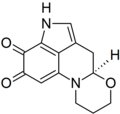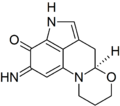Wikipedia talk:WikiProject Chemicals
| Chemicals NA‑class | |||||||
| |||||||
The discussion here concerns all parts of the Chemicals WikiProject, including the infoboxes, lists, standards, includes/excludes, tools, contributors, etc etc etc. Feel free to add your comments to any section here, or start a new topic. Older and closed discussions have been archived (2005, 2006, 2007, 2008, and 2009). Topics not specifically related to the Chemicals WikiProject would be better served at other wikipages.
Actual wikiproject info: statistics and alerts
| Article alerts |
|---|
|
Did you know
Articles for deletion
Redirects for discussion
Good article reassessments
Requested moves
Articles to be merged
Articles to be split
Articles for creation
|
| |||||||||||||||||||||||||||||||
The worklist shows the actual work to be done to achieve the goals of the Chemicals wikiproject. The choice of important compounds articles to work on has been finalized in an earlier stage of the wikiproject (around mid 2005), and no further articles are added, although we remain open for strong suggestions on this talkpage. The work these days focuses on improving the articles, from Chem Stub all the way to Chem A-Class articles. The table below shows that progress.
| Worklist historical status | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | ||||||
| Grade |
Jun | Oct | May | Oct | Mar | Oct | Feb | Aug | Apr | Dec |
| Template:Chem A-Class | 29 | 26 | 32 | 32 | 33 | 25 | 25 | 23 | 18 | 18 |
| Template:Chem B-Class | 71 | 84 | 101 | 130 | 148 | 156 | 158 | 180 | 185 | 188 |
| Template:Chem Start | 112 | 131 | 199 | 190 | 174 | 174 | 180 | 153 | 160 | 161 |
| Template:Chem Stub | 97 | 130 | 46 | 29 | 27 | 27 | 19 | 26 | 19 | 18 |
| unclassified | 76 | - | - | - | - | - | - | - | - | - |
| Total | 385 | 371 | 378 | 381 | 382 | 382 | 382 | 382 | 382 | 382 |
| percentage ≥Chem Start |
55.1 | 65.0 | 87.8 | 92.3 | 92.9 | 92.9 | 95.0 | 94.0 | 95.0 | 95.3 |
| weighted progress, % |
42.2 | 50.4 | 57.8 | 60.8 | 62.2 | 61.7 | 62.4 | 63.1 | 63.2 | 63.9 |
The percentage ≥ Chem Start was indicative of the initial effort. Now that we are progressing to more advanced progress, the weighted progress indicator is used, calculated as (Unclass*0 + Stub*1 + Start*2 + B-Class*3 + A-Class*4) / (Articles*4).
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
For the statistics for all chemicals, as registered by the bot, also see complete list
Moving actinoid
Apparently, only Lanthanoid was moved. Could an admin move Actinoid? Headbomb {talk / contribs / physics / books} 19:00, 17 March 2010 (UTC)
- Best wait for consensus - there are plenty of admins looking at this talk. Also it might be advisable once we have a good consensus to move protect the pages Ronhjones (Talk) 21:18, 17 March 2010 (UTC)
Phosphine vs phosphane
I am not sure why PH3 should be -ine when all the PR3 are already called -ines. Why is that? Nergaal (talk) 04:03, 12 February 2010 (UTC)
- This is similar to the discussion above about lanthanides/lanthanoids. Both phosphine and phosphane are IUPAC names, where 'phosphane' is the IUPAC recommended name. However, most people use 'phosphine' to describe PHxR3-x species. It is a matter of WP:COMMONNAME. I hope this explains. --Dirk Beetstra T C 07:07, 12 February 2010 (UTC)
- The discussion highlights a more difficult problem, i.e. that diphosphine refers to an obscure gas (P2H4_ and to a wildly popular class of ligands. I would guess that 99+% viewers of diphosphine really want to read about the organophosphorus ligands, not the gas. A similar problem with phosphine exists, see Phosphines#Phosphine ligands. We need to solve this glitch.--Smokefoot (talk) 00:48, 13 February 2010 (UTC)
- It would have been nicer, IMHO, if IUPAC had never put their boot into this one in the first place! Why should we say not say "phosphine", by analogy to "amine"? A quick search for "niccolate" on Google indicates that scholarly journals are not too bothered about this sort of obvious nomenclature change (it has been "nickelate" since 1976). A search on Google Scholar will find at least one member of the IUPAC Commission on Inorganic Nomenclature (as it was then) publishing papers entitled in contrary the Commission's recommendations. If the very people who write these rules cannot be bothered to enforce them in their own research groups, I feel that they have little or no value. As for "phosphane", it appears that the chemical community has spoken, at least for the time being. Physchim62 (talk) 15:58, 13 February 2010 (UTC)
- The discussion highlights a more difficult problem, i.e. that diphosphine refers to an obscure gas (P2H4_ and to a wildly popular class of ligands. I would guess that 99+% viewers of diphosphine really want to read about the organophosphorus ligands, not the gas. A similar problem with phosphine exists, see Phosphines#Phosphine ligands. We need to solve this glitch.--Smokefoot (talk) 00:48, 13 February 2010 (UTC)
- Physchim62 .. bad boy .. of course you are referring to 'azane' in analogy to 'phosphane' .. --Dirk Beetstra T C 13:44, 22 February 2010 (UTC)
- Beetstra, go on, just try ordering 100 ml of triethylazane and see what the stores manager says to you! I'll warn you, you might need to order a few hundred mils of a ≈5% v/v solution of ethyloxidane in oxidane to recover from his or her reaction! Physchim62 (talk) 14:10, 22 February 2010 (UTC)
- one that's been bugging me is iron(III) chloride
- anyone thoughts on this? —Preceding unsigned comment added by 170.170.59.133 (talk) 18:48, 10 March 2010 (UTC)
ENGVAR / solubility units.
I am opening a discussion here, since I think this needs a bit of discussion. 12.31.4.5 (talk · contribs) is changing two things on a couple of pages about chemicals, and with both I disagree:
- In the {{Chembox}} they changes 'colourless' to 'colorless'. In my opinion this goes against WP:ENGVAR, though they argues that all references and external links use the American English spelling.
- Also in the {{Chembox}}, they changes '0.015 g / 100 mL (20 °C)' to '0.015 g/100 g (20 °C)' (later to '0.015 g/100 g Water (20 °C)'). If I am correct, 0.015 g / 100 mL means that there is 15 mg of (in this case, ethylbenzene) dissolved in 100 mL total volume of solution, with the solvent being water. I presume here, that the reference that the value is taken from says it as '0.015 g / 100 mL (20 °C)', and hence that is what we report, but there is not a direct reference for this point of data). Though the density of this solution (of ethylbenzene) will be close to 1, that is not the same as 0.015 g / 100 g. They argues that it is 0.015 g ethylbenzene dissolved in 100 mL water, and hence, that 0.015 g / 100 mL is the same as 0.015 g / 100 g (ignoring the small deviation of the density of water, which is not '1.000' at 20 °C, but I can live with that small deviation).
The second point does brings a next question. IMHO, it would be good to have all values in the same unit. Solubility_table (as 12.31.4.5 argues) is in g / 100 g, and it would be nice to have all in the same unit (we might even consider to use an alternative parameter (e.g. Solubility_standard, like can be done with 'BoilingPt' -> 'BoilingPtC'), which would standardize our information a bit further (I did have a glance at WP:UF, though there is not a standard chemitry microformat ..).
Basically, we disagree on these points, but I think it is useful to discuss this in a wider community. Thanks. --Dirk Beetstra T C 08:32, 18 February 2010 (UTC)
- I've also crossed with some anon who claimed that g/100g is a standard on WP; I just reverted them because it is not - most WP articles are in g/100 mL. The solubility table is for water, and given the accuracy of those values, it is safe to change, IMHO, g/100g to g/100 mL, but. I was wondering why listing values in g/100 mL and not in g/L ? I don't see any problem with ENGVAR - the policy is quite clear on which spelling to keep. Materialscientist (talk) 08:46, 18 February 2010 (UTC)
- For 0.015 g / 100 g to 0.015 g / 100 mL, yes .. but if we are talking brine, I don't think that 26.4 g / 100g is 26.4 g / 100 mL there .. --Dirk Beetstra T C 08:50, 18 February 2010 (UTC)
GA reassessment of Vitamin C
I have conducted a reassessment of the above article as part of the GA Sweeps process. You are being notified as this project's banner is on the talk page. I have found some concerns which you can see at Talk:Vitamin C/GA1. I have placed the article on hold whilst these are fixed. Thanks. Jezhotwells (talk) 23:08, 19 February 2010 (UTC)
WP:ELEMENTS started creating books on each individual elements. Since there are a lot of them, any help would be very much appreciated. Headbomb {ταλκκοντριβς – WP Physics} 02:32, 28 February 2010 (UTC)
Hi guys
Can you guys do a sanity check of this image:
Before I put it up on the article? Thanks. --Rifleman 82 (talk) 13:40, 1 March 2010 (UTC)
- The addition of a Grignard reagent to a nitrile won't normally provide a methyl group. Either something is wrong in the image, or some very unusual chemistry is going on there. -- Ed (Edgar181) 14:44, 1 March 2010 (UTC)
Sorry, the paper (Hans Bock, Ilka Goebel, Zdenek Havlas, Siegfried Liedle, Heinz Oberhammer (1991). "Triisopropylamine: A Sterically Overcrowded Molecule with a Flattened NC3 Pyramid and a "p-Type" Nitrogen Electron Pair". Angew. Chem. Int. Ed. 30 (2): 187–190. doi:10.1002/anie.199101871.{{cite journal}}: CS1 maint: multiple names: authors list (link)) said CH3HgCl, not the Grignard. I suppose it was relatively unusual that I typed Mg in place of Hg. That aside, does it make sense? --Rifleman 82 (talk) 14:49, 1 March 2010 (UTC)
- Actually, now that I think about it a moment, the reaction will proceed through elimination of HCN, followed by addition of the methylmercury to the resulting imine. So, yes, this makes sense to me now. -- Ed (Edgar181) 14:59, 1 March 2010 (UTC)
- Ah, that's great. Thanks. I'm not familiar with mercury used this way. --Rifleman 82 (talk) 15:03, 1 March 2010 (UTC)
Hi,
Please, Excuse my english. In this article, it is question of 1,3,5-triaza-cyclohexane-2,4,6-trione (C6N3O3) which seems very strange. I already asked the French chemistry project here and for now, we think it should be 1,3,5-triazinane-2,4,6-trione C3H3N3O3, the tautomer of cyanuric acid which is still very different. The page 97 of the book referenced on the article isn't visible on googlebooks. We found about 1,3,5-triaza-cyclohexane-2,4,6-trione, the reference : Comments on inorganic chemistry, Volume 16, Number 6, 1994, page 104. One of you who have access to this journal in a good science library, could check what is the compound with this name and what is really his formula, please ? --tpa2067 (Allô...) 15:51, 4 March 2010 (UTC) —Preceding unsigned comment added by Tpa2067 (talk • contribs)
- If you type either of these names into ChemDraw, you get the same molecule, tautomer number 2 below (from cyanuric acid).
Ferrous sulfide = sulfanylideneiron?
PubChem lists the IUPAC name of ferrous sulfide as sulfanylideneiron. Is that correct? Almost nobody seems to use that term. But if it is correct we should definitely add it to our article. AxelBoldt (talk) 23:36, 12 March 2010 (UTC)
- No, it's PubChem being idiotic (again...) Sulfanylideneiron would be the Fe=S molecule, which is rather different chemical (and probably non-existent) from common-and-garden iron(II) sulfide. Physchim62 (talk) 09:05, 13 March 2010 (UTC)
- What about the vapour phase? I'm aware that in the solid phase, it is an ionic solid, but what is it's vapour phase does it convert to an covalent molecule--Plasmic Physics (talk) 07:16, 6 June 2010 (UTC)
There is some minor naming conflict around that article. Copper monosulfide argument is based on that copper is not simply in 2+ state there, as reflected in the article and its talk; however, this name is not popular on Google books, and copper (II) sulfide is more conventional there. An argument against monosulfide, expressed at my talk, is that prefixes, such as "mono-", should not be used to name ionic compounds. Thoughts? Materialscientist (talk) 04:22, 14 March 2010 (UTC)
- At times like this, we really miss editor User:Axiosaurus who was our chalcogen expert. If one look at the structure of covelite, it is mixed valence at the sulfur part as it contains both isolated S and S2 centers, which most reasonable people (i.e. those who agree with me) would assign as S2- and S22-. Hence the Cu's have two oxidation states. Copper sulfides are described as highly covalent in contrast to what the
idiot,other editor asserts.--Smokefoot (talk) 05:12, 14 March 2010 (UTC) - Maybe copper(I,II) sulfide in parallel with iron(II,III) oxide? Is the IUPAC name simply copper sulfide as the chembox states. Vsmith (talk) 14:00, 14 March 2010 (UTC)
- Yes, in principle copper sulfide refers unambiguously to the compound with formula CuS in stoichiometric nomenclature: obviously, in practice, it is better to provide a bit more information as "copper sulfide" can also refer to the group of CuxSy compounds, of which there are many. Iron(II,III) oxide should really be iron(II) diiron(III) oxide, or iron(2+) diiron(3+) tetra[oxide(2−)] (spinel type) if you want to be really pedantic. Physchim62 (talk) 23:47, 14 March 2010 (UTC)
Copper(I,II) sulfide isn't an ideal name because (according to the article), all the coppers are Cu(I) and the sulfurs are not all sulfides (i.e. S2−).
Copper(I,II) sulfide implies (Cu+)2(Cu2+)(S2−)2, i.e. Cu3S2.
Ben (talk) 15:57, 14 March 2010 (UTC)
- Just going on the structure, it looks like (Cu+)2(Cu2+)(S2−
2)(S2−), or copper(I,II) disulfide sulfide, but I'll bet there's a lot less actual charge separation than that. After all, since when did one see a trigonal planar Cu2+ ion? And shouldn't the sulfide ion be sufficiently reducing to reduce copper(II) to copper(I) in any case? I have no problems in using the stoichiometric name (copper monosulfide) to title the article: if we needed an alternative, we could use copper sulfide (covellite), although I don't think we have any other articles which are disambiguated in that manner. Physchim62 (talk) 17:13, 14 March 2010 (UTC)
- That covellite disamb. works for me (mineralogy background), but not likely supportable from usage by WP:RS. So, how is the compound named in those RS's? I find the IUPAC Name: sulfanylidenecopper [1] a bit much and seldom used (only 46 google hits). Vsmith (talk) 23:10, 14 March 2010 (UTC)
- Sulfanylidenecopper would be wrong, unless we happened to be talking about the Cu=S molecule in the gas phase! We do actually have some support from IUPAC for using the mineral name as a disambiguation: see International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 237. Electronic version., which allows the use of mineral names to define structural types. CAS uses the stoichiometric formula for disambiguation [2], although we would run into problems with displaying titles if we adopted that approach. Physchim62 (talk) 23:47, 14 March 2010 (UTC)
- That covellite disamb. works for me (mineralogy background), but not likely supportable from usage by WP:RS. So, how is the compound named in those RS's? I find the IUPAC Name: sulfanylidenecopper [1] a bit much and seldom used (only 46 google hits). Vsmith (talk) 23:10, 14 March 2010 (UTC)
Web of science hits:
- "Copper monosulfide" 3
- "Copper(II) sulfide" 23
- "Copper sulfide" 871
Looking through the last set, it refers almost equally to either CuS or Cu2S, suggesting that there is no specific name for CuS. Perhaps this is another area where the project can decide the WP convention. Materialscientist (talk) 23:36, 14 March 2010 (UTC)
- Well, I vote for copper monosulfide. Copper sulfide currently is a fleshed-out disambiguation article that discusses the range of compositions for CuSx, including cuprous phases (thanks to Axiosaurus). Alternatively we could rename copper sulfide diambig article as copper sulfides, and then assign copper sulfide to what we currently call copper monosulfide.--Smokefoot (talk) 00:27, 15 March 2010 (UTC)
- Technically, moving articles is not a problem - we can have Copper sulfide and Copper sulfide (disambiguation), as conventional for other topics on WP. Materialscientist (talk) 00:32, 15 March 2010 (UTC)
- On the other hand, we tend not to use names which are ambiguous between two chemical compounds except as the title for an article about a class of compounds (such as copper sulfide is at the moment). Copper monosulfide is unambiguous. The argument that you shouldn't use "mono-" for ionic compounds is completely back-to-front; you shouldn't use Stock notation (with the oxidation number in brackets) unless the compound is substantially ionic (so no manganese(VII) oxide). So "copper(II) sulfide" is wrong on two counts: it's the wrong oxidation state and it implies ionic bonding when the compound is substantially covalent. The downside with "copper monosulfide" as the article title is that we are just about the only people using that name. Physchim62 (talk) 02:13, 15 March 2010 (UTC)
- Technically, moving articles is not a problem - we can have Copper sulfide and Copper sulfide (disambiguation), as conventional for other topics on WP. Materialscientist (talk) 00:32, 15 March 2010 (UTC)
Oxiranes as Subcategory in epoxide
Just noticed that a separate cathegory exists for Category:Oxiranes which is a subcategory in Category:Epoxides. Any naming nuance I must have missed? If not, I put all oxirane-articles in the epoxide category... L.tak 19:19, 5 April 2010 (UTC)
- The two are synonymous. I agree that it would be best to move articles from Category:Oxiranes to Category:Epoxides. -- Ed (Edgar181) 20:42, 5 April 2010 (UTC)
- done, made a soft direct on Category:Oxiranes... L.tak 21:44, 5 April 2010 (UTC) —Preceding unsigned comment added by L.tak (talk • contribs)
Missing chemistry topics
I've updated my list of missing chemistry topics - Skysmith (talk) 13:24, 8 April 2010 (UTC)
2-Aminopurine structure
I discovered a small mistake in the 2-aminopurine structure, the hydrogen on the N7 should be on the N9 and the double bond should be between the C8 and the N7 instead of between the C8 and N9. I am not sure if this is the right place to report this kind of errors but I just registered to wiki and I have no clue how to change this structure myself
Kountraya 18:17, 9 April 2010 (UTC) —Preceding unsigned comment added by Kountraya (talk • contribs)
- It's a matter of convention rather than chemistry where you place the nitrogen, so it's not a serious (i.e., chemically wrong) error. I'm trying to find the recommendations for purine structures to see if we're following them: I'll report back! Physchim62 (talk) 18:28, 9 April 2010 (UTC)
- The "standard" tautomer of purine is 7H, see Panico, R.; Powell, W. H.; Richer, J. C., eds. (1993). A Guide to IUPAC Nomenclature of Organic Compounds. IUPAC/Blackwell Science. p. 168. ISBN 0-632-03488-2. Physchim62 (talk) 18:42, 9 April 2010 (UTC)
Thank you for your quick answer, I guess I have been mislead by the fact that the aminopurine used as a nuctleotide analogue is always bound to the sugar by its N9. Kountraya 19:30, 9 April 2010 (UTC) —Preceding unsigned comment added by Kountraya (talk • contribs)
classification of nitrogen heterocycles
I think category:nitrogen heterocycles needs to be kind of reformed. It's kind of disorganised. Right now I'm in the process of further breaking it down along the lines of aromatic, aliphatic (it's possible to have a compound which has both), simple or polycyclic, etc. with further information on whether there's a nitrogen that's imine-like, enamine-like, basic sp2, basic sp3, etc. I think this would be a little more informative. John Riemann Soong (talk) 19:22, 21 April 2010 (UTC)
- You might consider upgrading heterocyclic chemistry before the category business. IMHO, your implied talents would have greater impact focusing on this important and needful article. At present, it is a pretty good list, but lacks an overviews on construction approaches, patterns of reactivity, and occurrence. --Smokefoot (talk) 23:39, 21 April 2010 (UTC)
- I would recommend comparing with the categorization of heterocyclic compounds on Commons, which seems to be better organized. -- Ed (Edgar181) 17:18, 28 April 2010 (UTC)
Structure request
I was wondering if someone might be able to draw a structure for me. The chemical is haematopodin, a pigment found in the mushroom Mycena haematopus (currently at FAC). The structure may be found here, p. 603. Thanks in advance, Sasata (talk) 17:02, 28 April 2010 (UTC)
-
Haematopodin
-
Haematopodin B
Rifleman 82 (talk) 17:15, 28 April 2010 (UTC)
- Wow! That was fast... thanks so much! Sasata (talk) 17:20, 28 April 2010 (UTC)
- Can the pyrrole-nitrogen H be moved closer to the N? Looks like it's floating off a bit. But actually, this is just the easily-dectected compound...the "actual" primary pigment is haematopodin B, which has an NH imine instead of carbonyl at C7 (per quinoline ring-numbering)--see doi:10.1002/ejoc.200700739. It's all an interesting bit of chemical detective-work on those two. Rifleman, could you draw the other also, so we can have comparable structures of both for comparison there? We don't seem to have articles about anything directly related. DMacks (talk) 17:52, 28 April 2010 (UTC)
- Wow! That was fast... thanks so much! Sasata (talk) 17:20, 28 April 2010 (UTC)
About the floating hydrogen - I've looked through the settings and I can't figure out what to tweak. If you are using chemsketch perhaps you can show me? --Rifleman 82 (talk) 01:14, 29 April 2010 (UTC)
- I may have ChemSketch at work...will try tomorrow. I usually use ChemDraw, which puts the H in a less odd position. But when I use (what I think are) the WP:CHEM recommended settings, the atom labels look out-of-proportion small compared to the bonds and overall structure. See for example File:Ethylenetetracarboxylic_acid_dehydrations.png and File:Ullmann p-diphenoxybenzene.png. Would be great if WP could host actual template files rather than listing a pile of values to enter in a ton of preference panes in each editor program. DMacks (talk) 01:30, 29 April 2010 (UTC)
I found it simpler to rely on ACS settings. Just have to export them at high res? --Rifleman 82 (talk) 01:45, 29 April 2010 (UTC)
I just wanted to remind that we have the page Wikipedia:WikiProject Chemistry/Image Request. Unfortunately, there is however not much traffic… --Leyo 08:06, 29 April 2010 (UTC)
- I used the images and some of the info from Mycena haematopus to start Haematopodin and Haematopodin B. The articles are now stubs, and are in need of some more info on them. --Dirk Beetstra T C 12:20, 29 April 2010 (UTC)
List of commonly available chemicals
I think that brief ways to produce these chemicals should be listed along with the properties in the list of commonly available chemicals. --Chemicalinterest (talk) 18:50, 5 May 2010 (UTC)
- While well intentioned, the creation of list of commonly available chemicals is ill-advised. The topic is basically original research (who is to decide what is "common"?). What next, uncommon chemicals? --Smokefoot (talk) 22:28, 5 May 2010 (UTC)
Have you seen common chemicals? --Rifleman 82 (talk) 02:19, 6 May 2010 (UTC)
A common chemical is one that you do not have to buy from a chemical supply store or can be manufactured at home, such as chlorine(electrolysis of brine with carbon electrodes[platinum is not needed, carbon is available in Leclanche cells]), sodium sulfate(reaction of lye with battery acid), or calcium oxide (extreme heating of clamshell). --Chemicalinterest (talk) 10:37, 6 May 2010 (UTC)
- Hmm, still close to original research, a lot, if not everything, can be made at home, it may be a lot of work to construct a steroid, but starting from acetic acid, calcium carbide, sodium chloride, water, and maybe even sugar, one can get everywhere. --Dirk Beetstra T C 10:54, 6 May 2010 (UTC)
- Both common chemicals and list of commonly available chemicals are silly and appear to violate the guidelines. "Wikipedia is not a place to publish your own thoughts and analyses or to publish new information." The work is also a form of guidebook, also discouraged: "Wikipedia is not a directory of everything that exists or has existed" WP:NOTDIRECTORY, WP:NOTCATALOG, WP:NOTYELLOW. "The Wikipedia is not a manual, guidebook, textbook, or scientific journal" seeWP:NOTGUIDE etc. Oh well, I might think the effort is ill-advised, but it's just disk space and makes people feel productive.--Smokefoot (talk) 12:23, 6 May 2010 (UTC)
- If the article was so bad, why wasn't the nomination for deletion favored? --Chemicalinterest (talk) 11:43, 18 May 2010 (UTC)
- Both common chemicals and list of commonly available chemicals are silly and appear to violate the guidelines. "Wikipedia is not a place to publish your own thoughts and analyses or to publish new information." The work is also a form of guidebook, also discouraged: "Wikipedia is not a directory of everything that exists or has existed" WP:NOTDIRECTORY, WP:NOTCATALOG, WP:NOTYELLOW. "The Wikipedia is not a manual, guidebook, textbook, or scientific journal" seeWP:NOTGUIDE etc. Oh well, I might think the effort is ill-advised, but it's just disk space and makes people feel productive.--Smokefoot (talk) 12:23, 6 May 2010 (UTC)
Requested move
At Talk:Aluminium borohydride there is a request to move aluminium borohydride to aluminum borohydride, if anyone wishes to comment. ChemNerd (talk) 17:20, 7 May 2010 (UTC)
- Discussion closed.--Chemicalinterest (talk) 21:52, 19 May 2010 (UTC)
New articles
I'm no chemist, but the long name version of that chemical doesn't seem to follow correct hyphenation conventions. Can anyone take a look? Headbomb {talk / contribs / physics / books} 13:32, 11 May 2010 (UTC)
- most likely 2-(4-Biphenyl)-5-(4-t-butylphenyl)-1,3,4-oxadiazole or 2-biphenyl-4-yl-5-(4-t-butylphenyl)-1,3,4-oxydiazole buy it at Sial --Stone (talk) 14:58, 11 May 2010 (UTC)
- Thanks. Headbomb {talk / contribs / physics / books} 15:05, 11 May 2010 (UTC)
- No problem! --Stone (talk) 15:15, 11 May 2010 (UTC)
- You even have an article on it now! Physchim62 (talk) 10:36, 22 May 2010 (UTC)
- No problem! --Stone (talk) 15:15, 11 May 2010 (UTC)
- Thanks. Headbomb {talk / contribs / physics / books} 15:05, 11 May 2010 (UTC)
edit
{{editprotect}}
Please remove the protection and
Chemicals/Style guidelines Gnevin (talk) 08:18, 21 May 2010 (UTC)
- As far as I can see, it is not protected .. --Dirk Beetstra T C 08:20, 21 May 2010 (UTC)
- Sorry was redirected here . Wikipedia:WikiProject_Chemicals/Style_guidelines is the page Gnevin (talk) 08:24, 21 May 2010 (UTC)
 Unprotected — Martin (MSGJ · talk) 09:59, 21 May 2010 (UTC)
Unprotected — Martin (MSGJ · talk) 09:59, 21 May 2010 (UTC)
- Sorry was redirected here . Wikipedia:WikiProject_Chemicals/Style_guidelines is the page Gnevin (talk) 08:24, 21 May 2010 (UTC)
- Note that that page is historical, and no longer in use. The style guidelines are now Wikipedia:Manual_of_Style_(chemistry). Hence, I'm not sure if Wikipedia:WikiProject_Chemicals/Style_guidelines should be unprotected... --Dirk Beetstra T C 10:02, 21 May 2010 (UTC)
- I'm happy for you to revert that action, but I don't believe there is any precedent for fully protecting pages just because they are marked as {{historical}}. — Martin (MSGJ · talk) 10:05, 21 May 2010 (UTC)
4-[(4-dimethylaminophenyl)-phenyl-methyl]-N,N-dimethyl-aniline
There are three articles dealing with that chemical
- Crystal violet gives the synthesis but is a stub
- Methyl violet is the article about several similar compounds but the reference to the main article is not clear.
- Gentian violet is the main article, but is mainly focused on the medical applications. At the end it is a manual how to dye skin and jackets.
I will try to help later. All three have room for some improvement.--Stone (talk)
Nickel(III) oxide use in secondary cells
If [[nickel(III) oxide is used so commonly in secondary cells such as ni-cad and nickel metal hydride battery, why is it not well characterized? It doesn't show how it is made industrially either. --Chemicalinterest (talk) 12:49, 28 May 2010 (UTC)
- You don't have to know the composition and structure of a substance to use it. Methylalumoxane is another example of a very widely used substance (for making polythene and related products) whose structure is a bit elusive.
- Do they make it using the way shown in the article? It seemed more like a laboratory process, not an an industrial process. --Chemicalinterest (talk) 14:36, 28 May 2010 (UTC)
- No, I doubt they make it that way! For the electrodes in a secondary cell, the simplest way would be to stick a piece of nickel in a suitable acid solution and then charge it up as the anode of a electrolytic cell at a suitably high potential. That's roughly how you make lead dioxide in a lead–acid battery. Physchim62 (talk) 14:49, 28 May 2010 (UTC)
- Do they make it using the way shown in the article? It seemed more like a laboratory process, not an an industrial process. --Chemicalinterest (talk) 14:36, 28 May 2010 (UTC)
- By the way, is that "No Image" really necessary on Methylalumoxane? --shoy (reactions) 17:11, 28 May 2010 (UTC)
user:Plasmic Physics
keeps adding rare IUPAC names (some are never used and have almost zero Google hits) to chemistry articles. I would be glad to know the stance of the project on that. Materialscientist (talk) 01:50, 4 June 2010 (UTC)
- Well, it annoys me no end. Chris (talk) 09:16, 4 June 2010 (UTC)
I've tried to tell him a few times, but there is no reply. I think we should just revert it on sight. --Rifleman 82 (talk) 10:38, 4 June 2010 (UTC)
- I left a note on Plasma Physics's talk page, asking him/her to discuss things with us.
- Is failure to engage in discussion grounds for blocking?
- Ben (talk) 11:19, 4 June 2010 (UTC)
- Not really. His editing has to be disruptive to merit a block and, from what I've seen, the user is quite there yet. But by all means revert, under the BRD principle. Physchim62 (talk) 11:31, 4 June 2010 (UTC)
OK, I've asked him to come and discuss this here. For my part, whilst these edits are generally factually correct they do not add anything to the article (the IUPAC names can go in the infobox, by all means), and are distracting.... The thing is, nobody actually uses these names, and as far as I'm concerned we shouldn't go filling up the lead paragraph with lots of irrelevant information. Chris (talk) 13:31, 4 June 2010 (UTC)
- If they have a place, they could go into the 'othernames' field in the Chembox. That said, they are often too long, mostly unused etc., and for many compounds more IUPAC names are possible. The only one that would be of interest is the Preferred IUPAC name (new parameter, PIN field in the main body of the chembox), but I don't think there are definite decisions on that, yet. Although I do see the effort, and in a way the info is useful (if we want to be a source of chemical information (and we are, eyes are also upon us ..), then by all means, it should be possible to find chemicals by all possible means, and that includes as many 'identifiers' as possible, even if 'no-one' uses them.[citation needed] (but then still, it does not mean they need a prominent place in the page). --Dirk Beetstra T C 13:41, 4 June 2010 (UTC)
Here's a transclusion of the three-way multiple-talk-page discussion between Plasma Physics, Chris, and me:
| The original thread on Plasma Physics's talk page:
Dear Plasma Physics, Have you seen the latest discussion about your edits at Wikipedia talk:WikiProject Chemicals#user:Plasmic Physics? There are many editors who find you addition of (supposedly) IUPAC names unhelpful, but none (except you) who support them. It would benefit Wikipedia, and in particular its chemistry articles, if you would at least engage in discussion on the matter. Ben (talk) 11:16, 4 June 2010 (UTC)
|
- I have been trying to join the discussion here, but was unable due to edit conflicts. Mean while argument is already apparent. I must make a note, the IUPAC names are not explicitly stated in the IUPAC PRNOC (2004) as it is not a comprehensive catelogue, but simply a set of rules by which the names can be constructed. The names themselves are assembled by a third party following these rules. If said third party is not absolutely certain of the correctness of a name, then it does not qualify for addition to wikipedia. Google does not provide every result on a chemical search, sites of chemical suppliers uses the names mostly when Google doesn't. So Google should not be trusted so completely to provide all citations, it is not absolutely reliable for searching obscure IUPAC names.--Plasmic Physics (talk) 21:34, 4 June 2010 (UTC)
- Then please understand that you do appear as this third party, diligently trying to construct names, which nobody knows and nobody uses and which are therefore opposed because this contradicts the basic rule of wikipedia - document notable and reliable information. I believe we all here honestly aim to improve wikipedia, including yourself. Please, think constructively. Some conventions of this project are hard to understand, but are easy to live with, and the current consensus is clearly against those names. Materialscientist (talk) 23:33, 4 June 2010 (UTC)
- I have been trying to join the discussion here, but was unable due to edit conflicts. Mean while argument is already apparent. I must make a note, the IUPAC names are not explicitly stated in the IUPAC PRNOC (2004) as it is not a comprehensive catelogue, but simply a set of rules by which the names can be constructed. The names themselves are assembled by a third party following these rules. If said third party is not absolutely certain of the correctness of a name, then it does not qualify for addition to wikipedia. Google does not provide every result on a chemical search, sites of chemical suppliers uses the names mostly when Google doesn't. So Google should not be trusted so completely to provide all citations, it is not absolutely reliable for searching obscure IUPAC names.--Plasmic Physics (talk) 21:34, 4 June 2010 (UTC)
The other point to remember is that PRNOC(2004) is a proposal: it has not been adopted, and will not be adopted in exactly those words. The public draft is internally inconsistent: if you look carefully, it gives two different names for acetone (the second being propan-2-one). We don't know when the new rules are coming out – 2011 is a date I've heard from a very well placed source, but that is not an official announcement – until then, PRNOC2004 is just an idea as to the direction that things are going rather than a set of rules to be applied blindly. Physchim62 (talk) 01:34, 5 June 2010 (UTC)
- What about IUPAC names that are citable? That is what I'm most concerned with - if I can cite a IUPAC name, does it still not warrant a place in the article?--Plasmic Physics (talk) 02:52, 5 June 2010 (UTC)
- Put it in the box, not in the text. Though you might want to save your effort til the Preferred IUPAC names recommendations are approved. --Rifleman 82 (talk) 03:01, 5 June 2010 (UTC)
- Alright, so I will put it under the other names section and cite it. Although, I don't see any reason wait - there is no sense in allowing one IUPAC name, if the article has one, while not allowing another, especially if it is disallowing indefinitely. An addition of a cited alternative name does not harm wikipedia's integrity does it? When who knows when the PIN recommendations are approved, a switch can be carried out by choosing which of the IUPAC names to place in the IUPAC section for the template. By no means should the loser be removed, as it was still and maybe is still used in some sources.--Plasmic Physics (talk) 08:35, 5 June 2010 (UTC)
- I have a concern about citing: often, it is possible to find one-two obscure journal articles which use a certain rare name. This won't do. Materialscientist (talk) 08:40, 5 June 2010 (UTC)
- Alright, so I will put it under the other names section and cite it. Although, I don't see any reason wait - there is no sense in allowing one IUPAC name, if the article has one, while not allowing another, especially if it is disallowing indefinitely. An addition of a cited alternative name does not harm wikipedia's integrity does it? When who knows when the PIN recommendations are approved, a switch can be carried out by choosing which of the IUPAC names to place in the IUPAC section for the template. By no means should the loser be removed, as it was still and maybe is still used in some sources.--Plasmic Physics (talk) 08:35, 5 June 2010 (UTC)
- Apart from Materialscientist's and anyone else's concerns, Plasmic Physics: Can you please then fix this then? Specifically, move your text to the box. --Rifleman 82 (talk) 09:03, 5 June 2010 (UTC)
- Working on it.--Plasmic Physics (talk) 11:45, 5 June 2010 (UTC)
- Have a look at my latest edit to nitric acid, is this the general idea?--Plasmic Physics (talk) 13:03, 5 June 2010 (UTC)
The source you've provided for the name "oxazinic acid" for nitric acid doesn't look very reliable: [http://www.molport.com/buy-chemicals/moleculelink/nitric-acid-potassium/3930346 "oxoazinic acid potassium" at molport.com. There's some very questionable data there, notably the structure and formula, which seem to imply a 1:1 mixture of potassium hydride and nitric acid. Not a mixture that would hang around for very long:
- KH + HNO3 → KNO3 + H2
Have you read WP:RS?
Ben (talk) 13:31, 5 June 2010 (UTC)
- This editor has demonstrated a consistent disinterest in helpful editing at least with respect to names and chemistry. Instead, PlasmicPhysics is engaged in some sort of asinine, litigious game. I recommend that the community continue to revert this editor's work upon sight, as many here have done over the past few years. I am unconvinced that every possible ultra-obscure name even merit inclusions in the ChemBox.--Smokefoot (talk) 14:39, 5 June 2010 (UTC)
- To everyone who is actually interested in finding a solution rather than critising my motives: If it's a problem with my choice of citation, then what about [[3]]? It can be found under Chemical IUPAC Name. It is not uncommon for compounds to be discribed as a product of their reactants. The previous citation describes potassium nitrate as an implicit salt of potassium hydride and oxoazinic acid. This new citation descibes methylammonium nitrate as an implicit salt of methanamine and oxoazinic acid. The new source is a database as opposed to a chemical supplier's catalogue. I still need to know the answer to my earlier question: was my edit to nitric acid the general idea, Rifleman?--Plasmic Physics (talk) 00:15, 6 June 2010 (UTC)
- I'm not sure why you wish to expend so much effort in this direction, which IMHO, is futile. With regard to your edit to nitric acid in particular, I don't think it helps, but since our "other names" section is such a mess in general anyway, it probably does little harm. Better in that box than in the text. With regard to Ben's comment, if you're that adamant about doing the job which a computer is more suited for, please seek advise from him and PC about how to navigate IUPAC's rules to generate real IUPAC names according to the rules, not simply something you can find off the internet. Verifiability is not truth.--Rifleman 82 (talk) 03:16, 6 June 2010 (UTC)
- Oh it's no effort at all! BTW, I'm not generating the IUPAC names, I'm using a plugin from ChemAxon's Marvin. For more information about its generating ability, visit [4]. I'm not sure why Physchim reverted my edit to nitric acid, as I added oxoazinic acid to the other names section and removed it from the body of the article, just as you recommended.--Plasmic Physics (talk) 06:50, 6 June 2010 (UTC)
I don't think the database is a reliable source either.
The most appropriate source for IUPAC names is clearly IUPAC itself.
A quick Google search for "IUPAC nomenclature" gave the following relevant results:
- http://www.iupac.org/
- http://old.iupac.org/nomenclature/index.html
- http://www.chem.qmul.ac.uk/iupac/
- http://www.acdlabs.com/iupac/nomenclature/
- http://old.iupac.org/publications/books/seriestitles/nomenclature.html
- http://www.rsc.org/pdf/general/append.pdf
Try these first.
Ben (talk) 00:55, 6 June 2010 (UTC)
- That's just the thing, IUPAC itself does not provide any names, except for the purpose of proving examples of naming procedure, it is not a catalogue for names. IUPAC only provides the means of constructing approved names. These constructed names must be found elsewhere. I have the IUPAC names already, I just need proper online sources that I can cite the names.--Plasmic Physics (talk) 03:13, 6 June 2010 (UTC)
Right, so this all comes down to whether Marvin's automatic name generator is a reliable source of IUPAC names. What do you think?
Ben (talk) 08:23, 6 June 2010 (UTC)
- I can assure you that I have not intend to user Marvin's generator as a reliable source. There is no purpose if no one actually uses it, that's why I check online if anyone uses it before I decide to add it or not.--Plasmic Physics (talk) 08:28, 6 June 2010 (UTC)
That method (using Marvin for structure → name then using Google to check if name is used) is OK in principle, but you need to add a final step: discard any usage online in unreliable sources. Even then, the name might be used very little, in which case it's probably best to ignore it.
Maybe we can string together some guidelines on reliable sources for chemical nomenclature.
Ben (talk) 10:33, 6 June 2010 (UTC)
- That would be most useful, I'm honestly looking forward to continueing with my mission.--Plasmic Physics (talk) 11:20, 6 June 2010 (UTC)
- When should I be expecting a draft of the proposed guidelines?--Plasmic Physics (talk) 00:08, 8 June 2010 (UTC)
IUPAC name for urea
Speaking of IUPAC, there's a discussion at Talk:Urea#IUPAC_name that could use knowledgable input. Adrian J. Hunter(talk•contribs) 03:51, 4 June 2010 (UTC)
Expansion of Category:Inorganic compound stubs
Please take a look at what I did there and give guidelines for whether it is good to expand articles like that. Thank you. --Chemicalinterest (talk) 12:56, 4 June 2010 (UTC)
How do you verify your chembox data ?
Hi, I just wondering how do you verify your data in the chembox ? No links between data and books or database are provided. Biglama (talk) 13:11, 21 June 2010 (UTC)