Lepidoptera
| Lepidoptera Temporal range: Jurassic – Recent
| |
|---|---|

| |
| Clockwise from left to right, Emperor gum moth, Eggfruit Caterpillar moth (Sceliodes cordalis), Abantiades magnificus, Leopard Lacewing (Cethosia cyane) Giant Leopard Moth (Hypercompe scribonia), Willowherb Hawkmoth larva (Proserpinus proserpina) | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Subclass: | |
| Infraclass: | |
| Superorder: | |
| Order: | Lepidoptera |
| Suborders | |
Lepidoptera (Template:Pron-en) is a large order of insects that includes moths and butterflies (called lepidopterans). It is one of the most speciose orders in the world, encompassing moths and the three superfamilies of butterflies, skipper butterflies, and moth-butterflies and found virtually everywhere. The term was coined by Linnaeus in 1735 and is derived from Ancient Greek λεπίδος (scale) and πτερόν (wing).[1] Comprising an estimated 174,250 species,[2] in 126 families[3] and 46 superfamilies,[2] the Lepidoptera show many variations of the basic body structure which have evolved to gain advantages in lifestyle and distribution. Recent estimates suggest that the order may have more species,[4] and is among the four largest, most successful orders, along with the Hymenoptera, Diptera, and the Coleoptera.[5]
Species of lepidopteran species are characterized by more than 20 derived features, some of the most apparent being the scales covering their bodies and wings, and a proboscis. The scales are modified flattened hairs, and are how butterflies and moths get their extraordinary variety of colors. Almost all species have some form of membranous wings, except for a few species, of which have crossvein wings. Like most other insects, Butterflies and moths are holometabolists, or undergo complete metamorphosis. Mating and the laying of eggs are normally carried out by adults, normally near or on plants which would be their host plant as larvae. The larvae are called caterpillars and are completely different in form, having a cylindrical body with a well developed head, mandible mouth parts, and from 0–11 (usually 8) pairs of prolegs. These larvae will feed and grow and change in a series of stages called instars. Once fully matured as larvae, they undergo the pupal stage in their life cycle, which is commonly called a chrysalis for butterflies and a cocoon for moths. Most species spin a silk case for their pupal stage, while still many other may not, preferring to go underground.[6]: 559
The Lepidoptera have, over millions of years, evolved a wide range of wing patterns and coloration ranging from drab moths akin to the related order Trichoptera to the brightly colored and complex-patterned butterflies.[3] Accordingly, this is the most recognized and popular of insect orders with many people involved in the observing, study, collecting, rearing and commerce of these insects. A person who collects or studies this order is referred to as a lepidopterist. Many species of the order are of economic interest by virtue of their important natural role through pollination or the silk they produce.
Even though butterflies and moths play an important role in the natural ecosystem as pollinators, the larva are considered very problematic to vegetation in agriculture, as their main source of food is live plant matter. In many species, the female may be able to produce anywhere from 200 to 600 eggs, while in some others it may go as high as 30,000 eggs in one day. which leaves a large number of caterpillars, which in effect can mow down entire acres of crops.
Etymology
The word Lepidoptera comes from the Latin word for "scaly wing", from the Ancient Greek λεπίς (lepis) meaning scale and πτερόν (pteron) meaning wing.[1] Sometimes the term Rhopalocera is used to group the species that are butterflies, from the Ancient Greek ῥόπαλον (rhopalon) and κέρας (kæras) meaning club and horn respectively; coming from the shape of the antennae of butterflies.
The origins of the common names of many species vary. The English word butterfly is from Old English buttorfleoge, with many variations in spelling. Other than that, the origin is unknown, although it could be derived from the pale yellow color of many species' wings suggesting the color of butter.[1][7] The species of Heterocera are commonly called moths. The origins of the English word moth are more clear, which comes from Old English moððe" (cf. Northumbrian dialect mohðe) from Common Germanic (compare Old Norse motti, Dutch mot and German Motte all meaning "moth"). Perhaps its origins are related to Old English maða meaning "maggot" or from the root of "midge" which until the 16th century was used mostly to indicate the larva, usually in reference to devouring clothes.[1]: moth
The etymological origins of the word caterpillar, the larval form of butterflies and moths, are from the early 16th century, from Middle English catirpel, catirpeller, probably an alteration of Old North French catepelose: cate, cat (from Latin cattus) + pelose, hairy (from Latin pilōsus).[8]
Distribution and diversity
| Palearctic | Nearctic | Neotropic | Afrotropic | Indo-australian (comprising Indomalayan and Australian regions) | |
|---|---|---|---|---|---|
| Estimated number of species | 22,465 | 11,532 | 44,791 | 20,491 | 47,286 |
Lepidoptera are among the most successful groups of insects. They are to be found on all continents, except the Antarctic. Lepidoptera inhabit all terrestrial habitats ranging from desert to rain forest, from lowland grasslands to montane plateaus but almost always associated with higher plants, especially angiosperms (flowering plants).[9] Amongst the northern-most of butterflies and moths is the Arctic Apollo (Parnassius arcticus) which is found in the Arctic Circle in northeastern Yakutia, at an altitude of 1500 meters above sea level.[10] In the Himalayas, various Apollo species such as Parnassius epaphus, besides others, have been recorded to occur up to an altitude of 6,000 meters above sea level.[11]: 221
Some lepidopteran species exhibit symbiotic, phoretic or parasitic life-styles inhabiting the bodies of organisms rather than the environment. Coprophagous pyralid moth species, called as sloth moths, such as Bradipodicola hahneli and Cryptoses choloepi, are unusual in that they are exclusively found inhabiting the fur of the sloths, mammals found in central and South America.[12][13] Two species of Tinea moths have been recorded as feeding on horny tissue and have been bred from the horns of cattle. The larva of Zenodochium coccivorella is an internal parasite of the coccid Kermes species. Many species have been recorded as breeding in natural materials or refuse such as owl pellets, bat caves, honey-combs or diseased fruit.[13]
Out of the approximately 174,250 species described to date, it is estimated that butterflies and skippers comprise approximately 17,950 with moths making up the rest.[2][14] The vast majority of Lepidoptera are to be found in the tropics but a substantial biodiversity occurs on each continent. North America has over 700 species of butterflies and over 11,000 species of moths found in North America[15][16] while there are about 400 species of butterflies and 20,000 species of moths reported from Australia.[17]
The diversity of Lepidoptera in each faunal region has been estimated by John Heppner in 1991 based partly on actual counts from the literature, partly on the card indexes in the Natural History Museum (London) and the National Museum of Natural History (Washington), and partly on estimates:[4]: 726
External Morphology
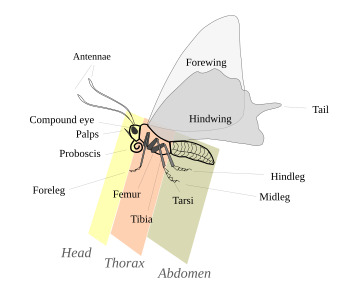

Lepidoptera are morphologically distinguished from other orders principally by the presence of scales on the external parts of the body and appendages, especially the wings. Butterflies and moths vary in size from microlepidoptera only a few millimeters long, to conspicuous animals with a wingspan of many inches, such as the Monarch butterfly and Atlas moth.[18]: 246 The Lepidoptera show many variations of the basic body structure which have evolved to gain advantages in lifestyle and distribution.
Head

The head is where many sensing organs and the mouth parts are found. Like the adults, the larvae also have a toughened, or sclerotized head capsule.[19] Here, there are two compound eyes, and, unique to Lepidoptera are raised spots or cluster of sensory bristles called chaetosema. even though many taxa have lost one or both of said spots. The antennae have a wide variation in the form amongst species and even between different sexes. The antennae of butterflies are usually filiform and shaped like clubs, while those of moths are with flagellar segments variously enlarged or branched. Some moths have a antennae that are enlarged, or tapered and hooked at the ends.[6]: 559–560
The mouth parts are elongated and joined in the form of a proboscis. The proboscis is made of up to one to five segments, usually kept coiled up under the head by small muscles when not being used to suck up nectar from flowers or other liquids. Some moths that are more primitive still have madibles, or separate moving jaws like their ancestors, these moths form the genus Micropterigidae.[19][20][6]: 560
The larvae, caterpillars, have a toughened (sclerotised) head capsule, with separate chewing mouthparts[19]. These mouthparts, called mandibles, are used to chew up the plant matter that the larvae eat, rather than a proboscis, which an adult uses to suck liquids. The lower jaw, or labium is weak buy may carry a spinneret, or an organ used to create silk. The head is made of large lateral lobes, each having an ellipse of up to six simple eyes.[6]: 562–563
Thorax
The thorax is made of three fused segments, the prothorax, mesothorax, and metathorax, each with a pair of legs. The first segment contains the first pair of legs. The males of some species in the butterfly family Nymphalidae, the fore-legs are greatly reduced and are not used for walking or perching.[6]: 586 The three pairs of legs are covered with scales. Lepidoptera also have olfactory organs on their feet which aid the butterfly in "tasting" or "smelling" out its food.[21] In the larva form there are 3 pairs of true legs, with 0–11 pairs of abdominal legs (usually 8) and hooklets, called apical crochets.[9]
The two pairs of wings are found on the middle and third segment, or mesothorax and metathorax respectively. In the more recent genera, the wings of the second segment or much more pronounce, however some, more primitive form, have similarly sized wings of both segments. The wings are covered in scales arranged like shingles, forming the extraordinary variety seen in color. The mesothorax is designed to have more powerful muscles to propel moth or butterfly through the air, with the wing of said segment having a stronger vein structure.[6]: 560 The largest superfamily, Noctuidae, has the wings modified to act as Tympanal or hearing organs[22]
The larvae have an elongated soft body that may have hair-like or other projections, 3 pairs of true legs, with 0–11 pairs of abdominal legs (usually 8) and hooklets, called apical crochets.[9] The thorax will usually have a pair of legs on each segment. The thorax is also lined with many sphericals on both the mesothorax and metathorax, except for a few aquatic species, who instead have a form of gills.[6]: 563
Abdomen
The abdomen consist of 10 segments which is less sclerotized than the thorax, and is not fused to the point of immobility, but rather has membranes in between meant for each section to be interdependent of the other. The sternum of the first segment is some families is small and other completely lost. The 7-10 or 8-10 segments are adapted to form the external parts of the species sex organs. The genetalia of Lepidoptera species are highly varied and are commonly use as a means of differentiating different families. In males, the valvae are usually large, as they are used to grasp the female during mating, while in females, there are three different types of genitalia. In more primitive moths, there is only one sex organ, which is used as a sex organ and as a ovipositor, or egg laying organ. While most others have a separate organ for copulation, or have an external duct that carries the sperm for male, which makes up 98% of species. [6]: 561
The abdomen usually has pairs of prolegs and a pair of prolegs by the anus. In some primitive moths, the these prolegs may be on every segment of the body, not just abdomen, but are normally restricted to the third to sixth segments, with the anal prolegs have a pair of tiny hooks called crotchets. These aid in griping and walking, especially in species that lack many prolegs (e.g. larvae of Geometridae). Prolegs may be lost completely in other groups, where they are more adapt to boring and living in sand (e.g., Prodoxidae and Nepticulidae respectively). [6]: 563
Scales

The wings, head parts of thorax and abdomen of Lepidoptera are covered with minute scales, from which feature the order 'Lepidoptera' derives its names, the word "lepteron" in Ancient Greek meaning 'scale'. Most scales are lamellar, or blade-like and attached with a pedicel, while other forms may be hair-like or specialized as secondary sexual characteristics.[23] The lumen or surface of the lamella, has a complex structure. It gives color either due to the pigmentary colors contained within or due to its three-dimensional structure.[24] Scales provide a number of functions, which include insulation, thermoregulation, aiding gliding flight, amongst others, the most important of which is the large diversity of vivid or indistinct patterns they provide which help the organism protect itself by camouflage, mimicry , and to seek mates.[23]
-
A patch of wing (×50).
-
Scales close up (×200).
-
A single scale (x1000).
-
Microstructure of a scale (x5000).
Internal morphology

In reproductive system of butterflies and moths, the male genitalia are complex and unclear. In females there are three types of genitalia based on the relating taxa: monotrysian, exoporian, and dytresian. In the monotrysian type there is an opening on the fused segments of the sterna 9 and 10, which act as insemination and oviposition. In the exoporian type (in Hepaloidae and Mnesarchaeoidea) there are two separate places for insemination and oviposition, both occurring on the same sterna as the monotrysian type, 9/10. In most species the genitalia are flanked by two soft lobes, although they may be specialized and sclerotized in some species for ovipositing in area such as crevices and inside plant tissue.[18] Hormones and the glands that produce them run the development of butterflies and moths as they go through their life cycle, called the endocrine system. The first insect hormone PTTH (Prothoracicotropic hormone) operates the species life cycle and diapause (see the relates section).[25] This hormone is produced by corpora allata, where it is also stored. Some glands are specialized to perform certain task such as producing silk or producing saliva in the palpi.[26]: 65, 75
In the digestive system, the anterior region of the foregut has been modified to form a pharyngial sucking pump as they need it for the food they eat, which are for the most part liquids. An esophagus follows and leads to the posterior of the pharynx and in some species forms a form of crop. The midgut is short and straight, with the hindgut being longer and coiled.[18] Ancestors of lepidopteran species, stemming from Hymenoptera, had midgut ceca, although this is lost in current butterflies and moths. Instead, all the digestive enzymes other then initial digestion, are immobilized at the surface of the midgut cells. In larvae, long-necked and stalked goblet cells are found in the anterior and posterior midgut regions, respectively. In insects, the goblet cells excrete positive potassium ions, which are absorbed from leaves ingested by the larvae. Most butterflies and moths display the usual digestive cycle, however species that have a different diet require adaptations to meet these new demands.[6]: 279
In the circulatory system, hemolymph, or insect blood, is used to circulate heat in a form of thermoregulation, where muscles contraction produces heat which is transferred to the rest of the body when conditions are unfavorable.[27]In lepidopteran species, hemolymph is circulated through the veins in the wings by some form of pulsating organ, either by the heart or by the intake of air into the trachea.[26]: 69 Air is taken in through spiracles along the sides of the abdomen and thorax supplying the trachea with oxygen as it goes through the lepidopteran's respiratory system. There are three different tracheae supplying oxygen diffusing oxygen throughout the species body: The dorsal, ventral, and visceral. The dorsal tracheae supply oxygen to the dorsal musculature and vessels, while the ventral tracheae supply the ventral musculature and nerve cord, and the visceral tracheae supply the guts, fat bodies, and gonads.[26]: 71, 72
Polymorphism


Polymorphism is appearance of forms or "morphs" differing in color and number of attributes within a single species.[9]: 163 [28] In Lepidoptera, polymorphism can be seen not only between individuals in a population, but also between the sexes as sexual dimorphism, between geographically separated populations in geographical polymorphism and also between generations flying at different seasons of the year (seasonal polymorphism). It also includes the phenomenon of mimicry when mimetic morphs fly alongside non-mimetic morphs in a population of a particular species. Polymorphism occurs both at specific level with heritable variation in the overall morphological design of individuals as well as in certain specific morphological or physiological traits within a species.[9]
Sexual dimorphism is the occurrence of differences between males and females in a species. In Lepidoptera, sexual dimorphism is widespread and almost completely determined by genetic determination.[29] Sexual dimorphism is present in all families of the Papilionoidoea and more prominent in the Lycaenidae, Pieridae and certain taxa of the Nymphalidae. Apart from color variation which may differ from slight to completely different color-pattern combinations, secondary sexual characteristics may also be present.[30]: 25 Different genotypes maintained by natural selection may also be expressed at the same time.[29] Polymorphic and/or mimetic females occur in the case of some taxa in the Papilionidae primarily to obtain a level of protection not available to the male of their species. The most distinct case of sexual dimorphism is that of adult females of many Psychidae species who have only vestigial wings, legs, and mouthparts as compared to the adult males who are strong fliers with well-developed wings and feathery antennae.[31]
Geographical polymorphism is where geographical isolation causes a divergence of a species into different morphs. A good example is the Indian White Admiral Limenitis procris which has five forms, each geographically separated from the other by large mountain ranges.[30]: 26 An even more dramatic showcase of geographical polymorphism is the Apollo butterfly (Parnassius apollo). Due to the Apollos living in small local populations, having no contact with each other, but because of the strong stenotopic species and weak migration ability interbreeding between populations of one species practically does not occur; they form over 600 different morphs, with the size of spots on the wings of which varies greatly.[32]
Environmental polymorphism, where genetic heritability plays no role, is often termed as polyphenism. Polyphenism in Lepidoptera is commonly seen in the form of seasonal morphs especially in the butterfly families of Nymphalidae and Pieridae. An Old World pierid butterfly, the Common Grass Yellow (Eurema hecabe) has a darker summer adult morph, triggered by a long day exceeding 13 hours in duration, while the shorter diurnal period of 12 hours or less induces a paler morph in the post-monsoon period.[29] Polyphenism also occurs in caterpillars, an example being the Peppered Moth, Biston betularia.[33]
Batesian and Müllerian mimicry complexes are commonly found in Lepidoptera. Genetic polymorphism and natural selection give rise to otherwise edible species (the mimic) gaining a survival advantage by resembling inedible species (the model). Such a mimicry complex is referred to as Batesian and is most commonly known by the mimicry by the limenitidine Viceroy butterfly of the inedible danaine Monarch. Later research has discovered that the Viceroy is, in fact more toxic than the Monarch and this resemblance should be considered as a case of Müllerian mimicry.[34]
In Müllerian mimicry, inedible species, usually within a taxonomic order, find it advantageous to resemble each other so as to reduce the sampling rate by predators who need to learn about the insects' inedibility. Taxa from the toxic genus Heliconius form one of the most well known Müllerian complexes.[35] The adults of the various species now resemble each other so well that the species cannot be distinguished without close morphological observation and, in some cases, dissection or genetic analysis.
Reproduction and development

Species of Lepidoptera undergo holometabolism or "complete metamorphosis". Their life cycle normally consists of an egg, larva, pupa, and an imago or adult.[9] The larvae are commonly called caterpillars, and the pupae of moths are called cocoons and that of butterflies are called chrysalides.
Mating
Males usually get a head start, and start eclosion or emergence, earlier then females and peak in numbers before females. Both of the sexes are sexually mature by the time of eclosion.[6]: 564 Butterflies and moths normally don't ever some together, except for migrating species, staying relatively asocial. Mating begins with an adult (female or male) attracting a mate, normally using visual stimuli, especially in diurnal species like most butterflies. However, most nocturnal female species, including almost all moth species, use pheromones to attract males, sometimes from long distances.[9] Some species engage in a form of acoustic courtship, or attract mates using sound or vibration such as the polka-dot wasp moth, Syntomeida epilais.[36]
Adaptations such as undergoing one seasonal generation, two, or even more, called voltinism (Univoltism, bivoltism, and multivism respectively). Most lepidoptera in temperate climates are univoltine, while most have two seasonal broods. Some others may take advantage of as much opportunity they can get, and mate continuously through out the year. These seasonal adaptations are controlled hormones, and these delays in reproduction are called diapause.[6]: 567 Many lepidopteran species, after mating and laying their eggs, die shortly after, having only lived for a few days after eclosion. Others may still be active for several weeks and then overwinter and become sexually active again in more favorable days, or diapause. The sperm of the mate that mated the most recent is most likely to have fertilized the eggs. While it is assumed that the sperm of the previous male may still prevail.[6]: 564
Life cycle

Lepidopteran species like all Endopterygota, they are holometabolic, or undergo complete metamorphosis, going through a four-stage life cycle: egg; larva / caterpillar; pupa / chrysalis; and imago (plural: imagines) / adult. The morphological characteristics which distinguish the order Lepidoptera from other insect orders are:[18]: 246
Eggs
Lepidoptera usually reproduce sexually and are oviparous (egg-laying), though some species give live birth in a process called ovoviviparity. There are a variety of differences in egg-laying and the number of eggs laid. Some species simply drop their eggs in flight (these species normally have polyphagous larvae, meaning they eat a variety of plants e.g., Hepialids and some nymphalids)[37] while most Lepidoptera will lay their eggs near or on the host plant that the larvae feed on, normally attracted by its odor. The number of eggs laid may vary from only a few to several thousand.[9]
The females of both butterflies and moths select the host plant primarily by chemical cues. The area and placement is made by what would be a proper habitat, and which area on the plant is best for oviposition. How the moth or butterfly knows is rather based on instinct which is Inherited genetically.[6]: 564
The eggs covered by a hard-ridged outer layer of shell, called the chorion, which in turn offers protection. This is lined with a thin coating of wax which prevents the egg from drying out before the larva has had time to fully develop. Each egg contains a number of micropyles, or tiny funnel-shaped openings at one end, the purpose of which is to allow sperm to enter and fertilize the egg. Butterfly and moth eggs vary greatly in size between species, but they are all either spherical or ovate.
The egg stage lasts a few weeks in most butterflies but eggs laid close to winter, especially in temperate regions, go through a diapause (see the diapause section), and the hatching may take place only in spring. Other butterflies may lay their eggs in the spring and have them hatch in the summer. These butterflies are usually northern species (e.g., Nymphalis antiopa).
Larvae

The larvae or caterpillars are the first stage in the life cycle after hatching, look very different from the adults and come in a variety of shapes and sizes. The larvae are herbivores, but a few are carnivores (some eat ants or other caterpillars) and detritivores.[38] Those that are herbivores, can eat every part of the plant, and are normally considered pests to their host plant; species have been found to lay their eggs on the fruit and other species lay their eggs on clothing or fur (e.g., Tineola bisselliella, the common clothes moth). Some species are carnivorous and others are even parasitic. A species of Geometridae from Hawaii has carnivorous larvae that catch and eat flies.[7] The larvae develop rapidly with several generations in a year; however, some species may take up to 3 years to develop.[9]
The Larval stage is where the feeding and growing stages occur, and periodically undergo hormone-induced ecdysis, developing further with each instar, until they undergo the final larval-pupal molt. Lepidoptera pupa, known as chrysalis, have functional mandibles and with appendages fused or glued to the body in most species, while the pupal mandibles are not functional in others. [19]
The larvae of both butterflies and moths mimicry to deter potential preditors. Some caterpillars have the ability to inflate parts of their head to appear snake-like. Many have false eye-spots to enhance this effect. Some caterpillars have special structures called osmeteria which are averted to produce smelly chemicals. These are used in defense. Host plants often have toxic substances in them and caterpillars are able to sequester these substances and retain them into the adult stage. This helps making them unpalatable to birds and other predators. Such unpalatability is advertised using bright red, orange, black or white warning colors. The toxic chemicals in plants are often evolved specifically to prevent them from being eaten by insects. Insects in turn develop countermeasures or make use of these toxins for their own survival. This "arms race" has led to the coevolution of insects and their host plants.[39]
Wing development
Any form of wings are externally visible on the larva, however when larvae are dissected, developing wings can be seen as disks, which can be found on the second and third thoracic segments, in place of the spiracles that are apparent on abdominal segments. Wing disks develop in association with a trachea that runs along the base of the wing, and are surrounded by a thin peripodial membrane, which is linked to the outer epidermis of the larva by a tiny duct. Wing disks are very small until the last larval instar, when they increase dramatically in size, are invaded by branching tracheae from the wing base that precede the formation of the wing veins, and begin to develop patterns associated with several landmarks of the wing.[40]
Near pupation, the wings are forced outside the epidermis under pressure from the hemolymph, and although they are initially quite flexible and fragile, by the time the pupa breaks free of the larval cuticle they have adhered tightly to the outer cuticle of the pupa (in obtect pupae). Within hours, the wings form a cuticle so hard and well-joined to the body that pupae can be picked up and handled without damage to the wings.[40]
Pupa
After about 5 to 7 instars,[41]: 26–28 or molts, certain hormones, like prothoracicotropic hormone, stimulate the production of ecdysone, which initiates insect molting. Then, the larva puparium, a sclerotized or hardened cuticle of the last larval instar, develops into the pupa. Depending on the species, the pupa may be covered in silk and attached with many different types of debris or may be covered with nothing at all. The pupa stays attached the leaf by silk spun by the caterpillar before spinning the full pupa.[6]: 566 All the features of the adult are easily recognizable in the pupa, externally. All the appendages that are found on the adult head and thorax are found cased inside the cuticle (antennae, mouthparts, ect.), with the wings wrapped around, adjacent to the antennae.[6]: 564
While encased, some of the lower segments are not fused, and are able to move using small muscles found in between the membrane. Moving may help the pupa, for example, escape the sun, which would otherwise kill it like the pupa of the Cydia deshaisiana, called Mexican jumping beans. The larvae cut a trapdoor in the bean (species of Sebastiania) and use the bean as shelter. When there is a sudden rise in temperature, the pupa inside twitches and spasms, pulling on the threads inside. Wiggling may also help to deter parasitoid wasp from laying eggs on the pupa. Other species of moths are able to make clicks to deter predators.[6]: 564, 566
The length in which the pupa ecloses, or emerges varies greatly. The Monarch butterfly may stay in its chrysalis for two weeks, while other species may need to stay for more than 10 months in diapause (see the section Lepidoptera#Diapause). The adult will emerge from the pupa either by using abdominal hooks or from projections located on the head. The mandibles found in the most primitive moth families are used to escape from their caccoon (e.g., Micropterigoidea).[9][6]: 564
Adult
Most lepidopteran species do not live long after eclosion, only living a few days to find a mate and then lay their eggs. Albeit, some may still be active from one to several weeks or go through diapause, overwintering like Monarch butterflies, or waiting out environmental stress. Some adult species of Microlepidoptera go through a stage where no reproductive-related activity lasting through summer and winter, followed by mating and oviposition, or egg laying, in the early spring. [6]: 564
While most butterflies and moths are terrestrial, many species of Pyralidae are truly aquatic with all stages except the adult occurring in water. Many species from other families such as Arctiidae, Nepticulidae, Cosmopterygidae, Tortricidae, Olethreutidae, Noctuidae, Cossidae and Sphingidae are aquatic or semi-aquatic.[42]: 22
Behavior
Flight
In butterflies and moths, flight is an important aspect in species, special for evading predators, searching for food and finding mates in a timely manor as lepidopteran species do not live long after eclosion. The main form of locomotion in most Lepidoptera species is flight. In Lepidoptera, forewings and hindwings are mechanically coupled and flap in synchrony. Flight is anteromotoric, or being driven primarily by action of the forewings. Although it has been reported that lepidopteran species can still fly when their hindwings are cut off, however, this reduces there linear flight and turning abilities.[43]
Lepidopteran species have to be warm, about 77 to 79°F (25 to 26°C) in order to fly. They depend on their body temperature, which, sense they can't regulate normally, is dependent on their environment. Butterflies living in cooler climates may use their wings to warm their bodies. They will bask in the sun, and spread out their wings, so they get maximum exposure to the suns light. In hotter climates butterflies can easily overheat, so they are usually active only during the cooler parts of the day, early morning, late afternoon, or early evening. During the heat of the day they rest in the shade. Some larger thick-bodied moths (e.g., Sphingidae) can generate their own heat to a limited degree by vibrating their wings. The heat generated by the flight muscles warms the thorax, but the abdomen does not need to be kept so warm. To avoid overheating some moths rely on hairy scales, internal air sacs, and other structures to separate the thorax and abdomen and keep the abdomen cooler. [44]
Some species of can reach fast speeds, such as the Southern Dart, which can go as fast as 48.4 km/h. Sphingids are some of the fastest flying insects, some are capable of flying at over 50 km/h (30 miles per hour), having a wingspan of 35-150 mm. [45][3] In some species, there is sometimes a gliding component to their flight. Flight occurs either as hovering, or as forward or backward motion.[22] In butterflies and in moths species, like hawk moths, hovering is important in that they need it to hover over flowers when feeding on the nectar. [3]
Navigation

Navigation is important to Lepidoptera species, specially for those that migrate. Butterflies, who have more species that migrate, have been shown to navigate using time compensated sun compasses. They can see polarized light and therefore orient even in cloudy conditions. The polarized light in the region close to the ultraviolet spectrum is suggested to be particularly important.[46] It is suggested that most migratory butterflies are those that belong to semi-arid areas where breeding seasons are short.[47] The life-histories of their host plants also influence the strategies of the butterflies.[48] Other theories include the use of landscapes. Lepidoptera may use coastal lines, mountains, but also man-made roads to orient themselves. Above sea it has been observed that the flight direction is much more accurate if the landscape on the coast is still visible.[49]
Moths also show navigation, as seen in many studies. One study showed that many moths may use Earth's magnetic field to navigate, as a study of the stray Heart and Dart suggests.[50] Another study, this time of the migratory behavior of the Silver Y, showed that this species, even at high altitudes, can correct its course with changing winds, and prefers flying with favourable winds, which suggests a great sense of direction.[51][52] Aphrissa statira in Panama loses its navigational capacity when exposed to a magnetic field, suggesting it uses the Earth’s magnetic field.[53]
Moths exhibit a tendency to circle artificial lights repeatedly. This suggests that these species use a technique of celestial navigation called transverse orientation. By maintaining a constant angular relationship to a bright celestial light, such as the Moon, they can fly in a straight line. Celestial objects are so far away, that even after traveling great distances, the change in angle between the moth and the light source is negligible; further, the moon will always be in the upper part of the visual field or on the horizon. When a moth encounters a much closer artificial light and uses it for navigation, the angle changes noticeably after only a short distance, in addition to being often below the horizon. The moth instinctively attempts to correct by turning toward the light, causing airborne moths to come plummeting downwards, and – at close range – which results in a spiral flight path that gets closer and closer to the light source.[54]
Other explanations have been suggested, such as the idea that moths may be impaired with a visual distortion called a Mach band by Henry Hsiao in 1972. He stated that they fly towards the darkest part of the sky in pursuit of safety and are thus inclined to circle ambient objects in the Mach band region.[55]
Migration

Lepidopteran migration is usually seasonal, moving to escape dry seasons or other disadvantageous conditions. Most lepidopteran that migrate are butterflies, varying from short to over long distances. Some butterflies that migrate include the Mourning Cloak, Painted Lady, American Lady, Red Admiral, and the Common Buckeye.[41]: 29–30 Particularly famous migrations are those of the Monarch butterfly from Mexico to northern USA and southern Canada, a distance of about 4,000–4,800 km (2,500–3,000 mi). Other well known migratory species include the Painted Lady and several of the danaine butterflies. Spectacular and large scale migrations associated with the Monsoons are seen in peninsular India.[56] Migrations have been studied in more recent times using wing tags and also using stable hydrogen isotopes.[57][58]
Moths also undergo migrations, such as the uraniids. Urania fulgens undergoes population explosions and massive migrations that may be not surpassed by any other insect in the Neotropics. In Costa Rica and Panama, the first population movements may begin in July and early August and, depending on the year, may be very massive, continuing unabated for as long as five months.[59]
Communication
Pheromones are commonly involved in mating rituals amongst species, especially moths, but pheromones are an important aspect of other forms of communication amongst species as well. Usually only one sex will produce the pheromones and the other would pick them up with its antennae.[52] In many female species, a gland between the eighth and ninth segment under the abdomen produces the pheromones.[9] Communication can also occur through stridulation, or producing sounds by rubbing various parts of the body together.[52]
Moths are known to engage in acoustic forms of communication; most often species engage use it in a form of acoustic courtship, or attract mates using sound or vibration. Like most other insects, moths pick up these sounds using tympanic membranes in the abdomen.[60] An example is that of the polka-dot wasp moth (Syntomeida epilais), which produce sounds that are above the normal range of humman hearing (~20kHZ). These sounds also function as tactile communication, or communication through touch, as they stradulate stimuli.[36]
Butterflies also engage in in visual communication. Though moths lack bright colors as they usually use the coloration as camouflage. Female cabbage butterflies, for example, Use ultraviolet light to communicate, with scales colored to this range on the dorsal wing surface. When they fly, each down stroke of the wing creates a brief flash of Ultraviolet that the males apparently recognize as the flight signature of a potential mate. These flashes from the wings may attract several males who engage in aerial courtship displays. [60]
Diapause
One of the most important adaptations is diapause, or delay in development in response to regularly and recurring periods of adverse environmental conditions (winter, dry season, ect.). [61] Diapause normally occurs in eggs, or as a reproductive delay in adults. Butterflies like the Monarchs may go under diapause during winter, where they under go a form of hibernation of sorts, laying dormant on trees, normally covering them as the migrate in large numbers. [62] Seasonal adaptations such as voltism, where they may reproduce one or more times annually are do to diapause. This response to environmental stress is controlled by hormones and is necessary to survive during unfavorable, specially in northern areas and high mountains here winter is regular and harsh. For example, in the Mediterranean, larval feeding is during the spring, where the vegetation flourish, however under go diapause in the summer where there is drought, and hibernation in the winter.[6]: 567
Ecology
Moths and Butterflies are important in the natural ecosystem. They are integral participants in the food chain, having co-evolved with flowering plants and predators, lepidopteran species have formed a network of trophic relationships between autotrophs and heterotrophs, which are included in the stages of Lepidoptera larvae, pupae and adults. Larvae and pupae are links in the diet of birds and parasitic entomophagous insects. The adults are included in food webs in a much broader range of consumers (including birds, small mammals, reptiles, etc.).[6]: 567
Defense and predation

Lepidopteran species are soft bodied, fragile and almost defenseless while the immature stages move slowly or are immobile, hence all stages are exposed to predation. Adult butterflies and moths are predated upon by birds, lizards, amphibians, dragonflies and spiders, besides others. Caterpillars and pupa fall prey, not only to birds but invertebrate predators, small mammals, as well as fungi and bacteria. Parasitoid and parasitic wasps and flies may lay eggs in the caterpillar which would eventually kill it as they hatch inside its body and eat its tissues. Insect-eating birds are probably the worst predators. Lepidoptera, especially the immature stages, are an ecologically important food to many insectivorous birds, such as the Great Tit in Europe.
An "evolutionary arms race" can be seen between predator and prey species. Lepidoptera have developed a number of strategies for defense and protection which include evolution of morphological characters, changes in ecological life-style and in behavior. These include aposematism, mimicry, camouflage, development of threat patterns and displays and so on.[63] Only a few birds, such as the nightjars, hunt nocturnal Lepidoptera and their main enemy are bats. Again, an "evolutionary race" exists which has led to numerous evolutionary adaptations of moths to escape from their main predators, such as the ability to hear ultrasonic sounds, or even to emit sounds in some cases. Lepidoptera eggs are also predated upon. Some caterpillars, such as the zebra swallowtail butterfly larvae, are cannibalistic and may eat other larvae of the same species. Lepidopteran species rely on a variety of strategies.
Some species of lepidoptera are poisonous to predators, such as the Monarch butterfly in the Americas, Atrophaneura species (roses, windmills etc.) in Asia, as well as Papilio antimachus and the birdwings, the largest butterflies in Africa and Asia respectively. They obtain their toxicity by sequestering the chemicals from the plants they eat into their own tissues. Some Lepidoptera manufacture their own toxins. Predators that eat poisonous butterflies and moths may become sick and vomit violently, learning not to eat those types of species. A predator who has previously eaten a poisonous lepidopteran may avoid other species with similar markings in the future, thus saving many other species as well.[63][64] Toxic butterflies and larvae tend to develop bright colors, striking patterns as an indicator to predators about their toxicity. This phenomenon is known as aposematism.[65] Other caterpillars emit bad smells to ward off predators.[63] Some caterpillars, especially members of Papilionidae, contain an osmeterium, a Y-shaped protrusible gland found in the prothoracic segment of the larvae. When threatened, the caterpillar emits unpleasant smells from the organ to ward off the predators.[66][67]
Camouflage and mimicry are also important defense strategies. Some lepidopteran species blend with its surroundings, making them difficult to be spotted by predators. Caterpillars can be shades of green that matches its host plant. Others look like inedible objects, such as twigs or leaves. The larvae of some species, such as the Common Mormon (Papilio polytes) and the Western Tiger Swallowtail look like bird droppings.[63][68] For example, adult Sesiidae species (also known as clearwing moths) have a general appearance that is sufficiently similar to a wasp or hornet to make it likely that the moths gain a reduction in predation by Batesian mimicry.[69] Eyespots are a type of automimicry used by some butterflies and moths. In butterflies, the spots are composed of concentric rings of scales of different colors. The proposed role of the eyespots is to deflect attention to predators. Their resemblance to eyes provokes the predator's instinct to attack these wing patterns.[70]
There is evidence moths are able to hear the range emitted by bats, which in effect causes flying moths to make evasive maneuvers because bats are a main predator of moths. Ultrasonic frequencies trigger a reflex action in the noctuid moth that cause it to drop a few inches in its flight to evade attack.[71] Tiger moths in a defense emit clicks with in the same range of the bats, which interfere with the bats, and foil their attempts to echolocate it.[72]
Pollination

Most species of Lepidoptera engage in some form of entomophily (more specifically psychophily and phalaenophily for butterflies and moths respectively), or the pollination of flowers.[73] Most adult butterflies and moths feed on the nectar inside flowers, using their proboscis to reach the nectar hidden at the base of the petals. In the process, the adult brushes against the flower's stamen, on which the flower's reproductive pollen is made and stored. The pollen is transferred on appendages on the adult, who flies to the next flower to feed and unwittingly deposits the pollen on the stigma of the next flower, where the pollen germinates and fertilizes the seeds.[6]: 813–814
Flowers pollinated by butterflies tend to be large and flamboyant, being pink or lavender in color, frequently having a landing area, and are usually scented, as butterflies are typically day-flying. Since butterflies do not digest pollen (except for Heliconid species[73]), more nectar is offered than pollen. The flowers have simple nectar guides with the nectaries usually hidden in narrow tubes or spurs, reached by the long tongue of the butterflies. Butterflies like the Thymelicus flavus have been observed to engage in flower constancy, which means that they are more likely to transfer pollen to other conspecific plants. This can be beneficial for the plants being pollinated, as flower constancy prevents the loss of pollen during different flights and the pollinators from clogging stigmas with pollen of other flower species.[74]
Among the more important moth pollinators are the hawk moths (Sphingidae). Their behavior is similar to hummingbirds: Using rapid wing beats to keep hovered in front of flowers. Most being nocturnal or crepuscular, so moth-pollinated flowers (e.g., Silene latifolia ) tend to be white, night-opening, large and showy with tubular corollas and a strong, sweet scent produced in the evening, night or early morning. A lot of nectar is produced to fuel the high metabolic rates needed to power their flight.[75] Other moths (e.g., Noctuids, Geometrids, Pyralids) fly slowly and settle on the flower. They do not require as much nectar as the fast-flying hawk moths, and the flowers tend to be small (though they may be aggregated in heads).[76]
Mutualism

Mutualism is a form of biological interaction where each individual involves benefits in some shape or form. An example of a mutualistic relationship would be the relationship shared by yucca moths (Tegeculidae) and their host, yucca flowers (Liliaceae). Female yucca moths enter the host flowers, collect the pollen into a ball using speciallized maxillary palps, then move to the apex of the pistil where pollen is depostied on the stigma, and lay eggs into the base of the pistil where seeds will develop. The larvae develop in the fruit pod and feed on a portion of the seeds. Thus, both insect and plant benefit, forming a highly mutualistic relationship.[6]: 814 Another form of mutual association is between some larvae of butterflies and with certain species of ants (e.g., Lycaenidae). They communicate with the ants using vibrations that are transmitted through a substrate, such as the wood of a tree or stems, as well as using chemical signals.[77] The ants provide some degree of protection to these larvae and they in turn gather honeydew secretions.[78]
Parasitism
There are only 41 known species of parasitoid lepidoptera (1-Pyralidae; 40-Epipyropidae).[6]: 748 The larvae of the Greater and Lesser wax moths feed on the honeycomb inside bee nests and may become pests; they are also found in bumblebee and wasp nests, albeit to a lesser extant. In northern Europe the wax moth is regarded as the most serious parasitoid of the bumblebee, and is found only in bumblebee nests. In some areas in southern England as many as eighty percent of nests can be destroyed.[79] Other parasitic larvae are know to prey upon cicadas or leaf hoppers.[80]

In reverse, moths and butterflies may be subject to parasitic wasps and flies, which may lay eggs on the caterpillar which will eventually hatch kill it as they hatch inside its body and eat its tissues. Although, In a form of parasitism called idiobiont, the adult paralyzes the host, so not to kill it but for it to live as long as possible, so the parasitic larvae may benefit the most. Another form of parasitism, is Koinobiont, where they are endoparasitic. These parasites may live inside the host caterpillar through out all its life cycle, or may affect it latter on as adult. Most species that are koinobionts are all dipteran, a majority of coleopteran, and many hymenopteran parasitoids.[6]: 748–749 Some species may be subjected to a variety of parasites, such as the Gypsy moth (Lymantaria dispar) is attacked by a series of 13 species, in 6 different taxa through out its whole life cycle.[6]: 750
In response to a parsitoid egg or larvae in the caterpillars body, the plasmatocytes, or simply the host's cells forms a multilayered capsule that would eventually case the endoparasite to asphyxiate, and die. The process is called encapsulation, and is one of the caterpillars only means of defense against threats as parasitoids.[6]: 748
Other biological interactions
A few species of Lepidoptera are secondary consumers, or predators. These species typically prey upon the eggs of other insects, aphids, scale insects, or ant larvae.[6]: 567 Some caterpillars are cannibals, and others prey on caterpillars of other species (e.g. Hawai'ian Eupithecia ). Those of the 15 species in Eupithecia that mirror inchworms, are the only known species of butterflies and moths that are ambush predators.[81] There are 4 known species that eat snails. For example, the Hawai'ian caterpillar, (H. molluscivora), uses silk traps, in a manner similar to that of spiders to capture certain species of snails (typically Tornatellides).[80]
Larvae of some species of moths of Tineidae, Gelechioidae and Noctuidae, besides others, feed on detritus, or organic material that is not living, such as fallen leaves and fruit, fungi, and animal products and turn it into humus. [6]: 567 Well known species include the cloth moths (Tineola bisselliella, T. pellionella, and T. tapetzella), which feed on detritus containing keratin, including hair, feathers, cobwebs, bird nests (particularly of Domestic Pigeons, Columba livia domestica) and fruits or vegetables. These species are important to the natural ecosystme as they remove substances that would otherwise take a long time to decompose.[82]
Evolution and systematics
History of study

Linnaeus in Systema Naturae (1758) recognized three divisions of the Lepidoptera: Papilio, Sphinx and Phalaena, with seven subgroups in Phalaena.[83] These persist today as 9 of the superfamilies of Lepidoptera. Other works on classification followed including those by Michael Denis & Ignaz Schiffermüller (1775), Johan Christian Fabricius (1775) and Pierre André Latreille (1796). Jacob Hübner described many genera, and the Lepidopteran genera were catalogued by Ferdinand Ochsenheimer and Georg Friedrich Treitschke in a series of volumes on the Lepidopteran fauna of Europe published between 1807 and 1835.[83] Gottlieb August Wilhelm Herrich-Schäffer (several volumes, 1843–1856), and Edward Meyrick (1895) based their classifications primarily on wing venation. Sir George Francis Hampson worked on the 'Microlepidoptera' during this period and Philipp Christoph Zeller published The Natural History of the Tineinae also on Microlepidoptera (1855).
Among the first entomologists to study fossil insects and their evolution was Samuel Hubbard Scudder (1837–1911), who worked on butterflies.[84] He published a study of the Florissant deposits of Colorado, including the exceptionally preserved Prodryas persephone. Andreas V. Martynov (1879–1938) recognized the close relationship between Lepidoptera and Trichoptera in his studies on phylogeny.[84]
Major contributions in the 20th century included the creation of the monotrysia and ditrysia (based on female genital structure) by Borner in 1925 and 1939.[83] Willi Hennig (1913–1976) developed the cladistic methodology and applied it to insect phylogeny. Niels P. Kristensen, E. S. Nielsen and D. R. Davis studied the relationships among monotrysian families and Kristensen worked more generally on insect phylogeny and higher Lepidoptera too.[83][84] While it is often found that DNA-based phylogenies differ from those based on morphology, this has not been the case for the Lepidoptera; DNA phylogenies correspond to a large extent to morphology-based phylogenies.[84]
Many attempts have been made to group the superfamilies of the Lepidoptera into natural groups, most of which fail because one of the two groups is not monophyletic: Microlepidotera and Macrolepidoptera, Heterocera and Rhopalocera, Jugatae and Frenatae, Monotrysia and Ditrysia.[83]
Fossil record
Not much is known about ancient Lepidoptera species because so few fossils have been found. The earliest known lepidopteran fossil, Archaeolepis mane is from the Jurassic period, about 190 million years ago. The fossil consists of a pair of wings with scales that are characteristically similar to the wing venation pattern found in Trichoptera (caddisflies). 2 other sets of Jurassic Lepidopteran fossils have been found, and 13 sets from the Cretaceous period.[84] The best preserved fossil lepidopteran is the Eocene Prodryas persephone from the Florissant Fossil Beds.
The fossil record for Lepidoptera is lacking in comparison to other winged species, and tending not to be as common as some other insects in the habitats that are most conducive to fossilization, such as lakes and ponds, and their juvenile stage has only the head capsule as a hard part that might be preserved. The location and abundance of the most common moth species are indicative that mass migrations of moths occurred over the Palaeogene North Sea, which is why there is a serious lack of moth fossils.[85] Yet there are fossils, some preserved in amber and some in very fine sediments. Leaf mines are also seen in fossil leaves, although the interpretation of them is tricky.[84]
The earliest fossil is Archaeolepis mane from the Jurassic, about 190 million years ago in Dorset, UK.[84] Being a small primitive moth-like species, consisting of wings and showing scales with parallel grooves under a scanning electron microscope and a characteristic wing venation pattern shared with Trichoptera (Caddisflies).[84] Only two more sets of Jurassic lepidopteran fossils have been found, and 13 sets in the Cretaceous.[84] From there, many more fossils are found from the Tertiary, and particularly the Eocene Baltic amber. These early species are believed to be related to the sister group, Trichoptera, with fossils being found from the Triassic, where the two are believed to have branched during the Mesozoic. [6]: 567
Phylogeny

Lepidoptera and Trichoptera (caddisflies) are more closely related than any other taxa, sharing many similarities that are lacking in other insect orders; for example the females of both orders are heterogametic, meaning they have two different sex chromosomes, whereas in most species the males are heterogametic and the females have two identical sex chromosomes. The adults in both orders display a particular wing venation pattern on their forewings. The larvae of both orders have mouth structures and gland with which they make and manipulate silk. Willi Hennig grouped the two sister orders into the Amphiesmenoptera superorder. This group probably evolved in the Jurassic, having split from the now extinct order Necrotaulidae.[84]
Micropterigidae, Agathiphagidae and Heterobathmiidae are the oldest and most basal lineages of Lepidoptera. The adults of these families do not have the curled tongue or proboscis that are found in most members order, but instead have chewing mandibles adapted for a special diet. Micropterigidae larvae feed on leaves, fungi, or liverworts (much like the Trichoptera).[83] Adult Micropterigidae chew the pollen or spores of ferns. In the Agathiphagidae, larvae live inside kauri pines and feed on seeds. In Heterobathmiidae the larvae feed on the leaves of Nothofagus, the southern beech tree. These families also have mandibles in the pupal stage, which help the pupa emerge from the seed or cocoon after metamorphosis.[83]
The Eriocraniidae have a short coiled proboscis in the adult stage, and though they retain their pupal mandibles with which they escaped the cocoon, their mandibles are non-functional thereafter.[83] Most of these non-ditrysian families, are primarily leaf miners in the larval stage. In addition to the proboscis, there is a change in the scales among these basal lineages, with later lineages showing more complex perforated scales.[84]
With the evolution of the Ditrysia in the mid-Cretaceous, there was a major reproductive change. The Ditrysia, which comprise 98% of the Lepidoptera, have two separate openings for reproduction in the females (as well as a third opening for excretion), one for mating, and one for laying eggs. The two are linked internally by a seminal duct. (In more basal lineages there is one cloaca, or later, two openings and an external sperm canal.) Of the early lineages of Ditrysia, Gracillarioidea and Gelechioidea are mostly leaf miners, but more recent lineages feed externally. In the Tineoidea, most species feed on plant and animal detritus and fungi, and build shelters in the larval stage.[84]
The Yponomeutoidea is the first group to have significant numbers of species whose larvae feed on herbaceous plants, as opposed to woody plants.[84] They evolved about the time that flowering plants underwent an expansive adaptive radiation in the mid-Cretaceous, and the Gelechioidea that evolved at this time also have great diversity. Whether the processes involved co-evolution or sequential evolution, the diversity of the Lepidoptera and the angiosperms increased together.
In the so-called "Macrolepidoptera", which constitutes about 60% of lepidopteran species, there was a general increase in size, better flying ability (via changes in wing shape and linkage of the forewings and hindwings), reduction in the adult mandibles, and a change in the arrangement of the crochets (hooks) on the larval prolegs, perhaps to improve the grip on the host plant.[84] Many also have tympanal organs, that allow them to hear. These organs evolved eight times, at least, because they occur on different body parts and have structural differences.[84] The main lineages in the Macrolepidoptera are the Noctuoidea, Bombycoidea, Lasiocampidae, Mimallonoidea, Geometroidea and Rhopalocera. Bombycoidea plus Lasiocampidae plus Mimallonoidea may be a monophyletic group.[84] The Rhopalocera, comprising the Papilionoidea (butterflies), Hesperioidea (skippers), and the Hedyloidea (moth-butterflies), are the most recently evolved.[83] There is quite a good fossil record for this group, with the oldest skipper dating from 56 million years ago.[84]
Taxonomy
Taxonomy is the classification of spieces in selected taxa, the process of naming being called nomenclature. There are over 120 families in lepidoptera, in 45 to 48 superfamilies. Lepidoptera have always been, historically, classified in five suborders, one of which is of primitive moths that never lost the morphological features of its ancestors. The rest o f the moths and butterflies make up ninety-eight percent of the other taxa, making Ditrysia. More recently, new findings of new taxa and larvae and pupa have aided in detailing the relationships of primitve taxa, phylogenetic analasys showing the primitive lineages to be paraphyletic compared to the rest of Lepidoptera lineages. Recently Lepidopterologist have abandoned clades like suborders, and those between orders and superfamilies. [6]: 569
Relationship to people
In culture

Artistic depictions of butterflies have been used in many cultures including as early as 3500 years ago, in Egyptian hieroglyphs.[86] Today, butterflies are widely used in various objects in art and jewelry: mounted in frames, embedded in resin, displayed in bottles, laminated in paper, and in some mixed media artworks and furnishings.[87] Butterflies have also inspired the "butterfly fairy" as an art and fictional character, including in the Barbie Mariposa film.
In many cultures the soul of a dead person is associated with the butterfly. As in Ancient Greece, where the word for butterfly ψυχή (psyche) also means soul and breath. In Latin, as in Ancient Greece, the word for "butterfly" papillio was associated with the soul of the dead.[88] The skull-like marking on the thorax of the Death's-head Hawkmoth has helped these moths, particularly A. atropos, earn a negative reputation, such as associations with the supernatural and evil. The moth has been prominently featured in art and movies such as Un Chien Andalou (by Buñuel and Dalí) and The Silence of the Lambs, and in the artwork of the Japanese metal band Sigh's album Hail Horror Hail. According to Kwaidan: Stories and Studies of Strange Things, by Lafcadio Hearn, a butterfly was seen in Japan as the personification of a person's soul; whether they be living, dying, or already dead. One Japanese superstition says that if a butterfly enters your guestroom and perches behind the bamboo screen, the person whom you most love is coming to see you. However, large numbers of butterflies are viewed as bad omens. When Taira no Masakado was secretly preparing for his famous revolt, there appeared in Kyoto so vast a swarm of butterflies that the people were frightened — thinking the apparition to be a portent of coming evil.[89]
In the ancient Mesoamerican city of Teotihuacan, the brilliantly colored image of the butterfly was carved into many temples, buildings, jewelry, and emblazoned on incense burners in particular. The butterfly was sometimes depicted with the maw of a jaguar and some species were considered to be the reincarnations of the souls of dead warriors. The close association of butterflies to fire and warfare persisted through to the Aztec civilization and evidence of similar jaguar-butterfly images has been found among the Zapotec, and Maya civilizations.[90]
As pests
The larvae of many Lepidopteran species are major pests in agriculture. Some of the major pests include Tortricidae, Noctuidae, and Pyralidae. The larvae of the Noctuidae genus Spodoptera (armyworms) and Helicoverpa (corn earworm) can cause extensive damage to certain crops.[83] Helicoverpa zea larvae (cotton bollworms or tomato fruitworms) are polyphagous, meaning they eat a variety of crops, including tomatoes and cotton.[91]
Butterflies and moths are one of the largest taxa to solely feed and be dependent on living plants, in terms of the number of species, and they are in many ecosystems make up the largest biomass to do so. In many species, the female may produce anywhere from 200 to 600 eggs, while in some others it may go as high as 30,000 eggs in one day. This creates many problems for agriculture, where many caterpillars can mow down acres of vegetation. Some reports estimate that there have been over 80,000 caterpillars of several different taxa feeding on a single oak tree. In some cases, phytophagous larvae can lead to the destruction of entire trees in relatively short periods of time.[6]: 567
Ecological ways of removing pest lepidoptera species are becoming more economically viable, as research has shown ways like introducing parasitic wasp and flies. For example Sarcophaga aldrichi, which the larvae feed upon the larvae of the Forest Tent Caterpillar Moth. Pesticides can affect other species other than the species they are targeted to eliminate, damaging the natural ecosystem. [92] Another good biological pest control method is the use of pheromone traps. A pheromone trap is a type of insect trap that uses pheromones to lure insects. Sex pheromones and aggregating pheromones are the most common types used. A pheromone-impregnated lure is encased in a conventional trap such as a Delta trap, water-pan trap, or funnel trap.[93]
Species of moths that are detrivores would naturally eat detritus containing keratin, such as hairs or feathers. Well known species are cloth moths (T. bisselliella, T. pellionella, and T. tapetzella), feeding on foodstuffs that people find economically important, such cotton, linen, silk and wool fabrics as well as furs; furthermore they have been found on shed feathers and hair, bran, semolina and flour (possibly preferring wheat flour), biscuits, casein, and insect specimens in museums.[82]
As beneficial
Even though most butterflies and moths affect the economy negatively, some species are a valuable economic resource. The most prominent example is that of the Domesticated silkworm moth (Bombyx mori), the larvae of which make their cocoons out of silk which can be spun into cloth. Silk is and has been an important economic resource throughout history. The species Bombyx mori has been domesticated to the point where it is completely dependent on mankind for survival.[94] A number of wild moths such as Bombyx mandarina, and Antheraea species, besides others, provide commercially important silks.[95]
The preference of the larvae of most Lepidopteran species to feed on a single species or limited range of plants is used as a mechanism for biological control of weeds in place of herbicides. The pyralid cactus moth was introduced from Argentina to Australia, where it successfully suppressed millions of acres of Prickly pear cactus.[6]: 567 Another species of the Pyralidae, called the alligator weed stem borer (Arcola malloi), was used to control the aquatic plant known as alligator weed (Alternanthera philoxeroides) in conjunction with the alligator weed flea beetle; in this case, the two insects work in synergy and the weed rarely recovers.[96]
Breeding butterflies and moths, or butterfly gardening, has become an ecologically viable process of introducing species into the ecosystem for the better of benefiting it. Butterfly ranching in Papua New Guinea permits nationals of that country to 'farm' economically valuable insect species for the collectors market in an ecologically sustainable manner.[97]
As food

Lepidoptera feature prominently in entomophagy as food items on almost every continent. While in most cases, adults, larvae or pupae are eaten as staples by indigenous people, beondegi or silkworm pupae are eaten as a snack in Korean cuisine[98] while Maguey worm is considered a delicacy in Mexico.[99] In the Carnia region of Italy, children catch and eat Zygaena moths in early summer. The ingluvies, despite having a very low cyanogenic content, serves as a convenient, supplementary source of sugar to the children who can include this resource as a seasonal delicacy at minimum risk.[100]
Health
Some larvae of both moths and butterflies have a form of hair that has been known to be a cause of human health problems. Caterpillar hairs sometimes have venomous toxins in them and species from approximately 12 families of moths or butterflies worldwide can inflict serious human injuries (Urticarial dermatitis and atopic asthma to osteochondritis, consumption coagulopathy, renal failure, and intracerebral hemorrhage).[101] Skin rashes are the most common, but there have been fatalities.[102] Lonomia is a frequent cause of economization in humans in Brazil, with 354 cases reported between 1989 and 2005. Lethality ranging up to 20% with death caused most often by intracranial hemorrhage.[103]
These hairs have also been known to cause kerato-conjunctivitis. The sharp barbs on the end of caterpillar hairs can get lodged in soft tissues and mucus membranes such as the eyes. Once they enter such tissues, they can be difficult to extract, often exacerbating the problem as they migrate across the membrane.[104] This becomes a particular problem in an indoor setting. The hairs easily enter buildings through ventilation systems and accumulate in indoor environments because of their small size, which makes it difficult for them to be vented out. This accumulation increases the risk of human contact in indoor environments.[105]
See also
- Taxonomy of the Lepidoptera
- Differences between butterflies and moths
- Societas Europaea Lepidopterologica
- McGuire Center for Lepidoptera and Biodiversity, University of Florida
Lists
- List of moths
- List of butterflies in Taiwan
- List of butterflies of Great Britain
- List of butterflies of Tobago
- List of butterflies of Menorca
- List of butterflies of India
- List of butterflies of North America
- List of Australian butterflies
References
- ^ a b c d Harper, Douglas. "lepidoptera". The Online Etymology Dictionary. Retrieved 8 February 2011. Cite error: The named reference "Etymology" was defined multiple times with different content (see the help page).
- ^ a b c Mallet, Jim (12 June 2007). "Taxonomy of Lepidoptera: the scale of the problem". The Lepidoptera Taxome Project. University College, London. Retrieved 8 February 2011.
- ^ a b c d Capinera, John L. (2008). "Butterflies and moths". Encyclopedia of Entomology. Vol. 4 (2nd ed.). Springer. pp. 626–672. ISBN 978140206242.
{{cite book}}: Check|isbn=value: length (help) - ^ a b Kristensen, Niels P.; Scoble, M. J. & Karsholt, Ole (2007). "Lepidoptera phylogeny and systematics: the state of inventorying moth and butterfly diversity". In Z.-Q. Zhang & W.A. Shear (ed.). Linnaeus Tercentenary: Progress in Invertebrate Taxonomy (Zootaxa:1668) (PDF). Magnolia Press. pp. 699–747. ISBN 9780126906479. Retrieved March 2, 2010.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Powell, Jerry A. (2009). "Lepidoptera". In Resh, Vincent H.; Cardé, Ring T. (eds.). Encyclopedia of Insects (2 (illustrated) ed.). Academic Press. p. 1132. ISBN 9780123741448. Retrieved 14 November 2010.
{{cite book}}: Cite has empty unknown parameter:|chapterurl=(help) - ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag Resh, Vincent H. (July 1, 2009). Encyclopedia of Insects (2 ed.). U.S.A.: Academic Press. ISBN 0123741440.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: year (link) - ^ a b Arnett, Ross H. (July 28, 2000). "Part I: 27". American insects: a handbook of the insects of America north of Mexico (2nd ed.). CRC Press. p. 631. ISBN 0849302129. Cite error: The named reference "Arnett" was defined multiple times with different content (see the help page).
- ^ "Caterpillar". Dictionary.com. The American Heritage Dictionary of the English Language, Fourth Edition. Houghton Mifflin Company, 2004. (accessed: March 26, 2008).
- ^ a b c d e f g h i j k Gullan, P. J. (September 13, 2004). "7". The insects: an outline of entomology (3 ed.). Wiley-Blackwell. pp. 198–199. ISBN 1405111135.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Stumpe, Felix. "Parnassius arctica Eisner, 1968". Russian-Insects.com. Retrieved 9 November 2010.
- ^ Mani, M. S. (1968). Ecology and Biogeography of High Altitude Insects. Volume 4 of Series entomologica. Springer. p. 530. ISBN 9789061931140. Retrieved 9 November 2010.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Sherman, Lee. ""An OSU scientist braves an uncharted rainforest in a search for rare and endangered species" in "Expedition to the Edge"". Terra, Spring 2008. Oregon State University. Retrieved 14 February 2011.
- ^ a b Rau, P (1941). "Observations on certain lepidopterous and hymenopterous parasites of Polistes wasps". Annals of the Entomological Society of America. 34: 355-366(12). Retrieved 14 February 2011.
- ^ Mallet, Jim (12 June 2007). "Taxonomy of butterflies: the scale of the problem". The Lepidoptera Taxome Project. University College, London. Retrieved 8 February 2011.
- ^ Eaton, Eric R. (2007). Kaufman field guide to insects of North America. Houghton Mifflin Harcourt. p. 228. ISBN 9780618153107. Retrieved 12 February 2011.
{{cite book}}: More than one of|pages=and|page=specified (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Tuskes, Paul M. (1996). The wild silk moths of North America: a natural history of the Saturniidae of the United States and Canada. The Cornell series in arthropod biology (illustrated ed.). Cornell University Press. p. 1. ISBN 9780801431302. Retrieved 12 February 2011.
{{cite book}}: More than one of|pages=and|page=specified (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Green, Ken (1994). Wildlife of the Australian snow-country: a comprehensive guide to alpine fauna (illustrated ed.). Reed. p. 162. ISBN 9780730104612.
{{cite book}}: More than one of|pages=and|page=specified (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d Gillot, C. (1995). "Butterflies and moths". Entomology (2 ed.). pp. 246–266. ISBN 9780306449673. Retrieved 14 November 2010.
{{cite book}}: Cite has empty unknown parameters:|chapterurl=and|coauthors=(help) - ^ a b c d Scoble (1995). Section The Adult Head - Feeding and Sensation, (pp 4 to 22).
- ^ Christopher, O'Toole. Firefly Encyclopedia of Insects and Spiders (1 ed.). ISBN 1-55297-612-2.
- ^ Heppner, J.B. (2008). "Butterflies and moths". In Capinera, John L. (ed.). Encyclopedia of Entomology. Gale virtual reference library. Vol. 4 (2 ed.). Springer Reference. p. 4345. ISBN 9781402062421. Retrieved 14 November 2010.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ a b Scoble, MJ. (1992). The Lepidoptera: Form, function, and diversity. Oxford Univ. Press. ISBN 9781402062421. Cite error: The named reference "Scoble" was defined multiple times with different content (see the help page).
- ^ a b Scoble (1995). Section Scales, (pp 63 - 66).
- ^ Vukusic, P. (2006). "Structural color in Lepidoptera" (PDF). Current Biology. 16 (16): R621–3. doi:10.1016/j.cub.2006.07.040. PMID 16920604. Retrieved 11 November 2010.
- ^ Williams, C.M. 1947. Physiology of insect diapause. II. Interaction between the pupal brain and prothoracic glands in the metamorphosis of the giant silkworm "Platysamia cecropia". Biol. Bull. 92:89-180.
- ^ a b c Cite error: The named reference
Gullanwas invoked but never defined (see the help page). - ^ Lighton J.R.B. & Lovegrove B.G. (1990) “A temperature-induced switch from diffusive to convective ventilation in the honeybee,” Journal of Experimental Biology, vol. 154, pp. 509-516
- ^ Ford, E. B. (1965). Genetic polymorphism. Oxford University Press. p. 11.
- ^ a b c Gullan & Cranston (2005). "Polymorphism and polyphenism". pp. 163–164.
{{cite book}}: Missing or empty|title=(help) - ^ a b Kunte, Krushnamegh (2000).Butterflies of Peninsular India. Part of Project lifescape.Orient Blackswan. ISBN 8173713545, ISBN 9788173713545.
- ^ "Psychidae at Bug Guide". Iowa State University. Retrieved January 19, 2010.
- ^ Ivy I. G., Morgun D. V., Dovgailo K. E., Rubin N. I., Solodovnikov I. A. Дневные бабочки (Hesperioidea and Papilionoidea, Lepidoptera) Восточной Европы." CD determinant, database and software package «Lysandra». Minsk, Kiev, Moscow: 2005. In Russian
- ^ Noor M. A., Parnell R. S., Grant B. S. (2008). "A reversible color polyphenism in American Peppered Moth (Biston betularia cognataria) caterpillars". PLoS ONE. 3 (9): e3142. doi:10.1371/journal.pone.0003142. PMC 2518955. PMID 18769543.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Ritland, D. B. (1991). "The viceroy butterfly is not a Batesian mimic". Nature. 350 (6318): 497–498. doi:10.1038/350497a0.
Viceroys are as unpalatable as monarchs, and significantly more unpalatable than queens from representative Florida populations.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Meyer, A. (2006). "Repeating patterns of mimicry". PLoS Biology. 4 (10): e341. doi:10.1371/journal.pbio.0040341. PMC 1617347. PMID 17048984.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Sanderford, M. V. (July, 1990). "Courtship sounds of the polka-dot wasp moth, Syntomeida epilais" (PDF). Naturwissenschaften. 77 (7). Berlin / Heidelberg: Springer: 345–347. doi:10.1007/BF01138395.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Wiklund, Christer (July, 1984). "Egg-laying patterns in butterflies in relation to their phenology and the visual apparency and abundance of their host plants" (PDF). Oecologia. 63 (1): 23–29. doi:10.1007/BF00379780. OCLC 0029-8549.
{{cite journal}}: Check|oclc=value (help); Check date values in:|date=(help) - ^ Dugdale, J.S. (1996). "Natural history and identification of litter-feeding Lepidoptera larvae (Insecta) in beech forests, Orongorongo Valley, New Zealand, with especial reference to the diet of mice (Mus musculus)" (PDF). Journal of the Royal Society of New Zealand. 26 (4): 251–274. doi:10.1080/03014223.1996.9517513. Retrieved 14 November 2010.
- ^ Ehrlich, P. R.; Raven, P. H. (1964). "Butterflies and plants: a study in coevolution". Evolution. 18 (4): 586–608. doi:10.2307/2406212.
- ^ a b Nijhout, H.Frederik (August 17 1991). The Development and Evolution of Butterfly Wing Patterns(Smithsonian Series in Comparative Evolutionary Biology) (1 ed.). Smithsonian Institution Scholarly Press. pp. 2–4. ISBN 0874749174.
{{cite book}}: Check date values in:|date=(help) - ^ a b Dole, Claire Hagen (May 28, 2003). The Butterfly Gardener's Guide. Brooklyn Botanic Garden. ISBN 1889538582.
- ^ Ward, JV & Peter. Insct Ecology.
- ^ Benjamin Jantzen*, Thomas Eisner (July 28, 2008). "". The National Academy of Sciences of the USA. 105 no. 43: summery.
{{cite journal}}: External link in|title= - ^ "Skippers Butterflies and Moths: Lepidoptera - Behavior And Reproduction". Net Industries and its Licensors. 2011. Retrieved 20 February 2011.
- ^ Alex Reisner. "Speed of animals". Retrieved 20 February 2011.
- ^ Sauman, Ivo (May 5, 2005). "Connecting the Navigational Clock to Sun Compass Input in Monarch Butterfly Brain". Neuron. 46 (3): 457–467. doi:10.1016/j.neuron.2005.03.014. PMID 15882645.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Southwood, T. R. E. (1962). "Migration of terrestrial arthropods in relation to habitat". Biological Reviews. 37 (2). Cambridge Philosophical Society: 171–211. doi:10.1111/j.1469-185X.1962.tb01609.x.
- ^ Dennis, Roger L. H. (September 2005). "Does diet breadth control herbivorous insect distribution size? Life history and resource outlets for specialist butterflies" (PDF). Journal of Insect Conservation. 9 (3). Springer Netherlands: 187–200. doi:10.1007/s10841-005-5660-x.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Made, J.G. van der (1989). Actie voor Vlinders, zo kunnen we ze redden (in Dutch). Weert: M & P cop. p. 192. ISBN 9065903038.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Baker, R. Robin (February 1987). "Integrated use of moon and magnetic compasses by the heart-and-dart moth, Agrotis exclamationis". Animal Behaviour. 35 (1): 94–101. doi:10.1016/S0003-3472(87)80214-2.
- ^ Breen, Amanda (May 7, 2008). "Scientists make compass discovery in migrating moths". University of Greenwich at Medway. p. 1. Retrieved December 9, 2009.
- ^ a b c Chapman, Jason W. (8 April 2008). "Wind selection and drift compensation optimize migratory pathways in a high-flying moth" (PDF). Current Biology. 18 (7): 514–518. doi:10.1016/j.cub.2008.02.080. PMID 18394893.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Cite error: The named reference "Chapman" was defined multiple times with different content (see the help page). - ^ Srygley, Robert B. (2005). "Experimental evidence for a magnetic sense in Neotropical migrating butterflies (Lepidoptera: Pieridae)" (PDF). The British Journal of Animal Behaviour. 71 (1): 183–191. doi:10.1016/j.anbehav.2005.04.013. ISSN 0003-3472.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Elliot, Debbie (August 18, 2007). "Why are Moths Attracted to Flame? (audio)". National Public Radio. p. 1. Retrieved December 12, 2009.
{{cite news}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hsiao, Henry S. (1972). Attraction of moths to light and to infrared radiation. San Francisco Press. ISBN 0911302212.
- ^ Williams, C. B. (1927). "A study of butterfly migration in south India and Ceylon, based largely on records by Messrs. G. Evershed, E. E. Green, J. C. F. Fryer and W. Ormiston". Transactions of the Royal Entomological Society of London. 75 (1): 1–33. doi:10.1111/j.1365-2311.1927.tb00054.x.
- ^ Urquhart, F. A. & N. R. Urquhart. 1977. Overwintering areas and migratory routes of the Monarch butterfly (Danaus p. plexippus, Lepidoptera: Danaidae) in North America, with special reference to the western population. Can. Ent. 109: 1583–1589
- ^ Wassenaar L. I. & K. A. Hobson (1998). "Natal origins of migratory monarch butterflies at wintering colonies in Mexico: new isotopic evidence". Proceedings of the National Academy of Sciences. 95 (26): 15436–15439. doi:10.1073/pnas.95.26.15436. PMC 28060. PMID 9860986.
- ^ Smith, N. G. (1983). Urania fulgens (Calipato Verde, Green Urania). Costa Rican Natural History. Chicago: University of Chicago Press. p. 816.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Meyer, John R. (2006). "Acoustic Communication". Department of Entomology, C State University. Retrieved 25 February 2011.
- ^ Chapman, R.F. (1998). The Insects; Structure and Function (4 ed.). Cambridge University Press. p. 403. ISBN 0521 57048.
{{cite book}}: Check|isbn=value: length (help) - ^ Tauber, M.J. (1986). Seasonal Adaptations of Insects (4 ed.). Oxford University Press. p. 414. ISBN 0521 57048.
{{cite book}}: Check|isbn=value: length (help) - ^ a b c d "Caterpillar and Butterfly Defense Mechanisms". EnchantedLearning.com. Retrieved December 7, 2009.
- ^ Kricher, John (August 16, 1999). "6". A Neotropical Companion. Princeton University Press. pp. 157–158. ISBN 9780691009742.
- ^ Santos, J.C.; Cannatella, D.C. (2003). "Multiple, recurring origins of aposematism and diet specialization in poison frogs". Proceedings of the National Academy of Sciences. 100 (22). http://www.pnas.org: 12792–12797. doi:10.1073/pnas.2133521100. Retrieved 21 November 2010.
{{cite journal}}: External link in|publisher= - ^ "osmeterium". Merriam-Webster, Incorporated. Retrieved December 9, 2009.
- ^ Hadley, Debbie. "Osmeterium". About.com Guide. Retrieved December 9, 2009.
- ^ Latimer, Jonathan P. (May 30, 2000). Butterflies. Houghton Mifflin Harcourt Trade & Reference Publis. p. 12. ISBN 0395979447.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Insects and Spiders of the World,. Vol. 10. Marshall Cavendish Corporation. Marshall Cavendish. January 2003. pp. 292–293. ISBN 0761473440.
{{cite book}}: CS1 maint: others (link) - ^ Carroll, Sean (2005). Endless forms most beautiful: the new science of evo devo and the making of the animal kingdom. W. W. Norton & Co. pp. 205–210. ISBN 0393060160.
- ^ Jones, G (2000). "Moth hearing in response to bat echolocation calls manipulated independently in time and frequency". Proceedings of the Royal Society B Biological Sciences. 267 (1453): 1627. doi:10.1098/rspb.2000.1188. PMC 1690724. PMID 11467425.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ratcliffe1, John M. (2009). "". Biology Letters. 5: 368–371. doi:[https://doi.org/doi%3A10.1098%2Frsbl.2009.0079 doi:10.1098/rsbl.2009.0079.
{{cite journal}}:|access-date=requires|url=(help); Check|doi=value (help); External link in|title= - ^ a b Gilbert L. E. (1972). "Pollen feeding and reproductive biology of Heliconius butterflies". Proceedings of the National Academy of Sciences. 69: 1402–1407. doi:10.1073/pnas.69.6.1403.
- ^ "Foraging strategies in the small skipper butterfly, Thymelicus flavus: when to switch?". Animal Behavior. 53: 1009–1016. 1997.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Helen J. Young and Lauren Gravitz (2002). "The effects of stigma age on receptivity in Silene alba (Caryophyllaceae)". American Journal of Botany. 89: 1237–1241.
- ^ Oliveira PE, PE Gibbs, and AA Barbosa (2004). "Moth pollination of woody species in the Cerrados of Central Brazil: a case of so much owed to so few?". Plant Systematics and Evolution. 245 (1–2): 41–54. doi:10.1007/s00606-003-0120-0.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Devries, P.J. (1988). "The larval ant-organs of Thisbe irenea (Lepidoptera: Riodinidae) and their effects upon attending ants". Zoological Journal of the Linnean Society. 94: 379. doi:10.1111/j.1096-3642.1988.tb01201.x.
- ^ Devries, Pj (1990). "Enhancement of Symbioses Between Butterfly Caterpillars and Ants by Vibrational Communication". Science. 248 (4959): 1104–1106. doi:10.1126/science.248.4959.1104. PMID 17733373.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Benton, Frank (1895). The honey bee: a manual of instruction in apiculture. Vol. 1–6, 33. Oestergaard Verlag. pp. 113–114.
{{cite book}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) - ^ a b Rubinoff, D; Haines, WP (2005). "Web-spinning caterpillar stalks snails". Science. 309 (5734): 575. doi:10.1126/science.1110397. PMID 16040699.
{{cite journal}}: More than one of|author=and|last1=specified (help) - ^ Pierce, N.E. (1995). "Predatory and parasitic Lepidoptera: Carnivores living on plants". Journal of the Lepidopterist's Society. 49 (4): 412–453.
- ^ a b Grabe, Albert (1942). Eigenartige Geschmacksrichtungen bei Kleinschmetterlingsraupen ("Strange tastes among micromoth caterpillars") (PDF). 27 (in German). pp. 105–109.
- ^ a b c d e f g h i j Scoble, Malcolm J. (September 1995). "2". The Lepidoptera: Form, Function and Diversity (1 ed.). Oxford University: Oxford University Press. pp. 4–5. ISBN 0198549520.
- ^ a b c d e f g h i j k l m n o p q Grimaldi, D. and Engel, M. S. (2005). Evolution of the Insects. Cambridge University Press. ISBN 0-521-82149-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Rust, Jest (2000). "Palaeontology: Fossil record of mass moth migration". Nature. 405: 530–531. doi:doi:10.1038/35014733. Retrieved 22 February 2011.
{{cite journal}}: Check|doi=value (help) - ^ Larsen, Torben B. (1994). "Butterflies of Egypt". Saudi Aramco world. 45 (5). Saudi Aramco world: 24–27. Retrieved December 18, 2009.
- ^ "Table complete with real butterflies embedded in resin". Mfjoe.com. December 18, 2009. Retrieved March 30, 2003.
- ^ Rabuzzi, Matthew (November 1997). "Butterfly Etymology Cultural Entomology Digest 4". Cupertino, California: Bugbios. p. 4. Retrieved December 18, 2009.
- ^ Hearn, Lafcadio (1904). Kwaidan: Stories and Studies of Strange Thing. Dover Publications, Inc. ISBN 0-486-21901-1.
- ^ Miller, Mary (1993). The Gods and Symbols of Ancient Mexico and the Maya. Thames & Hudson. ISBN 978-0-500-27928-1.
- ^ Cook, Kelly A. (2004). "IPM: Field Crops: Corn Earworm (Heliothis Zea)". IPM. p. 1. Retrieved January 17, 2009.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Jeff Hahn (June 15, 2003). "Friendly Flies: Good News, Bad News". Yard & Garden Line News. 5 (9). University of Minnesota.
- ^ R. Weinzierl, T. Henn, P. G. Koehler and C. L. Tucker (June 2005). "Insect Attractants and Traps". Alternatives in Insect Management. Entomology and Nematology Department, University of Florida. Office of Agricultural Entomology, University of Illinois at Urbana-Champaign.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Goldsmith M. R., T. Shimada & H. Abe (2005). "The genetics and genomics of the silkworm, Bombyx mori". Annual Review of Entomology. 50: 71–100. doi:10.1146/annurev.ento.50.071803.130456. PMID 15355234.
- ^ Yoshitake, N. (1968). "Phylogenetic aspects on the origin of Japanese race of the silkworm, Bombyx mori". Journal of Sericological Sciences of Japan. 37: 83–87.
- ^ Coombs, E. M. (2004). Biological Control of Invasive Plants in the United States. Corvallis: Oregon State University Press. p. 146.
- ^ http://www.butterfliesandart.com/Butterfly_Farms/Butterfly_Farms.htm
- ^ Martin Robinson, Ray Bartlett, Rob Whyte. Korea (2007). Lonely Planet publications, ISBN 9781741045581. (pg 63)
- ^ http://www.insectia.com/beta/e/dr_c2508724.html
- ^ Mika Zagrobelny, Angelo Leandro Dreon, Tiziano Gomiero, Gian Luigi Marcazzan, Mikkel Andreas Glaring, Birger Lindberg Møller & Maurizio G. Paoletti (2009). "Toxic moths: source of a truly safe delicacy". Journal of Ethnobiology. 29 (1): 64–76. doi:10.2993/0278-0771-29.1.64.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Diaz, HJ (2005). "The evolving global epidemiology, syndromic classification, management, and prevention of caterpillar envenoming". Am. J. Trop. Med. Hyg. 72 (3): 347–357. PMID 15772333.
- ^ Redd, JT; Voorhees, RE; Török, TJ (2007). "Outbreak of lepidopterism at a Boy Scout camp". Journal of the American Academy of Dermatology. 56 (6): 952–955. doi:10.1016/j.jaad.2006.06.002. PMID 17368636.
{{cite journal}}: More than one of|author=and|last1=specified (help) - ^ Kowacs, PA; Cardoso, J; Entres, M; Novak, EM; Werneck, LC (2006). "Fatal intracerebral hemorrhage secondary to Lonomia obliqua caterpillar envenoming: case report" (Free full text). Arquivos de neuro-psiquiatria. 64 (4): 1030–2. doi:10.1590/S0004-282X2006000600029. PMID 17221019.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Patel RJ, Shanbhag RM (1973). "Ophthalmia nodosa – (a case report)". Indian J Ophthalmol. 21 (4): 208.
- ^ Corrine R Balit, Helen C Ptolemy, Merilyn J Geary, Richard C Russell and Geoffrey K Isbister, CR (2001). "Outbreak of caterpillar dermatitis caused by airborne hairs of the mistletoe browntail moth (Euproctis edwardsi)" (Free full text). The Medical journal of Australia. 175 (11–12): 641–3. ISSN 0025-729X. PMID 11837874.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help)CS1 maint: multiple names: authors list (link)
Further reading
- Kristensen, N. P. (Ed.) 1999. Lepidoptera, Moths and Butterflies. Volume 1: Evolution, Systematics, and Biogeography. Handbuch der Zoologie. Eine Naturgeschichte der Stämme des Tierreiches / Handbook of Zoology. A Natural History of the phyla of the Animal Kingdom. Band / Volume IV Arthropoda: Insecta Teilband / Part 35: 491 pp. Walter de Gruyter, Berlin, New York.
- Nye, I. W. B. & Fletcher, D. S. 1991. Generic Names of Moths of the World. Volume 6: xxix + 368 pp. Trustees of the British Museum (Natural History), London.
- O'Toole, Christopher. 2002. Firefly Encyclopedia of Insects and Spiders. ISBN 1-55297-612-2.
- Nemos, F. (c. 1895). Europas bekannteste Schmetterlinge. Beschreibung der wichtigsten Arten und Anleitung zur Kenntnis und zum Sammeln der Schmetterlinge und Raupen (PDF). Berlin: Oestergaard Verlag.
{{cite book}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) - Walsh, P. M., T. Boyd, D. W. Nash, E. Rolston & A. Tyner (2009). "Report on migrant and notable Lepidoptera in Ireland, 2006". Irish Naturalists' Journal. 30: 40–50.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Lepidoptera Report 2008.p. 19 - 20. in Annual Report for 2008 Copeland Bird Observatory.
External links
- European Butterflies and Moths by Christopher Jonko
- Polish Butterflies and Moths by motylpodlaski.pl
- Norwegian Butterflies and Moths (Huge picture archive)
- Moths and Butterflies of Europe and North Africa
- British Butterflies and Moths
- Butterflies of Bulgaria
- Photography of European Butterflies and Moths
- Butterflies and Moths in the Netherlands
- Swedish Moths and Butterflies Lepidoptera.se
- Butterflies of Asturias – Spain
- Lepidoptera of French Antilles
- Butterflies of Asian Russia
- Butterflies from Indo China
- Butterflies of Turkey
- Moths of Jamaica
- Historic Moth illustrations
- "Lepidoptera". Integrated Taxonomic Information System.
- Caught Between the Pages: Treasures from the Franclemont Collection Online virtual exhibit featuring a selection of historic entomological writings and images from the Comstock Library of Entomology at Cornell University
- Japmoth Japanese moths. Access images via the numbers on the left.
- Literaturatenbank Free downloads






