Sulfur
 | ||||||||||||||||||||||||||||||||||||
| Sulfur | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alternative name | Sulphur (British spelling) | |||||||||||||||||||||||||||||||||||
| Allotropes | see Allotropes of sulfur | |||||||||||||||||||||||||||||||||||
| Appearance | Lemon yellow sintered microcrystals | |||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(S) | ||||||||||||||||||||||||||||||||||||
| Sulfur in the periodic table | ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 16 | |||||||||||||||||||||||||||||||||||
| Group | group 16 (chalcogens) | |||||||||||||||||||||||||||||||||||
| Period | period 3 | |||||||||||||||||||||||||||||||||||
| Block | p-block | |||||||||||||||||||||||||||||||||||
| Electron configuration | [Ne] 3s2 3p4 | |||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 6 | |||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||||
| Melting point | alpha (α-S8): 388.36 K (115.21 °C, 239.38 °F) | |||||||||||||||||||||||||||||||||||
| Boiling point | 717.8 K (444.6 °C, 832.3 °F) | |||||||||||||||||||||||||||||||||||
| Density (near r.t.) | alpha (α-S8): 2.07 g/cm3 beta (β-S8): 1.96 g/cm3 gamma (γ-S8): 1.92 g/cm3 | |||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 1.819 g/cm3 | |||||||||||||||||||||||||||||||||||
| Critical point | 1314 K, 20.7 MPa | |||||||||||||||||||||||||||||||||||
| Heat of fusion | beta (β-S8): 1.727 kJ/mol | |||||||||||||||||||||||||||||||||||
| Heat of vaporization | beta (β-S8): 45 kJ/mol | |||||||||||||||||||||||||||||||||||
| Molar heat capacity | 22.75 J/(mol·K) | |||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||
| Oxidation states | common: −2, +2, +4, +6 −1,[3] 0, +1,[3] +3,[3] +5[3] | |||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.58 | |||||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||||
| Covalent radius | 105±3 pm | |||||||||||||||||||||||||||||||||||
| Van der Waals radius | 180 pm | |||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||||||||||
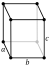
| Crystal structure | alpha (α-S8): orthorhombic (oF128) | |||||||||||||||||||||||||||||||||||
| Lattice constants | a = 1.0460 nm b = 1.2861 nm c = 2.4481 nm (at 20 °C)[4] | |||||||||||||||||||||||||||||||||||
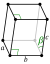
| Crystal structure | beta (β-S8): monoclinic (mP48) | |||||||||||||||||||||||||||||||||||
| Lattice constants | a = 1.0923 nm b = 1.0851 nm c = 1.0787 nm β = 95.905° (at 20 °C)[4] | |||||||||||||||||||||||||||||||||||
| Thermal conductivity | 0.205 W/(m⋅K) (amorphous) | |||||||||||||||||||||||||||||||||||
| Electrical resistivity | 2×1015 Ω⋅m (at 20 °C) (amorphous) | |||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[5] | |||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | alpha (α-S8): −15.5×10−6 cm3/mol (298 K)[6] | |||||||||||||||||||||||||||||||||||
| Bulk modulus | 7.7 GPa | |||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.0 | |||||||||||||||||||||||||||||||||||
| CAS Number | 7704-34-9 | |||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||
| Discovery | before 2000 BCE[7] | |||||||||||||||||||||||||||||||||||
| Recognized as an element by | Antoine Lavoisier (1777) | |||||||||||||||||||||||||||||||||||
| Isotopes of sulfur | ||||||||||||||||||||||||||||||||||||
34S abundances vary greatly (between 3.96 and 4.77 percent) in natural samples. | ||||||||||||||||||||||||||||||||||||
Sulfur or sulphur (/[invalid input: 'icon']ˈsʌlfər/ SUL-fər; see spelling below) is the chemical element that has the atomic number 16. It is denoted with the symbol S. It is an abundant, multivalent non-metal. Sulfur, in its native form, is a bright yellow crystalline solid. In nature, it can be found as the pure element and as sulfide and sulfate minerals. It is an essential element for life and is found in two amino acids: cysteine and methionine. Its commercial uses are primarily in fertilizers, but it is also widely used in black gunpowder, matches, insecticides and fungicides. Elemental sulfur crystals are commonly sought after by mineral collectors for their brightly colored polyhedron shapes. In nonscientific contexts, it can also be referred to as brimstone.[8]
Characteristics

Physical
At room temperature, sulfur is a soft, bright-yellow solid with only a faint odor, similar to that of matches (the strong "smell of sulfur" usually refers to the odor of hydrogen sulfide (H
2S) or organosulfur compounds). Sulfur is an electrical insulator. It melts slightly above 100 °C and easily sublimes.[8]
Chemical
Sulfur burns with a blue flame that emits sulfur dioxide, notable for its peculiar suffocating odor (this is the odor of burnt matches). Sulfur is insoluble in water, but soluble in carbon disulfide — and to a lesser extent in other non-polar organic solvents such as benzene and toluene. Sulfur in the solid state ordinarily exists as cyclic crown-shaped S8 molecules. The crystallography of sulfur is complex. Depending on the specific conditions, the sulfur allotropes form several crystal structures, with rhombic and monoclinic S8 best known.[8]
Unlike most other liquids, molten sulfur increases in viscosity with temperatures of 200 °C (392 °F) due to the formation of polymers. The molten sulfur assumes a dark red color above this temperature. At still higher temperatures, however, the viscosity is decreased as depolymerization occurs.
Amorphous or "plastic" sulfur can be produced through the rapid cooling of molten sulfur. X-ray crystallography studies show that the amorphous form may have a helical structure with eight atoms per turn. This form is metastable at room temperature and gradually reverts to crystalline form. This process happens within a matter of hours to days but can be rapidly catalyzed.
Allotropes

Sulfur forms more than 30 solid allotropes, more than any other element.[9] Besides S8, several other rings are known.[10] Removing one atom from the crown gives S7, which is more deeply yellow than S8. HPLC analysis of "elemental sulfur" reveals an equilibrium mixture of mainly S8, but also S7 and small amounts of S6.[11] Larger rings have been prepared, including S12 and S18.[12][13] By contrast, sulfur's lighter neighbor oxygen only exists in two states of allotropic significance: O2 and O3. Selenium, the heavier analogue of sulfur, can form rings but is more often found as a polymer chain.
Isotopes
Sulfur has 25 known isotopes, four of which are stable: 32S (95.02%), 33S (0.75%), 34S (4.21%), and 36S (0.02%). Other than 35S, the radioactive isotopes of sulfur are all short lived. 35S is formed from cosmic ray spallation of 40argon in the atmosphere. It has a half-life of 87 days.
When sulfide minerals are precipitated, isotopic equilibration among solids and liquid may cause small differences in the δS-34 values of co-genetic minerals. The differences between minerals can be used to estimate the temperature of equilibration. The δC-13 and δS-34 of coexisting carbonates and sulfides can be used to determine the pH and oxygen fugacity of the ore-bearing fluid during ore formation.
In most forest ecosystems, sulfate is derived mostly from the atmosphere; weathering of ore minerals and evaporites also contribute some sulfur. Sulfur with a distinctive isotopic composition has been used to identify pollution sources, and enriched sulfur has been added as a tracer in hydrologic studies. Differences in the natural abundances can also be used in systems where there is sufficient variation in the 34S of ecosystem components. Rocky Mountain lakes thought to be dominated by atmospheric sources of sulfate have been found to have different δ34S values from lakes believed to be dominated by watershed sources of sulfate.
Natural occurrence


The most common sulfur isotope in nature, sulfur-32, is created in extremely large, extremely hot (over 2.5 billion kelvin) stars. This requires fusion of one nucleus of silicon plus one nucleus of helium.[14] For details of the process, see silicon burning. Partly because this so-called alpha process produces elements in abundance, sulfur is the 10th most common element in the universe.
The distinctive colors of Jupiter's volcanic moon, Io, are from various forms of molten, solid and gaseous sulfur.
There is also a dark area near the crater Aristarchus on Earth's Moon, that has been suggested to be a sulfur deposit.
Sulfur is present in many types of meteorites. Ordinary chondrites contain on average 2.1% sulfur, and carbonaceous chondrites may contain as much as 6.6%. Sulfur in meteorites is normally present as troilite (FeS), but other sulfides are found in some meteorites, and carbonaceous chondrites contain free sulfur, sulfates, and possibly other sulfur compounds.[15]

On Earth, elemental sulfur can be found near hot springs and volcanic regions in many parts of the world, especially along the Pacific Ring of Fire. Such volcanic deposits are currently mined in Indonesia, Chile, and Japan. Sicily is also famous for its sulfur mines. Sulfur deposits are polycrystalline, and the largest documented single crystal measured 22×16×11 cm.[16][17]
Significant deposits of elemental sulfur also exist in salt domes along the coast of the Gulf of Mexico, and in evaporites in eastern Europe and western Asia. The sulfur in these deposits is believed to come from the action of anaerobic bacteria on sulfate minerals, especially gypsum, although apparently native sulfur may be produced by geological processes alone, without the aid of living organisms (see below). However, fossil-based sulfur deposits from salt domes have, until recently, been the basis for commercial production in the United States, Poland, Russia, Turkmenistan, and Ukraine.[18] As noted below, such sources are now of secondary commercial importance, and most are no longer worked.
Common naturally-occurring sulfur compounds include the sulfide minerals, such as pyrite (iron sulfide), cinnabar (mercury sulfide), galena (lead sulfide), sphalerite (zinc sulfide) and stibnite (antimony sulfide); and the sulfates, such as gypsum (calcium sulfate), alunite (potassium aluminium sulfate), and barite (barium sulfate). It occurs naturally in volcanic emissions, such as from hydrothermal vents, and from bacterial action on decaying sulfur-containing organic matter.
Production
Sulfur may be found as a pure mineral ("native" sulfur) and historically was usually obtained in this way. However, today's sulfur production is as a side product of other industrial processes, such as oil refining. In these processes, sulfur often occurs as sulfur compounds, which are then converted to elemental sulfur.
As a mineral, native sulfur under salt domes is thought to be a fossil mineral resource, produced by the action of ancient bacteria on sulfate deposits. It was removed from such salt-dome mines mainly by the Frasch process.[18] In this method, superheated water was pumped into a native sulfur deposit to melt the sulfur, and then compressed air returned the relatively pure (99.5%) melted product to the surface. Throughout the 20th century this procedure produced elemental sulfur which required no further purification. Howevever, due to a limited number of such sulfur deposits and the high cost of working them, this process for mining sulfur has not been employed in a major way anywhere in the world, since 2002.

Today, sulfur is produced from petroleum, natural gas, and related fossil resources, from which it is obtained mainly as hydrogen sulfide (a gas). organosulfur compounds, which are undesirable impurities in petroleum, may be upgraded by subjecting them to hydrodesulfurization, which cleaves the C-S bonds:
- R-S-R + 2 H2 → 2 RH + H2S
The resulting hydrogen sulfide from this process, and also as it occurs in natural gas, is converted into elemental sulfur by the Claus process This process entails oxidation of some hydrogen sulfide to sulfur dioxide and then the comproportionation of the two:
- 1.5 O2 + H2S → SO2 + H2O
- SO2 + 2 H2S → 3 S + 2 H2O
Many sour gas streams (gases containing some H2S) are treated to remove the sulfur, e.g. Shell-Paques sulfide removal/sulfur recovery process. Owing to the high sulfur content of the Athabasca Oil Sands, stockpiles of elemental sulfur from this process now exist throughout Alberta, Canada.
Compounds
- See also Category: sulfur compounds
Common oxidation states of sulfur range from −2 to +6. Sulfur forms stable compounds with all elements except the noble gases.
Sulfides
Treatment of sulfur with hydrogen gives hydrogen sulfide. When dissolved in water, hydrogen sulfide is mildly acidic:[8]
- H2S HS- + H+
Reduction of elemental sulfur gives polysulfides, which consist of chains of sulfur atoms terminated with S- centres:
- 2 Na + S8 → Na2S8
This reaction highlights arguably the single most distinctive property of sulfur: its ability to catenate (bind to itself by formation of chains). Protonation of these polysulfide anions gives the polysulfanes, H2Sx where x = 2, 3, and 4.[19] Ultimately reduction of sulfur gives sulfide salts:
- 16 Na + S8 → 8 Na2S
The interconversion of these species is exploited in the sodium-sulfur battery. The radical anion S2- gives the blue color to the mineral lapis lazuli.

Elemental sulfur can also be oxidized, for example to give bicyclic S82+.
Oxides and oxyanions
The principal sulfur oxides are obtained by burning sulfur:
- S + O2 → SO2
- SO2 + 1/2 O2 → SO3
Other oxides are known, e.g. sulfur monoxide and disulfur mono- and dioxides, but they are unstable.
The sulfur oxides form numerous oxyanions with the formula SOn2-. Sulfur dioxide and sulfites (SO2−
3) are related to the unstable sulfurous acid (H2SO3). Sulfur trioxide and sulfates (SO2−
4) are related to sulfuric acid. Sulfuric acid and SO3 combine to give oleum, a solution of pyrosulfuric acid (H2S2O7) in sulfuric acid.
Peroxides convert sulfur into unstable such as S8O, a sulfoxide. Peroxymonosulfuric acid (H2SO5) and peroxydisulfuric acids (H2S2O8), made from the action of SO3 on concentrated H2O2, and H2SO4 on concentrated H2O2 respectively.
4
Thiosulfate salts (S
2O2−
3), sometimes referred as "hyposulfites", used in photographic fixing (HYPO) and as reducing agents, feature sulfur in two oxidation states. Sodium dithionite, (S
2O2−
4), is more highly reducing dianion. Sodium dithionate (Na2S2O6) is the first member of the polythionic acids (H2SnO6), where n can range from 3 to many.
Halides and oxyhalides
Sulfur hexafluoride, SF6 is a dense gas that is used as nonreactive and nontoxic propellant. In contrast, sulfur tetrafluoride is a rarely used reagent that is highly toxic. The two main sulfur chlorides are sulfur dichloride (SCl2) and sulfur monochloride (S2Cl2). Sulfuryl chloride (SO2Cl2) and chlorosulfuric acid (ClSO3H) are derivatives of sulfuric acid. Thionyl chloride (SOCl2) is a reagent in organic synthesis.
Pnictnides
The most important S-N compound is the cage tetrasulfur tetranitride (S4N4). Heating this compound gives Polymeric sulfur nitride ((SN)x), which has metallic properties even though it does not contain any metal atoms. Thiocyanates contain the SCN− group. Oxidation of thiocyanate gives thiocyanogen, (SCN)2 with the connectivity NCS-SCN. Phosphorus sulfides are numerous, the most important commercially being the cages P4S10 and P4S3.[20][21]
Metal sulfides
Many if not most minerals occur as sulfides. The principal ores of copper, zinc, nickel, cobalt, molybdenum and others are sulfides. These materials tend to be dark-colored semiconductors that are not readily attacked by water or even many acids. They are formed, both geochemically and in the laboratory, by the reaction of hydrogen sulfide with metal salts to form the metal sulfides. The mineral Galena (PbS) was the first demonstrated semiconductor and found a use as a signal rectifier in the cat's whiskers of early crystal radios. The iron sulfide called pyrite, the so-called "fool's gold," has the formula Fe>S2.[22] The upgrading of these ores, usually by roasting, is costly and environmentally hazardous. Sulfur corrodes many metals via the process called tarnishing.
Organic compounds
- Illustrative organosulfur compounds
-
Allicin, the active ingredient in garlic
-
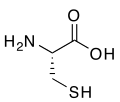
R-cysteine, an amino acid containing a thiol group
-
Methionine, an amino acid containing a thioether
-
Diphenyl disulfide, a representative disulfide
-
Perfluorooctanesulfonic acid, a controversial surfactant
-
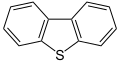
Dibenzothiophene, a component of crude oil
Some of the main classes of sulfur-containing organic compounds include the following (R, R', and R are organic groups such as CH3):[23]
- Thioethers have the form R-S-R′. These compounds are the sulfur equivalents of ethers.
- Sulfonium ions have the formula RR'R"S+, i.e. where three groups are attached to the cationic sulfur center. Dimethylsulfoniopropionate (DMSP; (CH3)2S+CH2CH2COO−) is a sulfonium ion, which is important in the marine organic sulfur cycle.
- Thiols (also known as mercaptans) have the form R-SH. These are the sulfur equivalents of alcohols. Treatment of thiols with base gives thiolates ions (R-S-.
- Sulfoxides and Sulfones have the form R-S(=O)-R′ and R-S(=O)(=O)-R′. The simplest sulfoxide, DMSO, is a common solvent. A common sulfone is sulfolane C4H8SO2.
- Sulfonic acids (R-SO3- are used in many detergents.
Organosulfur compounds are responsible for the unpleasant odors of decaying organic matter. Thiols and sulfides are used in the odoration of natural gas, notably, t-butyl mercaptan. The odor of garlic and "skunk stink" are also caused by organosulfur compounds. Not all organic sulfur compounds smell unpleasant; for example, grapefruit mercaptan, a sulfur-containing monoterpenoid is responsible for the characteristic scent of grapefruit. It should be noted that this thiol is present in very low concentrations. In larger concentrations, the odor of this compound is that typical of all thiols.
Inorganic carbon-sulfur compounds are also well known. Carbon disulfide (CS2) is a volatile liquid that is structurally similar to carbon dioxide. It is used to make polymers. Whereas carbon monoxide is a highly stable gas, carbon monosulfide (CS) is a laboratory curiosity with only a fleeting existence.
History
Antiquity
Being abundantly available in native form, sulfur (Sanskrit, गन्धक sulvari; Latin Sulphurium) was known in ancient times and is referred to in the Torah (Genesis). English translations of the Bible commonly referred to burning sulfur as "brimstone", giving rise to the name of 'fire-and-brimstone' sermons, in which listeners are reminded of the fate of eternal damnation that await the unbelieving and unrepentant. It is from this part of the Bible that Hell is implied to "smell of sulfur" (likely due to its association with volcanic activity). According to the Ebers Papyrus, a sulfur ointment was used in ancient Egypt to treat granular eyelids. Sulfur was used for fumigation in preclassical Greece;[24] this is mentioned in the Odyssey.[25] Pliny the Elder discusses sulfur in book 35 of his Natural History, saying that its best-known source is the island of Melos. He also mentions its use for fumigation, medicine, and bleaching cloth.[26]
A natural form of sulfur known as shiliuhuang was known in China since the 6th century BC and found in Hanzhong.[27] By the 3rd century, the Chinese discovered that sulfur could be extracted from pyrite.[27] Chinese Daoists were interested in sulfur's flammability and its reactivity with certain metals, yet its earliest practical uses were found in traditional Chinese medicine.[27] A Song Dynasty military treatise of 1044 AD described different formulas for Chinese black powder, which is a mixture of potassium nitrate (KNO
3), charcoal, and sulfur. Early alchemists gave sulfur its own alchemical symbol which was a triangle at the top of a cross.
In traditional medical skin treatment which predates modern era of scientific medicine, elemental sulfur has been used mainly as part of creams to alleviate various conditions such as scabies, ringworm, psoriasis, eczema and acne. The mechanism of action is not known, although elemental sulfur does oxidize slowly to sulfurous acid, which in turn (through the action of sulfite) acts as a mild reducing and antibacterial agent.
Modern times
In 1777, Antoine Lavoisier helped convince the scientific community that sulfur was an element and not a compound. The Sicilian process was used in ancient times to obtain sulfur from rocks present in volcanic regions of Sicily. In this process, the sulfur deposits are piled and stacked in brick kilns built on sloping hillsides, and with airspaces between them. Then powdered sulfur is put on top of the sulfur deposit and ignited. As the sulfur burns, the heat melts the sulfur deposits, causing the molten sulfur to flow down the sloping hillside.

In 1867, sulfur was discovered in underground deposits in Louisiana and Texas. The highly successful Frasch process was developed to extract this resource.[28]
In the late 18th century, furniture makers used molten sulfur to produce decorative inlays in their craft. Because of the sulfur dioxide produced during the process of melting sulfur, the craft of sulfur inlays was soon abandoned. Molten sulfur is sometimes still used for setting steel bolts into drilled concrete holes where high shock resistance is desired for floor-mounted equipment attachment points. Pure powdered sulfur was also used as a medicinal tonic and laxative.[18]
Spelling and etymology
The element was traditionally spelt sulphur in the United Kingdom (since the 14th century),[29] most of the Commonwealth including India, Malaysia, South Africa, and Hong Kong, along with the rest of the Caribbean and Ireland. Sulfur is used in the United States, while both spellings are used in Canada and the Philippines.
However, the IUPAC adopted the spelling sulfur in 1990, as did the Royal Society of Chemistry Nomenclature Committee in 1992.[30] The Qualifications and Curriculum Authority for England and Wales recommended its use in 2000,[31] and it now appears in GCSE exams.[32] The Oxford Dictionaries note that "In chemistry... the -f- spelling is now the standard form in all related words in the field in both British and US contexts"[33]
In Latin, the word is variously written sulpur, sulphur, and sulfur (the Oxford Latin Dictionary lists the spellings in this order). It is an original Latin name and not a Classical Greek loan, so the ph variant does not denote the Greek letter φ (phi). Sulfur in Greek is thion (θείον), whence comes the prefix thio-. The simplification of the Latin words p or ph to an f appears to have taken place towards the end of the classical period.[34][35]
Applications
Sulfuric acid
Elemental sulfur is mainly used as a precursor to other chemicals. Approximately 85% (1989) is converted to sulfuric acid (H2SO4):
- 2 S + 3 O2 + 2 H2O → 2 H2SO4
With sulfuric acid being central importance to the world's economies, its production and consumption is an indicator of a nation's industrial development.[36] For example with 36.1 million metric tons in 2007, more sulfuric acid is produced in the United States every year than any other inorganic industrial chemical.[37] The principal use for the acid is the extraction of phosphate ores for the production of fertilizer manufacturing. Other applications of sulfuric acid include oil refining, wastewater processing, and mineral extraction.[18]

Other large scale sulfur chemicals
Sulfur reacts directly with methane to give carbon disulfide, which is used to manufacture cellophane and rayon.[18] One of the direct uses of sulfur is in vulcanization of rubber, where polysulfides crosslink organic polymers. Sulfites are heavily used to bleach paper. Sulfites are also used as preservatives in dried fruit. Many surfactants and detergents, e.g. sodium lauryl sulfate, are produced are sulfate derivatives. Calcium sulfate, gypsum, (CaSO4.2H2O) is mined on the scale of 100 million tons each year for use in Portland cement and fertizers.
When silver-based photography was widespread, sodium and ammonium thiosulfate were widely used as "fixing agents." Sulfur is a component of gunpowder.
Fertilizer
Sulfur is increasingly used as a component of fertilizers. The most important form of sulfur for fertilizer is the mineral calcium sulfate. Elemental sulfur is hydrophobic (that is, it is not soluble in water) and therefore cannot be taken up by the plants instantly. Soil bacteria convert it to soluble derivatives. Sulfur also improves the use efficiency of other essential plant nutrients, particularly nitrogen and phosphorus.[38] Biologically produced sulfur particles are naturally hydrophilic due to a biopolymer coating. This sulfur is therefore easier to disperse over the land (via spraying as a diluted slurry), and results in a faster release.
Plant requirements for sulfur are equal to or exceed those for phosphorus. It is one of the major nutrients essential for plant growth, root nodule formation of legumes and plants protection mechanisms. Sulfur deficiency has become widespread in many countries in Europe.[39][40][41] Because atmospheric inputs of sulfur will continue to decrease, the deficit in the sulfur input/output is likely to increase, unless sulfur fertilizers are used.
Fine chemicals

Organosulfur compounds are also used in pharmaceuticals, dyestuffs, and agrichemicals. Many drugs contain sulfur, early examples being the sulfa drugs. Sulfur is a part of many bacterial defense molecules. Most beta lactam antibiotics, including the penicillins, cephalosporins, and monolactams contain sulfur.[23]
Magnesium sulfate, better known as Epsom salts, can be used as a laxative, a bath additive, an exfoliant, magnesium supplement for plants, or a desiccant.
Fungicide and pesticide
Elemental sulfur is one of the oldest fungicides and pesticides. Dusting sulfur, elemental sulfur in powdered form, is a common fungicide for grapes, strawberry, many vegetables and several other crops. It has a good efficacy against a wide range of powdery mildew diseases as well as black spot. In organic production, sulfur is the most important fungicide. It is the only fungicide used in organically farmed apple production against the main disease apple scab under colder conditions. Biosulfur (biologically produced elemental sulfur with hydrophilic characteristics) can be used well for these applications.
Standard-formulation dusting sulfur is applied to crops with a sulfur duster or from a dusting plane. Wettable sulfur is the commercial name for dusting sulfur formulated with additional ingredients to make it water miscible.[42] It has similar applications, and is used as a fungicide against mildew and other mold-related problems with plants and soil.
Sulfur is also used as an "organic" (i.e. "green") insecticide (actually an acaricide) against ticks and mites. A common method of use is to dust clothing or limbs with sulfur powder. Some livestock owners set out a sulfur salt block as a salt lick. [citation needed]
Biological role
Protein and organic cofactors
Sulfur is an essential component of all living cells. In plants and animals the amino acids cysteine and methionine contain sulfur, as do all polypeptides, proteins, and enzymes that contain these amino acids. Disulfide bonds (S-S bonds) formed between cysteine residues in peptide chains are very important in protein assembly and structure. These covalent bonds between peptide chains confer extra toughness and rigidity.[43] For example, the high strength of feathers and hair is in part due to their high content of S-S bonds and their high content of cysteine and sulfur. Eggs are high in sulfur because large amounts of the element are necessary for feather formation, and the characteristic odor of rotting eggs is due to hydrogen sulfide. The high disulfide bond content of hair and feathers contributes to their indigestibility, and also to their characteristic disagreeable odor when burned.
Homocysteine and taurine are other sulfur-containing acids that are similar in structure, but which are not coded by DNA, and are not part of the primary structure of proteins. Many important cellular enzymes use prosthetic groups ending with -SH moieties to handle reactions involving acyl-containing biochemicals: two common examples from basic metabolism are coenzyme A and alpha-lipoic acid.[43] Sulfur also plays and imporant part as a carrier of reducing hydrogen and its electrons, for cellular repair of oxidation. Reduced glutathione, a sulfur-containing tripeptide, is a reducing agent through its sulfhydryl (-SH) moiety derived from cysteine. The thioredoxins are essential classes of small proteins acting as general reducing agents in cells, that use pairs of reduced cysteines to similar effect.
Methanogenesis, the route to most of the world's methane, is a multistep biochemical transformation of carbon dioxide. This conversion requires several organosulfur cofactors. These include coenzyme M, CH3SCH2CH2SO3-, the immediate precursor to methane.[44]
Metalloproteins and inorganic cofactors
Inorganic sulfur forms a part of iron-sulfur clusters as well as many copper, nickel, and iron proteins. Most pervasive are the ferrodoxins, which serve as electron shuttles in cells. Nitrogenase, an Fe-Mo-S cluster, ia a catalyst that converts atmospheric nitrogen to ammonia, required by plants and microorganisms.[45]
Sulfur metabolism
Sulfur may also serve as energy (chemical food) source for bacteria that use hydrogen sulfide (H2S) in the place of water as the electron donor in a primitive photosynthesis-like process in which oxygen is the electron receptor. The photosynthetic green and purple sulfur bacteria and some chemolithotrophs use elemental oxygen to carry out such oxidization of hydrogen sulfide to produce elemental sulfur (So), oxidation state = 0. Primitive bacteria which live around deep ocean volcanic vents oxidize hydrogen sulfide in this way with oxygen: see giant tube worm for an example of large organisms (via bacteria) making metabolic use of hydrogen sulfide as food to be oxidized.
The so-called sulfur bacteria, by contrast, "breathe sulfate" instead of oxygen. They use sulfur as the electron acceptor, and reduce various oxidized sulfur compounds back into sulfide, often into hydrogen sulfide. They also can grow on a number of other partially oxidized sulfur compounds (e. g. thiosulfates, thionates, polysulfides, sulfites). The hydrogen sulfide produced by these bacteria is responsible for the smell of some intestinal gases and decomposition products.
Sulfur is absorbed by plants via the roots from soil as the sulfate and transported as a phosphate ester. Sulfate is reduced to sulfide via sulfite before it is incorporated into cysteine and other organosulfur compounds.[46]
- SO42- → SO32- → H2S → cysteine
Precautions
Elemental sulfur is non-toxic, but it can burn, producing sulfur dioxide. Although sulfur dioxide is sufficiently safe to be used as a food additive in small amounts, at high concentrations it harms the lungs, eyes or other tissues. In organisms without lungs such as insects or plants, it otherwise prevents respiration. Sulfur trioxide and sulfuric acid are similarly highly corrosive, due to the strong acids that form on contact with water.

The burning of coal and/or petroleum by industry and power plants generates sulfur dioxide (SO2), which reacts with atmospheric water and oxygen to produce sulfuric acid (H2SO4) and sulfurous acid (H2SO3). These acids are components of acid rain, which lower the pH of soil and freshwater bodies, sometimes resulting in substantial damage to the environment and chemical weathering of statues and structures. Fuel standards increasingly require sulfur to be extracted from fossil fuels to prevent the formation of acid rain. This extracted sulfur is then refined and represents a large portion of sulfur production. In coal fired power plants, the flue gases are sometimes purified. In more modern power plants that use syngas the sulfur is extracted before the gas is burned.
Hydrogen sulfide is as toxic as hydrogen cyanide and kills by the same mechanism, although hydrogen sulfide is less likely to result in surprise poisonings from small inhaled amounts, due to its more disagreeable warning odor. However, although very pungent at first awareness to the human nose, hydrogen sulfide quickly deadens the sense of smell, so potential victims breathing larger and larger quantities of it may be unaware of its presence until severe symptoms occur (these can then quickly lead to death).
See also
References
- ^ "Standard Atomic Weights: Sulfur". CIAAW. 2009.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c d Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 28. ISBN 978-0-08-037941-8.
- ^ a b Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ "Sulfur History". Georgiagulfsulfur.com. Retrieved 2022-02-12.
- ^ a b c d Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ^ Steudel, Ralf; Eckert, Bodo (2003). "Solid Sulfur Allotropes Sulfur Allotropes". Topics in Current Chemistry. 230: 1–80. doi:10.1007/b12110.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Steudel, R. (1982). "Homocyclic Sulfur Molecules". Topics Curr. Chem. 102: 149. doi:10.1007/3-540-11345-2_10.
- ^ Tebbe, F. N.; Wasserman, E.; Peet, W. G.; Vatvars, A. and Hayman, A. C. (1982). "Composition of Elemental Sulfur in Solution: Equilibrium of S
6, S7, and S8 at Ambient Temperatures". J. Am. Chem. Soc. 104: 4971. doi:10.1021/ja00382a050.{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Beat Meyer (1964). "Solid Allotropes of Sulfur". Chem. Rev. 64 (4): 429–451. doi:10.1021/cr60230a004.
- ^ Beat Meyer (1976). "Elemental sulfur". Chem. Rev. 76: 367–388. doi:10.1021/cr60301a003.
- ^ A.G.W. Cameron (1957). "Stellar Evolution, Nuclear Astrophysics, and Nucleogenesis" (PDF). CRL-41.
- ^ B. Mason (1962). Meteorites. New York: John Wiley & Sons. p. 160.
- ^ Rickwood, P. C. (1981). "The largest crystals" (PDF). American Mineralogist. 66: 885–907.
- ^ "The giant crystal project site". Retrieved 2009-06-06.
- ^ a b c d e Nehb, Wolfgang (2006). "Sulfur". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag. doi:10.1002/14356007.a25_507.pub2.
{{cite encyclopedia}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 421.
- ^ Heal, H. G. (1980). The Inorganic Heterocyclic Chemistry of Sulfur, Nitrogen, and Phosphorus. London: Academic Press. ISBN 0123356806.
- ^ Chivers, T. (2004). A Guide To Chalcogen-Nitrogen Chemistry. Singapore: World Scientific. ISBN 9812560955.
- ^ Vaughan, D. J.; Craig, J. R. "Mineral Chemistry of Metal Sulfides" Cambridge University Press, Cambridge (1978) ISBN 0-521-21489-0
- ^ a b R. J. Cremlyn “An Introduction to Organosulfur Chemistry” John Wiley and Sons: Chichester (1996). ISBN 0 471 95512 4.
- ^ George Rapp Archaeomineralogy, 2nd ed., Springer: 2009, ISBN 978-3-540-78593-4 p. 242.
- ^ Odyssey, book 22, lines 480–495.
- ^ Pliny the Elder on science and technology, John F. Healy, Oxford University Press, 1999, ISBN 0198146876 pp. 247–249.
- ^ a b c Zhang Yunming (1986). "The History of Science Society: Ancient Chinese Sulfur Manufacturing Processes". Isis. 77: 487. doi:10.1086/354207.
- ^ Botsch, Walter (2001). "Chemiker, Techniker, Unternehmer: Zum 150. Geburtstag von Hermann Frasch". Chemie in unserer Zeit (in German). 35 (5): 324–331. doi:10.1002/1521-3781(200110)35:5<324::AID-CIUZ324>3.0.CO;2-9.
- ^ Sulphur or Sulfur?, accessed March 30, 2010
- ^ Spelling of Sulfur (PDF)
- ^ http://www.worldwidewords.org/topicalwords/tw-sul1.htm Worldwidewords
- ^ [1], accessed 12 November 2010.
- ^ Ask Oxford, accessed 12 November 2010.
- ^ Vanderkrogt.net.
- ^ Kelly DP (1995). "Sulfur and its Doppelgänger". Arch. Microbiol. 163: 157–158. doi:10.1007/BF00305347.
- ^ Sulfuric Acid Growth
- ^ Ober, Joyce A. "Mineral Yearbook 2007: Sulfur" (PDF). United States Geological Survey.
- ^ Sulfur as a fertilizer
- ^ Zhao, F (1999). "Sulphur Assimilation and Effects on Yield and Quality of Wheat". Journal of Cereal Science. 30: 1. doi:10.1006/jcrs.1998.0241.
- ^ Blake-Kalff, M.M.A. (2000). Plant and Soil. 225: 95. doi:10.1023/A:1026503812267.
{{cite journal}}: Missing or empty|title=(help) - ^ Ceccotti, S. P. (1996). "Plant nutrient sulphur-a review of nutrient balance, environmental impact and fertilizers". Fertilizer Research. 43: 117. doi:10.1007/BF00747690.
- ^ Every, Richard L.; et al. (1968-08-20). "Method for Preparation of Wettable Sulfur" (PDF). Retrieved 2010-05-20.
{{cite web}}: Explicit use of et al. in:|author=(help) - ^ a b Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ^ Thauer, R. K., "Biochemistry of Methanogenesis: a Tribute to Marjory Stephenson", Microbiology, 1998, volume 144, pages 2377-2406.
- ^ S. J. Lippard, J. M. Berg “Principles of Bioinorganic Chemistry” University Science Books: Mill Valley, CA; 1994. ISBN 0-935702-73-3.
- ^ Hans-Walter Heldt: Pflanzenbiochemie. Spektrum Akademischer Verlag, Heidelberg 1996, ISBN 3-8274-0103-8, S. 321-333