Friedländer synthesis
The Friedländer synthesis is the chemical reaction of 2-aminobenzaldehydes[1] with ketones to form quinoline derivatives.[2][3] It is named after German chemist Paul Friedländer (1857-1923).

This reaction has been catalyzed by trifluoroacetic acid[4], toluenesulfonic acid[5], iodine[6], and Lewis acids[7].
Several reviews have been published.[8][9][10][11]
Mechanism
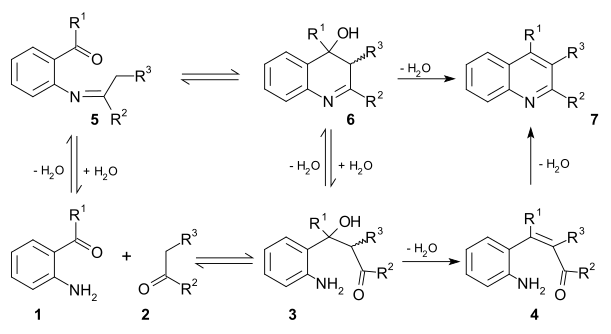
Two viable reaction mechanisms exist for this reaction. In the first mechanism 2-amino substituted carbonyl compound 1 and carbonyl compound 2 react in a rate-limiting step to aldol adduct 3. This intermediate loses water in an elimination reaction to unsaturated carbonyl compound 4 and then loses water again in imine formation to quinoline 7. In the second mechanism the first step is Schiff base formation to 5 followed by Aldol reaction to 6 and elimination to 7 [12].
The Pfitzinger reaction and the Niementowski quinoline synthesis are variations.
References
- ^ Organic Syntheses, Coll. Vol. 3, p.56 (1955); Vol. 28, p.11 (1948). (Article)
- ^ Friedländer, P. Ber. 1882, 15, 2572.
- ^ Friedländer, P.; Gohring, C. F. Ber. 1883, 16, 1833.
- ^ Shaabani, A.; Soleimani, E.; Badri, Z. Synth. Commun. 2007, 37, 629-635. (doi:10.1080/00397910601055230)
- ^ Jia, C.-S.; Zhang, Z.; Tu, S.-J.; Wang, G.-W. Org. Biomol. Chem. 2006, 4, 104-110.
- ^ Wu, J.; Xia, H.-G.; Gao, K. Org. Biomol. Chem. 2006, 4, 126-129.
- ^ Varala, R.; Enugala, R.; Adapa, S. R. Synthesis 2006, 3825-3830.
- ^ Cheng, C.-C.; Yan, S.-J. Org. React. 1982, 28, 37. (doi: 10.1002/0471264180.or028.02)
- ^ Manske, R. H. Chem. Rev. 1942, 30, 113. (Review)
- ^ Bergstrom, F. W. Chem. Rev. 1944, 35, 77. (Review)
- ^ Cheng, C. C.; Yan, S. J. Org. React. 1982, 28, 37. (Review)
- ^ Recent Advances in the Friedlander Reaction Jose Marco-Contelles, Elena Perez-Mayoral,Abdelouahid Samadi, Marıa do Carmo Carreiras, and Elena Soriano Chem. Rev. 2009 doi:10.1021/cr800482c