride? Does sulfuric acid displace hydrochloric acid from sodium chloride? If it does, then hydrochloric acid is weaker than sulfuric acid. --Chemicalinterest (talk) 00:29, 11 May 2010 (UTC)
- The answer to your first three examples is "yes": hydrochloric acid will create acetic, hydrofluoric, and boric acid from the appropriate salts. This is because those three are "Weak acids", and do exist as single species in water. However, the answer to your last question (hydrochloric and sulfuric acid in water) is NO. Both of those are "Strong acids" that will completely dissociate in water: there will not exist either HCl molecules, nor H2SO4 molecules, in the water. Instead, there will exist H3O+, Cl-, and HSO4-. I think your confused about "acid strength" and the table of standard reduction potentials. An acid-base reaction is not an oxidation-reduction reaction. The trouble is, many acids are also oxidizing agents: so called "oxidizing acids" (that should be an article. Edit: and now it is!). That is, the anion acts as an oxidizing agent stronger than the H+. These include nitric acid, chloric acid, Chromic acid, and to a lesser extent sulfuric acid, among others. While they may seem similar, the "strength" of an acid refers to it's ability to dissociate, not it's strength as an oxidizing agent. Many highly oxidizing acids are also strong acids: nitric, perchloric, and sulfuric, for example, so it's easy to confuse the two different properties. Buddy431 (talk) 04:27, 11 May 2010 (UTC)
- See Hydrogen chloride#Laboratory methods: they produced hydrogen chloride from sulfuric acid by its reaction with sodium chloride. (But can sulfuric acid be produced from hydrochloric acid?) --Chemicalinterest (talk) 10:49, 11 May 2010 (UTC)
- I see. What they're doing is taking dry sodium chloride (i.e. salt) and adding concentrated sulfuric acid to it. The HCl is then released as a gas (where it's usually called "hydrogen chloride" rather than "hydrochloric acid"). You couldn't do the same with sulfuric acid only because sulfuric acid isn't volatile: it won't bubble out as a gas. Additionally, sulfuric acid can be prepared as a nearly pure liquid, while hydrochloric acid, when a liquid, always exists as a solution. So if you tried adding even the most concentrated hydrochloric acid to a sulfate, you would just dissolve the sulfate and get an acidic solution. Buddy431 (talk) 14:55, 11 May 2010 (UTC)
- See Hydrogen chloride#Laboratory methods: they produced hydrogen chloride from sulfuric acid by its reaction with sodium chloride. (But can sulfuric acid be produced from hydrochloric acid?) --Chemicalinterest (talk) 10:49, 11 May 2010 (UTC)
I'm searching for the name of a possible "fallacy"
I've seen an article here on Wikipedia, but cannot remember the name. I've just searched the list of fallacies, but could not find it there. It is about a scenario when the situation is getting worse, nearly everyone knows about it and about possibilities to avoid it, but they don't do anything against it because that would lead to short-term loss of comfort or competitiveness... so they doom themselves on the long term. There was a name for this, and even scientific game theory -related explanations, if I remember correctly. --131.188.3.21 (talk) 12:10, 10 May 2010 (UTC)
- I think you may somewhat misremember the tragedy of the commons. The problem there is not a loss of comfort or competitiveness (albeit that might be a result of cooperative action), but that at any time the optimal individual behavior is to stress the commons as much as possible, even if that leads to a degradation that's bad for everyone. The two standard ways out of this are to privatize the commons (hard to do with oceans or the air, for example), or to enforce rational behavior from all participants via regulation with punishment. --Stephan Schulz (talk) 12:44, 10 May 2010 (UTC)
- Thanks, but I know this dilemma, and think this is not the one I'm looking for. This is about acting to max out one's own benefit and disregarding the common goals. What I'm searching for is when, for example, decision makers could solve a situation (or at least keep it from worsening) but refuse to act out of fear that the temporary loss of comfort might make them lose power or not win the next election. --131.188.3.20 (talk) 13:03, 10 May 2010 (UTC)
- Sounds a lot like Prisoners dilemma. Googlemeister (talk) 13:16, 10 May 2010 (UTC)
- Not at all. I'm sorry if that sentence was not clear enough, but by "This is about acting to max out one's own benefit and disregarding the common goals" I meant the example given above (tragedy of the commons) was about it, but that's not the one I'm looking for. --131.188.3.21 (talk) 13:30, 10 May 2010 (UTC)
- Sounds a lot like Prisoners dilemma. Googlemeister (talk) 13:16, 10 May 2010 (UTC)
- Thanks, but I know this dilemma, and think this is not the one I'm looking for. This is about acting to max out one's own benefit and disregarding the common goals. What I'm searching for is when, for example, decision makers could solve a situation (or at least keep it from worsening) but refuse to act out of fear that the temporary loss of comfort might make them lose power or not win the next election. --131.188.3.20 (talk) 13:03, 10 May 2010 (UTC)
- I think the kind of situation our OP is thinking about is like if you had an oil leak on your car. You can either spend $10 to top up the oil once a week - or $300 to fix the problem properly. On any given week, topping up the oil is easiest and cheapest - but over a year, you've spent $520 and a dozen visits to the store to buy oil and 52 x 10 minutes = 8 hours of your time dealing with it...when you could have spent $300 and 6 hours and fixed it properly. Sadly, I don't know of a convenient name for this behavior. It's very similar to the tragedy of the commons...but not exactly that. How about "short term thinking" - or "horizon thinking"? SteveBaker (talk) 15:20, 10 May 2010 (UTC)
- Drifting off topic, but I'm not sure those terms best describe your scenario, Steve. You might be well aware of the long term disadvantage of repeatedly topping up, but simply not have $300 available for the better solution, a sort of individual variation on the Poverty trap.
- Re the OP's question, Endowment effect isn't quite what is described but is perhaps related. 87.81.230.195 (talk) 15:53, 10 May 2010 (UTC)
- It might be that my memory is failing me, but I still think I've seen an article about it here on Wikipedia. I think the examples were more politically oriented, for example decision makers avoiding to do something just because they fear their ratings will drop, or that society itself became to value its comfort too much and let things get worse over time. --131.188.3.20 (talk) 16:13, 10 May 2010 (UTC)
- The situation you described reminds me of groupthink. Vranak (talk) 16:28, 10 May 2010 (UTC)
- Is there a name for the fallacy of believing that all fallacies have names? --Tagishsimon (talk) 16:33, 10 May 2010 (UTC)
- Or the fallacy that all forms of wrong-mindedness are indeed fallacies. Vranak (talk) 17:17, 10 May 2010 (UTC)
- I'm just searching for an article I've once seen. It might not even have "fallacy" in its name, because I already searched that list. --131.188.3.21 (talk) 16:50, 10 May 2010 (UTC)
- You are describing a situation like a Nash equilibrium in mathematical game theory. This is where any change in strategy by any player (competitor) will produce (short-term) losses. In such a situation, people tend to do nothing, even though this may be bad for all parties. Staecker (talk) 17:16, 10 May 2010 (UTC)
- I'm surprised we don't have an article on the monkey trap. The monkey puts its hand into a transparent jar to grab the bait, but can't withdraw its clenched fist from the jar; it would have to let go of the food in order to free itself, but refuses to do so. It "prefers" to remain trapped.--Shantavira|feed me 08:15, 11 May 2010 (UTC)
- I think you might be looking for dynamic inconsistency. Maedin\talk 11:36, 11 May 2010 (UTC)
Physical fitness, health, and running
I've tried looking online for advice on general physical health and fitness, but I find pretty much nothing that I could consider to be a reliable source - they're all either:
- People trying to sell something, or
- People who have learned everything from people trying to sell them something.
So I've turned to the Wikipedians for some fitness advice. I'm considering going running, twice a week (my current fitness regime consists of sitting in front of my computer every day). Now, most websites seem to recommend doing it more than that, but they mention doing it specifically to increase things like muscle development, endurance, etc. Thing is, I'm not interested in any of that - I just want to live a little longer (and maybe get some free endorphins); I want to be healthier for no more reason than to be healthier. A friend told me that, if that was the case, I may as well just walk the same distance instead.
So; is running twice a week enough to achieve anything? Is walking really just as good (and what's the difference)? And why is it so hard to find objective sources for this kind of info? Vimescarrot (talk) 12:47, 10 May 2010 (UTC)
- There is no way to predict what your optimal training regime should be. There are some important guidelines that you should stick to, though. If you are older than 35 years old and you have not been physically active since a very long time, then you should not start any intensive physical activity like running straight away. You should then start with walking and then very gradually build up your fitness over the course of several months by gradually increase walking pace and duration, before you start intensive physical training like running. Even then, it is advisable to check with your doctor before you switch to running.
- Even if you are younger than 35, it is still advisable to start with walking. This is because the frequency and duration of trainings are important. If you are not fit, there is no way you could run for half an hour, five times per week. But it is likely that you can start with brisk walking every other day for 30 minutes. As your fitness level improves, you can replace one of these brisk walking trainings by a jogging exercise. Some time later you can replace all the brisk walking excercises by jogging exercises. At that time you can also replace one of the jogging exercises with running.
- This gradual increase in intensity has the advantage that when exercising you get can interpret the feedback your body gives you better. If you were to start running straight away, then you may get out of breath after 2 minutes. You don't have yet developed any feeling to adjust your pace to the right level. Most people in this situation tend to run way too fast.
- Depending on your age, you may be able to get into a 5 times per week running routine within perhaps half a year or one year. So, the short term goal should actually be to get fit enough to be able to train intensively. I have been excercising at a five times per week half an hour fast running routine for many years now, and all I can say is that the results are very good. Count Iblis (talk) 14:21, 10 May 2010 (UTC)
- I agree with that advice. Walking for 20 minutes every evening is better than running for 40 minutes twice a week. Get a dog...they won't let you forget or duck out of your training schedule! SteveBaker (talk) 15:14, 10 May 2010 (UTC)
- If you want to improve your general fitness then you need to do something that gets your heart rate up. It doesn't need to be very strenuous, as long as it increases your heart rate a bit. If you don't do much exercise at all now then a brisk walk would probably do it. I think the usual advice is that you should do it for about 30 minutes three times a week as the minimum to be effective. If you want more detailed advice then you could join a gym - they will usually have trainers that will help you put together a training regime (or you can hire a freelance trainer for a session or two). --Tango (talk) 15:25, 10 May 2010 (UTC)
- If you want an exercise programme that wasn't designed to extract money from you, consider the Royal Canadian Air Force plans 5BX (for men) or XBX (for women), which were published in an inexpensive paperback by Penguin Books titled Physical Fitness from 1964. Note that, as our articles say, some of the exercises involved are now thought not to be ideal if performed unsupervised, but the programmes' general outline might be useful to you. 87.81.230.195 (talk) 15:44, 10 May 2010 (UTC)
- Actually, when we had this conversation, I meant walk once a day or something, because we had been talking about certain inconveniences Vimes has of running every day and there only really being the option to do it twice a week. --KägeTorä - (影虎) (TALK) 17:45, 10 May 2010 (UTC)
- Having fluctuated all my life between periods of intense physical activity and periods of torpor, I disagree about walking being better than running. Running even once a week is far more useful to your heart, lungs, energy level, and weight than any amount of walking (unless you walk up steep hills), especially if you can work up to covering five miles at a moderately brisk pace. Looie496 (talk) 22:24, 10 May 2010 (UTC)
Thanks for the responses, everyone. Vimescarrot (talk) 05:45, 11 May 2010 (UTC)
methane hydrate ignition
Would heating methane hydrates to 25C cause them to ignite or detonate if they are at 150 bara pressure and surrounded by water, or would they just melt? Googlemeister (talk) 15:28, 10 May 2010 (UTC)
- No. They can't ignite/detonate without a source of oxygen...and there isn't one because you're underwater. Since you're obviously thinking about the gulf oil spill - I should point out that the oil that's coming out of the well-head is up at 150C - so if heat was enough, it would already have exploded! They might melt with the heat of oil collected beneath the dome - but that doesn't seem to be happening. The water down there is extremely cold! SteveBaker (talk) 15:35, 10 May 2010 (UTC)
- Yeah, I was wondering why they couldn't rig their collector unit with electric heating or something since it looks like the methane hydrates are forming and blocking their pipe. I mean it's not like putting that in would be cheap, but its got to be cheaper then their clean up costs. Googlemeister (talk) 15:39, 10 May 2010 (UTC)
- I believe they considered pumping hot water into it to do that - but the problem is that the thing filled up so quickly that now it's bouyant! This thing is the size of a three storey house and it's made of steel and concrete...it takes a LOT of ice to make something that big and heavy (200 tons!) bouyant! SteveBaker (talk) 16:23, 10 May 2010 (UTC)
- The different in density between oil and water over 1.5 km height all the way up the pipe has got a lot of buoyancy... but they should have thought of that. --BozMo talk 17:22, 10 May 2010 (UTC)
- I'm sure they knew that - that's the reason the thing had to weigh 200 tons! But the clathrate buildup was evidently either not expected or not expected to be so serious. SteveBaker (talk) 22:39, 10 May 2010 (UTC)
- The different in density between oil and water over 1.5 km height all the way up the pipe has got a lot of buoyancy... but they should have thought of that. --BozMo talk 17:22, 10 May 2010 (UTC)
- I believe they considered pumping hot water into it to do that - but the problem is that the thing filled up so quickly that now it's bouyant! This thing is the size of a three storey house and it's made of steel and concrete...it takes a LOT of ice to make something that big and heavy (200 tons!) bouyant! SteveBaker (talk) 16:23, 10 May 2010 (UTC)
- Yeah, I was wondering why they couldn't rig their collector unit with electric heating or something since it looks like the methane hydrates are forming and blocking their pipe. I mean it's not like putting that in would be cheap, but its got to be cheaper then their clean up costs. Googlemeister (talk) 15:39, 10 May 2010 (UTC)
Evian bottle ring
whats the ring at the top after you take off the cap off made out of on Evian water bottles made of of ? it dosent feel like plastic it feels like acrylic or something. it gets like sticky and leaves a gross residue on your hands after its been open for a day or 2 or if it gets wet. just that ring thou not the whole bottle. heres a pic of a bottle. im taking about the part circled in red. (make sure you click to maximize the pic size)
http://img96.imageshack.us/img96/5116/water1i.jpg —Preceding unsigned comment added by Tom12350 (talk • contribs) 20:45, 10 May 2010 (UTC)
- It's just a part of the cap - when you get an unopened bottle of water, it's attached to the cap - as you unscrew the cap, you break the connection between ring and cap. This is done so that you can tell that the water bottle hasn't been tampered with. I don't understand why it would leave a residue or anything though. SteveBaker (talk) 22:34, 10 May 2010 (UTC)
- It's made of polypropylene. And water should not affect it at all. So I don't know what the residue you mention is. Ariel. (talk)
how do u know its made from that? the ring feels like a differnt material than the cap —Preceding unsigned comment added by Tom12350 (talk • contribs) 02:58, 11 May 2010 (UTC)
- It'll feel weaker and smoother different because it's not as thick and it doesn't have the cap's structure keeping it rigid, or the grooves for grip. If the cap was as smooth as the ring, you'd never be able to grip it to open it. Vimescarrot (talk) 05:47, 11 May 2010 (UTC)
- If you examine one, you'll see that it's moulded as an integral part of the cap, and you break the link between them when you open the bottle. It's therefore made from the same material as the cap. If it gets sticky and disgusting over time, you may like to think about whether you ever drink from the bottle directly, and leave a residue of your mouth on th ring? --Phil Holmes (talk) 09:06, 11 May 2010 (UTC)
Can Crusher
For my science class I have to build an aluminum can crusher. It has to crush cans consistently, into a thin piece of metal. It has to be based on a simple machine. I'm debating between dropping a piece of wood onto the can from a height, and using a pulley to keep it up until I need it to drop, and using a lever to crush it. Which one would work better? I'm planning to build it using wood, but if anyone has a better idea, I'd be open to it, and the same with another idea for the concept of the machine.
tl;dr version: What's the best way to build a can crushing machine?
RefDeskAnon (talk) 21:11, 10 May 2010 (UTC)
- Well, I would expect a lever would be faster to reset after you crush a can. Googlemeister (talk) 21:13, 10 May 2010 (UTC)
- Very true. RefDeskAnon (talk) 21:51, 10 May 2010 (UTC)
- "Which one would work better" depends on a jillion variables (weights, distances, acceptible cost, etc.). It's a science class, you say? Do the experiment! Pick an approach, build it an test it. Change it a little to see if it gets better or worse. Each test doesn't have to be the fully built machine, just the key parts. How high a drop does it take to crush? How well can you aim from that distance? How long a lever does it take to crush? How well can your materials tolerate that much force? etc. DMacks (talk) 21:15, 10 May 2010 (UTC)
- The problem is that the project is due pretty soon, and I still need to get the wood. But I hadn't thought of just doing the key parts. Thanks, both of you. Any more ideas would be appreciated. RefDeskAnon (talk) 21:51, 10 May 2010 (UTC)
- I just stomped the can flat with my boot, very fast, no special tools needed. Nowadays the recyclers don't care. Graeme Bartlett (talk) 22:02, 10 May 2010 (UTC)
- The problem is that the project is due pretty soon, and I still need to get the wood. But I hadn't thought of just doing the key parts. Thanks, both of you. Any more ideas would be appreciated. RefDeskAnon (talk) 21:51, 10 May 2010 (UTC)
- I actually have a can crusher - it bolts to the wall, has a hopper for the cans and a large vertical lever that pushes on a plunger to crush the can end-to-end. Of course the classy "science fair" way to do it would be to "pinch" them like this! SteveBaker (talk) 22:31, 10 May 2010 (UTC)
- How are you planning on building your lever? The strength of that could offer some creative options. Dropping the wood seems the easiest option but basically that's it, you drop the wood and hope it's heavy/fast enough to crush the can. —Preceding unsigned comment added by 87.114.95.229 (talk) 22:43, 10 May 2010 (UTC)
- Measuring the least amount of weight you need to reliably crush a can would be a part of the science in this. Do experiments with a variety of cans. Do some math - figure out the most weight you needed to crush each can so you know which kind are toughest. Then plot graphs of the amount by which the can gets shorter for a given amount of weight. That will allow you to decide the point at which adding more weight doesn't make much difference to how much the can gets crushed. Machines that my son and I built for science fairs that required dropping something in a controlled way benefitted from using a few feet of 2" or 3" PVC pipe (buy it from any DIY store). This guides the weight precisely onto the target and helps you to always drop it from the same height in a repeatable way that gets you more reliable data. Once you have the data - you can think how to build the machine. (How about a weight on the end of a pendulum arm that swings down and crushes a can laid horizontally in front of the bottom-most part of the swing? You could raise the weight by pulling on a lever mounted to the top of the pendulum.) SteveBaker (talk) 01:01, 11 May 2010 (UTC)
- In case you didn't already Google for "Can Crusher", you could look at this web site has a bunch of different can crushers for sale. (The one I have is this one). SteveBaker (talk) 01:04, 11 May 2010 (UTC)
- A lever will work a LOT better than dropping a weight. An average person with a lever can easily impart 1000 pounds of force on something. You are unlikely to get anywhere near that by dropping something. (You might have a bit of an impact force, but that will only dent the can, you need longer duration force to crush it totally.) Ariel. (talk) 03:52, 11 May 2010 (UTC)
- ... and avoid using soft wood for the part in contact with the can. If it has to be wood, use a hardwood, but metal would be better. Dbfirs 07:52, 11 May 2010 (UTC)
Do wormholes violate the 2nd 1st law of thermodynamics?
I know wormholes are theoretical, but lets assume they are real for the purposes of a thought experiment.
Lets take two mouths of a wormhole. Mouth A and Mouth B. Mouth B is stationary, but you accelerate Mouth A to 99% the speed of light. Now any object you send into Mouth B will come out of Mouth A and should now be moving at 99% the speed of light as well right? Unless Mouth A slows down as a result of accelerating the object, wouldn't this be a violation of the 2nd 1st law of thermodynamics since the object would have been accelerated without energy being conserved. ScienceApe (talk) 22:00, 10 May 2010 (UTC)
- Are you sure you're talking about the second law? You don't seem to mention entropy anywhere in your thought experiment, so I don't really see how the second law comes into play. --Trovatore (talk) 22:03, 10 May 2010 (UTC)
- Indeed, I think ScienceApe probably means the first law. The second law is also a problem with wormholes, though (they mess with causality, since one end can be time dilated relative to the other, allowing time travel). --Tango (talk) 22:05, 10 May 2010 (UTC)
- These are the kinds of reason why very few people think that wormholes are 'real'...and even if they are, what their properties might be. One rather likely possibility is that you can't actually move them...or perhaps one end cannot move with respect to the other (so they both have to move together). If those kinds of restrictions are imposed then there is there still a problem with either thermodynamics or time travel? SteveBaker (talk) 22:23, 10 May 2010 (UTC)
- That might fix it, I'm not sure how it would work, though. You can't say the ends can't move, since that would require everything in the universe to stay still since motion is relative. Not move relative to each other might be manageable, but I'm not sure how. Wormholes simply not lasting long enough for anything to pass through them is quite likely, if you can't get rid of them all together. --Tango (talk) 22:48, 10 May 2010 (UTC)
- I was thinking that you might not be able to move them...because there is no way to grab a hold of them - or that there is no way to apply forces to them maybe. Since they are "connected" it seems somewhat plausible that you might not be able to move one end without also moving the other. Dunno - I think it's vastly more likely that the either don't exist - or are too narrow to allow things to pass through them - or (as you say) that they have such a short life that it's irrelevent...I dunno...they just seem like so much wishful thinking with zero evidence behind them. SteveBaker (talk) 01:09, 11 May 2010 (UTC)
- That might fix it, I'm not sure how it would work, though. You can't say the ends can't move, since that would require everything in the universe to stay still since motion is relative. Not move relative to each other might be manageable, but I'm not sure how. Wormholes simply not lasting long enough for anything to pass through them is quite likely, if you can't get rid of them all together. --Tango (talk) 22:48, 10 May 2010 (UTC)
- These are the kinds of reason why very few people think that wormholes are 'real'...and even if they are, what their properties might be. One rather likely possibility is that you can't actually move them...or perhaps one end cannot move with respect to the other (so they both have to move together). If those kinds of restrictions are imposed then there is there still a problem with either thermodynamics or time travel? SteveBaker (talk) 22:23, 10 May 2010 (UTC)
- Indeed, I think ScienceApe probably means the first law. The second law is also a problem with wormholes, though (they mess with causality, since one end can be time dilated relative to the other, allowing time travel). --Tango (talk) 22:05, 10 May 2010 (UTC)
- I never understood why people (ScienceApe: you are not the only one) assume that if one end of the wormhole is moving and the other stationary the object sent inside would take on the properties of the exit hole. If the exit hole is moving, then the exit location would constantly change, but the exit velocity of the object traveling inside it wouldn't. Unless you assume the object is bouncing off the walls of the wormhole (like water in a tube), but then by traveling in it you are slowing down the wormhole. Also, a two dimensional wormhole entrance (like the opening of a pipe) has problems with conservation of momentum. You can cause an object to change direction, which violates that law. I think if a worm hole exists, it would have to be three dimensional - meaning you can enter it from any direction, and you leave in the same direction, with the same velocity you entered. Ariel. (talk) 01:38, 11 May 2010 (UTC)
- The equivalence principle implies that you can't put these sorts of restrictions on ends of a wormhole. Any object, including a wormhole mouth, can be manipulated by gravitational tugging, and there's no way for the wormhole to "compensate" for that. Given both ends of a wormhole, I think you can always create a closed causal loop by luring them along worldlines of different lengths. Pipe-like wormholes like the one in Star Trek: Deep Space 9 are unrealistic even by wormhole standards. I think some people are misled by illustrations like this into thinking that black holes are "flat". It's actually the accretion disc that's flat. -- BenRG (talk) 09:28, 11 May 2010 (UTC)
- I never understood why people (ScienceApe: you are not the only one) assume that if one end of the wormhole is moving and the other stationary the object sent inside would take on the properties of the exit hole. If the exit hole is moving, then the exit location would constantly change, but the exit velocity of the object traveling inside it wouldn't. Unless you assume the object is bouncing off the walls of the wormhole (like water in a tube), but then by traveling in it you are slowing down the wormhole. Also, a two dimensional wormhole entrance (like the opening of a pipe) has problems with conservation of momentum. You can cause an object to change direction, which violates that law. I think if a worm hole exists, it would have to be three dimensional - meaning you can enter it from any direction, and you leave in the same direction, with the same velocity you entered. Ariel. (talk) 01:38, 11 May 2010 (UTC)
Yes I meant 1st law, my bad. I'll change the topic name. ScienceApe (talk) 22:32, 10 May 2010 (UTC)
- Energy, as we classically understand it, is not globally conserved in general relativity. Instead a compound entity, the stress-energy-momentum pseudotensor is conserved. In essence this says that you can change the total energy in the universe if and only if you change the configuration of space-time as you do it. So, yes, if you accelerate matter through your wormhole then there must be a counter-reaction in the wormhole itself, presumably causing it to slow down. Dragons flight (talk) 01:26, 11 May 2010 (UTC)
ScienceApe, by changing what you've written previously you're making some of the responses to your question appear nonsensical. I've changed it so that your old version appears, crossed out, next to your new version - hope you don't mind - it just makes the contributors who responded look slightly less foolish. Vimescarrot (talk) 05:40, 11 May 2010 (UTC)
May 11
Minimum energy for brute force attack
In the article for Brute force attack it says
The so-called Von Neumann-Landauer Limit implied by the laws of physics sets a lower limit on the energy required to perform a computation of ln(2)kT per bit erased in a computation, where T is the temperature of the computing device in kelvins, k is the Boltzmann constant, and the natural logarithm of 2 is about 0.693
So how can the minimum energy required be 0.693? 0.693 is just a number. Shouldn't it has a unit of J? 139.130.1.226 (talk) 00:22, 11 May 2010 (UTC)
- The Boltzmann constant has units of J K−1. Looie496 (talk) 00:27, 11 May 2010 (UTC)
- Also, it is easy to see that this limit can be violated, so it is not a fundamental limit at all. Count Iblis (talk) 01:40, 11 May 2010 (UTC)
- That paper seems like a meaningless moving of the goalposts. If their bit reservoir is thermal then their bit-clearing protocol doesn't work. Whatever is forcing the reservoir into a non-thermal state ought to be treated as part of the system. To put it another way, a reservoir of N bits each with an independent probability p of being zero contains N (1 + p log p + (1−p) log (1−p)) bits of known value. Their "erasure" protocol swaps the memory bit with a known bit from the reservoir, which just foists the erasure off on whatever device is replenishing the known bits in the reservoir. (And if nothing is replenishing the bits then the reservoir might as well be treated as part of the demon's internal RAM.) The only way to get p ≠ ½ in a thermal state is to have an energy difference between 0 and 1, which takes us back to square one. Maybe I'm missing something. -- BenRG (talk) 08:42, 11 May 2010 (UTC)
- I'm not a mathy guy but it seems to me you are completely misreading the sentence. It's saying that "the natural logarithm of 2 is about .693". Which is true. It's not saying that the total energy required is .693; it's still defining all of the variables. The next sentence discusses the actual energy that this equation would imply (in joules and gigawatts and etc.). --Mr.98 (talk) 13:27, 11 May 2010 (UTC)
Help required with this code
Hey! Can anyone please explain me this matlab code for uniform quantization?
computer code
|
|---|
%quantize_uniform.m (Fig.4.1)
% gives boundary vector b, quantization level vector c,
% mean-square quantization error(MSQE)
clear, clf
%Gaussian probability density function of x
pdf='exp(-(x-m).^2/2/sigma^2)/sqrt(2*pi)/sigma';
%pdf='exp(-(x-m).^2/2/sigma^2)';
xf=inline(['x.*' pdf],'x','m','sigma');
f=inline(pdf,'x','m','sigma');
m=0; sigma=1; % Mean and variance of the random variable x
b0=-3; bN=3; % Given least/greatest value of the random variable x
for N=5:6 % the number of quantization intervals
delta=(bN-b0)/N; b=b0+[0:N]*delta;
msqe=0; %Mean-Square Quantization Error
for i=1:N %centroid of each interval
tmp1=quad(xf,b(i),b(i+1),0.01,[],m,sigma);
tmp2=quad(f,b(i),b(i+1),0.01,[],m,sigma);
tmp=tmp1/tmp2; c(i)=tmp;
x2f=inline(['(x-tmp).^2.*' pdf],'x','m','sigma','tmp');
msqe=msqe+quad(x2f,b(i),b(i+1),0.01,[],m,sigma,tmp);
end
b,c
x=b0+[0:1000]*(bN-b0)/1000; N1=N+1;
%ind0=find(x<b(1)); x(ind0)=b(1)*ones(size(ind0)); %left-most interval
%indN=find(x>b(N1)); x(indN)=b(N1)*ones(size(indN)); %right-most interval
y(find(x<b(1)))=c(1); y(find(x>=b(N1)))=c(N);
for i=1:N
y(find(b(i)<=x&x<b(i+1)))=c(i);
end
subplot(2,2,N-4), plot(x,y) %quantization graph
hold on, grid on
fx=feval(f,x,m,sigma); %probability density ftn
plot(x,fx,'r:')
axis([-3 3 -3 3])
msqe
end
|
Thanks in advance.--111.68.97.146 (talk) 04:11, 11 May 2010 (UTC)
- I've taken the liberty of formatting your source-code as MATLAB code and fixing some mis-leading indentation. This code runs two separate trials, with 5 and 6 quantization intervals, seeking to quantize a function. It appears that the quantization intervals are designed to be non-uniform width - but uniform in the number of inputs that get binned into that interval, based on an input probability distribution function. The code then demonstrates this by computing the quantization intervals (results are stored in the vector c), and applying it to quantize the input function, then plotting the results. Such quantization (with non-uniform intervals) makes optimum use of the number of bits stored, but at the expense of complexity and (deliberate) variable precision of each interval. The hard part was noting what "uniform" refers to. In this case, what is uniform is not the width of the interval, but the probability that a given input will bin into that interval. Nimur (talk) 10:35, 11 May 2010 (UTC)
Lasso Physics
Okay, so this is a homework question, but I've managed to get an answer myself that I wanted to confirm here. If I'm wrong, then hopefully you can identify where I made my error. The question goes as follows:
A thin loop of mass M and radius R is suspended from a string through a point on the trim of the hoop (the setup looks like a lariat trick/lasso). If the support is spun with a high angular velocity ω, the hoop will spin with its plane nearly horizontal and its center nearly on the axis of the support (the support here refers to what the string is attached to). The sring makes an angle α with the verticle.
- (a) Find approximately the small angle β between the plane of the hoop and the horizontal.
- (b) Find approximately the radius of the circle traced out by the center of mass around the vertical axis.
Here are the answers I found. If I'm wrong, let me know and I'll provide my reasoning so that you can perhaps let me know where I've made any incorrect assumptions. For (a), I got β = g/(Rω2), and for (b) I got r = √(Mgtanα/ω). 173.179.59.66 (talk) 07:21, 11 May 2010 (UTC)
- Can you clarify what you mean by "the support is spun". Do you mean the support is twisted like a drill? And the whole setup is rigid? Or do you mean spun like a lasso is spun - i.e. the loop makes a circle, and the string (support) traces out a cone shape? Ariel. (talk) 09:43, 11 May 2010 (UTC)
- The former, but the cone opens downward. 173.179.59.66 (talk) 16:08, 11 May 2010 (UTC)
Can a continent be subducted?
Is it possible for one continent to be subducted beneath another? As far as I can tell from Geology of India, India has slowed down a lot since colliding with Asia, but what will is likely to happen in the future? Will it simply stop eventually or end up disappearing altogether? I've checked plate tectonics, plate reconstruction and obduction but can't find any information. Continental collision looks to be the place to look but doesn't make it clear what the ultimate fates of the continents are. 131.111.30.21 (talk) 09:17, 11 May 2010 (UTC)
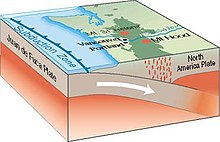
"Clean" subduction of an oceanic plate under a continental plate - because oceanic crust is much denser than the continent. - Usually, oceanic crust subducts, because it is denser than continental crust. In the case where two continental crust zones are colliding, as in India, the two plates have comparable density, so neither is "easily" forced down relative to the other. Eventually, given enough geological time, it seems plausible that some continental crust might be forced downward, but it would be a lot messier, with more deformation of both plates, than your typical picture of a nice, contiguous sheet as you see in the classic diagrams. In the short term, the deformation of the plates results in the Himalayan orogeny zone - more of an inelastic deformation of both plates than a clean subduction. Nimur (talk) 09:34, 11 May 2010 (UTC)
- Followup - this letter from the journal Nature, The possible subduction of continental material to depths greater than 200 km (2000), puts a little bit more quantitative spin on the problem. The continental crust has to overcome a density gradient, so it can only be forced downward if the collision gives enough energy and downward momentum to the continent to overcome its buoyant force. (The momentum-, energy-, and time-scales for these sort of inelastic collisions are all huge). This letter indicates some geochemical and mineralogical observations that might put some bounds on how deep continental crust can be forced down. They claim continental crust has been subducted to depths of "150 km", and up to 300 km using "indirect observations". This paper, The Himalayan Arc: large-scale continental subduction, oroclinal bending and back-arc spreading (1986), which I'm having a hard time finding a PDF of, might be of interest. They seem to suggest that paleomagnetic data indicate a rotation and subduction of the entire Indian subcontinent. This paper, Cenozoic Volcanism in Tibet: Evidence for a Transition from Oceanic to Continental Subduction, presents geochemical evidence for subduction. They present the idea that, in India, the only two ways to relieve the strain are continental subduction (India goes under Asia), or convective thinning (India "melts" from the bottom up, and its continental crust is slowly absorbed back into the asthenosphere). Their claim is that the geochemical evidence suggests subduction. Nimur (talk) 09:39, 11 May 2010 (UTC)
- Cheers for that, one reason I was asking was because I wondered if any continents have disappeared in the past - reconstructions of plate movements often look like they have big gaps in them making me wonder if other continents have been subducted, taking all their fossils with them. I guess we'll never be able to tell. Try this DOI link it gives me a pdf of the paper you couldn't find. Gotta love that someone modelled the collision with plasticene! 131.111.30.21 (talk) 10:25, 11 May 2010 (UTC)
- Followup - this letter from the journal Nature, The possible subduction of continental material to depths greater than 200 km (2000), puts a little bit more quantitative spin on the problem. The continental crust has to overcome a density gradient, so it can only be forced downward if the collision gives enough energy and downward momentum to the continent to overcome its buoyant force. (The momentum-, energy-, and time-scales for these sort of inelastic collisions are all huge). This letter indicates some geochemical and mineralogical observations that might put some bounds on how deep continental crust can be forced down. They claim continental crust has been subducted to depths of "150 km", and up to 300 km using "indirect observations". This paper, The Himalayan Arc: large-scale continental subduction, oroclinal bending and back-arc spreading (1986), which I'm having a hard time finding a PDF of, might be of interest. They seem to suggest that paleomagnetic data indicate a rotation and subduction of the entire Indian subcontinent. This paper, Cenozoic Volcanism in Tibet: Evidence for a Transition from Oceanic to Continental Subduction, presents geochemical evidence for subduction. They present the idea that, in India, the only two ways to relieve the strain are continental subduction (India goes under Asia), or convective thinning (India "melts" from the bottom up, and its continental crust is slowly absorbed back into the asthenosphere). Their claim is that the geochemical evidence suggests subduction. Nimur (talk) 09:39, 11 May 2010 (UTC)
I, Ant
I've been killing a lot of ants recently. A) Are ants certain to be too tiny-brained to have any sense of self? B) Would a modern computer be capable of mimicing an ant brain in real time? Although I expect the ant-brain circuitry has not been perfectly mapped yet. 78.146.87.143 (talk) 11:26, 11 May 2010 (UTC)
- List of animals by number of neurons says that ants have 10000 to 100000 neurons, certainaly a modern PC could do a fair job of simulating that --Digrpat (talk) 11:41, 11 May 2010 (UTC)
- Brains are funny things. So funny that there are guidelines as to what does and what does not deserve the moniker, and the central control of insect nervous systems are called ganglia as opposed to a brain for a reason. I don't have first hand knowledge of every organism type, but I believe that invertebrates as a group are said to have ganglia as opposed to brains -- this could very well be incorrect, though. It's likely that rules and regulations of what we term psychology do not traverse into the realm of invertebrates in any sense other than instinct and the like (except for some exceptions, such as high level cephalapods, etc.) DRosenbach (Talk | Contribs) 11:54, 11 May 2010 (UTC)
- It might be possible with the computational power we have (but keep in mind that a neuron is more complex than a node in an artificial neural network), but I've never heard of any research that has even started mapping out insect central nervous systems (I bet this is because it's impossible with current technology), and we understand so little about the way that brains work that simulating an ant is an impossible dream for now. Paul (Stansifer) 14:00, 11 May 2010 (UTC)
- List of animals by number of neurons says that ants have 10000 to 100000 neurons, certainaly a modern PC could do a fair job of simulating that --Digrpat (talk) 11:41, 11 May 2010 (UTC)
- Any animal that is physically capable of biting itself (as an ant is) needs to have enough self-recognition to prevent that from happening. Ants can do a lot more than that: they can distinguish ants belonging to their own colony from ants belonging to other colonies. The ant system for passing chemical messages is quite sophisticated. Their brains are small but they pack a lot of computation into that tiny space. Looie496 (talk) 14:56, 11 May 2010 (UTC)
- When we think of how to get a universal computer to do something like determine when not to bite something, or how to distinguish some detected creature from another, it often ends up being very hard to do. Now consider if you had to make a device that wasn't universal, but instead had only one task to do (like tell you if a room is light or dark). This sort of specialized device can exist in nature, too, so it's not always fair to compare a computer to anything that demonstrates some level of intelligence. Consider, as a more direct example, the recent work being done on calculations at the atomic level: this article is about how we can use an iodine molecule to perform a fairly sophisticated math equation in a very short amount of time. Are we going to replace a general purpose CPU in a computer with a single iodine molecule any time soon? Its utility, while amazing, is incredibly narrow. --Jmeden2000 (talk) 15:27, 11 May 2010 (UTC)
- As to A, it's really not possible to say without you yourself being an ant. They do not take kindly to harassment though, so I think that qualifies them for (very tiny and minor) personhood. Vranak (talk) 15:21, 11 May 2010 (UTC)
- One method used to see whether something has a sense of self is to show it a mirror - if it recognizes that this is a reflection then this is meant to be proof that the creature has a sense of self (rather than the more obvious proof that the creature has a sense of 'mirrors'). Do this with ants. If they can't recognize themselves, stomp away! :) --KägeTorä - (影虎) (TALK) 15:50, 11 May 2010 (UTC)
- That seems like a rather limited assessment. Apparently blind people have no sense of self, either! --Mr.98 (talk) 16:37, 11 May 2010 (UTC)
- The actual experiment that KageTora is referring to here is a lot more complex than that - but it's certainly flawed. Animals like Bats that "see" with sonar may well not recognize that they are even looking at a bat because it has the wrong three dimensional curvature, etc. Dogs are often the same - most (but not all) dogs are really unimpressed by their own reflection in a mirror...presumably because it doesn't smell anything like a dog. On the other hand, birds are completely taken in by mirrors - we had a Red Cardinal in our backyard a few years ago who evidently thought that his own reflection was another male impinging on his terratory - and spend weeks and weeks battering himself against the window trying to scare himself off. If the reaction to the mirror itself is so variable, it's hard to say that an ant that fails the test is somehow lacking a sense of "self" - when the lack of pheromones coming from the reflection mean that the reflection doesn't seem like an ant at all - self or otherwise. It would be like saying that humans are not self-aware because we cannot recognize the smell from a vial of our own sweat as being our own...a dog would find that test pretty convincing! SteveBaker (talk) 17:04, 11 May 2010 (UTC)
- Exactly the point I was trying to make - that the mirror experiment is designed from a very human perspective and cannot tell us much if anything about the existence or lack of a creature's sense of self. --KägeTorä - (影虎) (TALK) 17:22, 11 May 2010 (UTC)
- Just FYI, it would seem that the European Magpie (ref in article) is aware that the bird in the mirror is its own reflection. I'm not exactly sure if it's the same for some of the larger parrot species (e.g. macaws, African Greys) - but some of them do certainly seem to become aware with time that the reflection is not another bird - in a way that say, Budgerigars or Cockatiels do not. --Kurt Shaped Box (talk) 17:19, 11 May 2010 (UTC)
- The actual experiment that KageTora is referring to here is a lot more complex than that - but it's certainly flawed. Animals like Bats that "see" with sonar may well not recognize that they are even looking at a bat because it has the wrong three dimensional curvature, etc. Dogs are often the same - most (but not all) dogs are really unimpressed by their own reflection in a mirror...presumably because it doesn't smell anything like a dog. On the other hand, birds are completely taken in by mirrors - we had a Red Cardinal in our backyard a few years ago who evidently thought that his own reflection was another male impinging on his terratory - and spend weeks and weeks battering himself against the window trying to scare himself off. If the reaction to the mirror itself is so variable, it's hard to say that an ant that fails the test is somehow lacking a sense of "self" - when the lack of pheromones coming from the reflection mean that the reflection doesn't seem like an ant at all - self or otherwise. It would be like saying that humans are not self-aware because we cannot recognize the smell from a vial of our own sweat as being our own...a dog would find that test pretty convincing! SteveBaker (talk) 17:04, 11 May 2010 (UTC)
- That seems like a rather limited assessment. Apparently blind people have no sense of self, either! --Mr.98 (talk) 16:37, 11 May 2010 (UTC)
- One method used to see whether something has a sense of self is to show it a mirror - if it recognizes that this is a reflection then this is meant to be proof that the creature has a sense of self (rather than the more obvious proof that the creature has a sense of 'mirrors'). Do this with ants. If they can't recognize themselves, stomp away! :) --KägeTorä - (影虎) (TALK) 15:50, 11 May 2010 (UTC)
- E.O. Wilson's recent piece in the New Yorker, "Trailhead" explores, to some extent, the question of what ants "think" about. It's labeled as a work of fiction, appropriately, but given that Wilson is probably the world's foremost expert on ants, it is safe to assume that it is rooted pretty heavily in fact. His ant is basically a "programmed" creature—it does not think in a way that is analogous with humans (or mammals), though it is not "dumb". I don't know what a "sense of self" is supposed to mean in a scientific setting but I don't see much evidence for ants having one. They are instinctual automatons, albeit ones that can learn and do rather complicated things:.
- If the Trailhead Colony could not understand the history of its own species, how much did it understand of its current condition? How could it make the right decisions for survival? In fact, the Trailhead Colony knew a great deal. Worker ants are far more than automated specks running around on the ground. Even with a brain one-millionth the size of a human’s, an ant can learn a simple maze half as fast as a laboratory rat, and remember the directions to as many as five different destinations when she forages away from the nest. After exploring a new terrain, a worker can integrate all the seemingly haphazard twists and loops she made and, amazingly, return to the nest in a straight line. She can learn and recall the special odor of the colony to which she belongs. The Trailhead Colony, when all the learning and thought of its workers came together, was very smart, by insect standards—and, with the unifying power of its Queen lost and its population growth plummeting, it needed to call on that group intelligence to regain its balance.
- Which goes along with a lot of what Wilson has long said about ants—the colony, taken as a superorganism, is really the unit to be worried about, not the individual ant. --Mr.98 (talk) 16:37, 11 May 2010 (UTC)
- Better yet, consider the whole ant colony as a being. An individual ant is just a cell of it: drop a chemical that means "I'm dead" on one ant, and all the others will quickly carry it out to the "burial place/dump". It hurries back to the nest, and the whole process is repeated again and again, the carriers never realizing that the kicking and is not quite dead. I doubt that individual ant are capable of learning anything. I'm curious weather someone can give counterexamples. --131.188.3.20 (talk) 17:10, 11 May 2010 (UTC)
Fox's diet
Do foxes eat weasels?Jameslpeterson (talk) 11:28, 11 May 2010 (UTC)
- Weasels' main predators are raptors, but foxes would eat weasels. DRosenbach (Talk | Contribs) 11:56, 11 May 2010 (UTC)
Pitta bread and heat
Why is pitta bread so hot to the touch after coming out of the toaster? I'm assuming it has something to do with the air pocket inside it, but would like a proper explanation. Your regular slice of toast, for example, doesn't even get remotely near as hot. Hammer Raccoon (talk) 12:59, 11 May 2010 (UTC)
- The air pocket sounds reasonable. I'd also think the texture and density have a lot to do with it. Pita bread is denser than standard toast (thus carrying more energy at a given temperature), but perhaps more importantly, has a reasonably solid surface. When you pick up a piece of toast, most of the surface is actually exposed air pockets, which can quickly cool (and which make a good insulator anyway). Contrast with pita, where all you touch is hot bread. — Lomn 13:20, 11 May 2010 (UTC)
- Mostly agree with Lomn, but also consider that steam will get trapped inside those air pockets, while toast's open structure will allow more of the steam to escape. Same mechanism, it's just that steam holds an awful lot of heat. Matt Deres (talk) 13:31, 11 May 2010 (UTC)
In addition to more moisture in the pita. A pita is also much denser than a piece of white bread. the more holes the more heat dissipates quicker. The denser it is the more heat it retains.165.212.189.187 (talk) 14:24, 11 May 2010 (UTC)
- Before jumping to a conclusion about steam, I'd like to pose a question — is the pita hotter, or does it just seem hotter? In other words (and Lomn's implicitly response touched on this) is there a difference in the heat conductivity and heat transfer properties of pita bread versus regular toast? If the pita can transfer energy to your fingers more rapidly (due to greater density, differences in water content, more surface in contact with your skin, or what-have-you) it will feel hotter even if it is at the same surface temperature as the toast. Consider the perceived 'hotness' of the air in an oven with the perceived temperature of a metal rack inside the oven — similar temperature, but very different heat transfer properties.
- Incidentally, if you're curious about the effects of the air pocket inside, you can repeat the experiment with the pita sliced in half (across its diameter) so that the inner pocket is unsealed. TenOfAllTrades(talk) 14:27, 11 May 2010 (UTC)
Read our articles on specific heat and humours. I am not going to put two and two together for you, for fear of angering the locals, but if you reflect on what emotional personality a representative sample of a cross section of Americans are statistically likely to attribute to an anthropomorphic cartoon representation of pita bread and a piece of toast, respectively, and how this relates to the two articles, I think you will find yourself no nearer to, and perhaps even inexorably estranged from the answer you seek. 92.224.204.126 (talk) 14:34, 11 May 2010 (UTC)
- My experience is that when you pick up a toasted pita, it's hard to avoid squeezing it a bit, which causes steam to come out, and it is the steam that burns you if you aren't careful. Looie496 (talk) 14:59, 11 May 2010 (UTC)
The flat surface of pita transfers heat efficiently to your fingertips, especially since heat causes a little vegetable oil to migrate out to the surface. Vranak (talk) 15:19, 11 May 2010 (UTC)
Thanks for the responses everyone! Very informative. Hammer Raccoon (talk) 17:25, 11 May 2010 (UTC)
Giant King Grass
when i search for giant king grass, the article under wikipedia does not appear but a totally unrelated article. —Preceding unsigned comment added by 75.172.158.69 (talk) 13:25, 11 May 2010 (UTC)
:The help desk might be a better place to ask your question. --Chemicalinterest (talk) 13:34, 11 May 2010 (UTC
- Giant King Grass is a red link. Page 8 of this presentation says that China Giant King Grass is a hybrid between elephant grass and another grass. Miscanthus giganteus is also a hybrid between two species, but I'm not sure if it shares all the characteristics in the presentation about CGKG. If someone can find more information (there is none on google scholar) a redirect should be made to a relevant article (maybe just to Miscanthus if nothing else can be found. 131.111.30.21 (talk) 14:41, 11 May 2010 (UTC)
- This says that Giant King Grass is a trademark, that's why we won't be able to find out what actual species it is a hybrid of. Probably shouldn't have an article yet as most info looks like press releases but maybe a redirect to Miscanthus wouldn't go a miss. 131.111.30.21 (talk) 14:48, 11 May 2010 (UTC)
- Giant King Grass is a red link. Page 8 of this presentation says that China Giant King Grass is a hybrid between elephant grass and another grass. Miscanthus giganteus is also a hybrid between two species, but I'm not sure if it shares all the characteristics in the presentation about CGKG. If someone can find more information (there is none on google scholar) a redirect should be made to a relevant article (maybe just to Miscanthus if nothing else can be found. 131.111.30.21 (talk) 14:41, 11 May 2010 (UTC)
PoCl2 oxidation
This is purely out of curiosity. In the polonium dichloride article, it says that polonium dichloride reacts with nitric acid to form a dark red solution and a flaky white precipitate of unknown composition. This may be the reaction: 4 HNO3 + 6 PoCl2 → 2 H2O + 4 NO + 3 PoCl4 + 3 PoO2 Any suggestions whether it is or not? --Chemicalinterest (talk) 13:32, 11 May 2010 (UTC)
- The statement is referenced to a 1955 article in J. Chem Soc. Did you check out that article? It may have more info in the article. Furthermore, there are analytical techniques which are commonplace now which were unavailible in 1955. A simple literature search may turn up more recent studies on polonium halide salts where the nitric acid reaction has been more thoroughly analyzed. --Jayron32 16:35, 11 May 2010 (UTC)
- A quick google taught me that PoCl4 is a yellow solid that seems to be fairly water-soluble (or susceptible to hydrolyis) and eaily forms soluble complexes. The Po+2/Po+4 electrode potential is 1.1 V (0.72 V in HCl as an ion complex) and Po+4/Po+6 1.5 V. How does that fit with your proposal of nitric acid oxidizing Po+2 to Po+4? DMacks (talk) 17:01, 11 May 2010 (UTC)


