Risk of Missed PRRS PCR Detection
Risks of false-negative results: real-time PCR testing for PRRS Virus
Introduction:
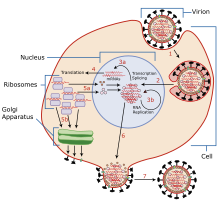
Porcine reproductive and respiratory syndrome virus (PRRS) is an RNA virus that causes reproductive and respiratory disease in swine. PRRS was first recognized in midwestern United States in the late 1980's.[1] Pork producers and veterinarians use vaccination, test-and-removal, and other farm-management practices as strategies to control spread of the virus. Although clinical signs of PRRSV infection are now well known, a number of other common respiratory and reproductive diseases may appear clinically similar which has made laboratory diagnostic testing important. Testing populations of pigs for presence of the virus requires taking blood or oral fluid[2] samples and sending them to a diagnostic lab.
History of PRRS Virus Testing
A brief overview below of PRRS diagnostic tests focuses only on tests which detect the virus itself or its genetic material from ante-mortem samples (from live animals). More comprehensive discussions of PRRS tests exist, which include tests that detect antibody to the virus. [3]
Virus Isolation (Culture)
Laboratory-based diagnostic tests have evolved significantly since initial discovery of the PRRS virus in the late 1980's. Initially viral culture was used to confirm PRRSV in serum or tissue samples. This process involves growing the virus in-vitro on cell lines over a period of 3-14 days or longer. If cytopathic effect is observed during culture, the culture is confirmed as the PRRS virus by direct fluorescent antibody or other confirmation method prior to reporting the sample as positive for presence of PRRSV.
Polymerase Chain Reaction (PCR)
Polymerase chain reaction (PCR) assays came into use in the mid to late 1990's. Reduced turnaround time (1-2 days) and improved sensitivity were gained from applying this new technology and it quickly became the predominant means of ante-mortem diagnosis as well as confirmation of viral culture. PCR was originally developed for DNA targets but the application of reverse-transcriptase enzymes RT-PCR allowed the method to extend to RNA targets.
Nested RT-PCR
Nested PCR is a 2-step approach which targets outer and inner portions of a sequence of DNA. Initial PCR assays used by diagnostic labs in the late 1990's tended to be nested assays as they showed improved sensitivity over non-nested PCR.[4] Because the nested PCR requires handling of amplified PCR products during testing, it suffers from false-positives if not done carefully.
Real-time PCR
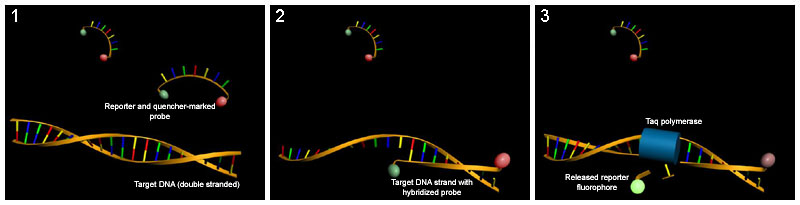
The innovation of flourescent-labeled DNA probes and high-throughput instrumentation to read them brought Real-time PCR to diagnostic labs. Real-time PCR assays offered as-good or better sensitivity than nested PCR, fast turnaround time in the lab, and lower rates of cross-contamination via closed-tube amplification. The specific chemistry used in diagnosis of PRRSV is commonly Taqman probes which emit light when they bind to the target sequence. Taqman probes focus on a small, roughly 100 base-pair, fragment of the PRRS genome.
PRRS Virus Mutation and Evolution
The RNA of any given RNA virus changes over time as the virus mutates due (in part) to the infidelity of the RNA polymerase it uses to replicate.[1] As an RNA virus with a 15 kb genome, PRRS mutates at a relatively high rate as it is transmitted from pig-pig over time.[5] The calculated rate of PRRSV nucleotide substitution is the highest reported so far for an RNA virus. It is estimated as 4.7-9.8 x 10-2 / site / year.[1]
Hazard to Detection from Viral Mutation
Though the real-time PCR tests used now have high sensitivity and specificity, these improvements have come with some hazards as well. Real-time PCR using Taq-man chemistry is prone to false-negative results when the virus mutates.[6] [7] [8] A false negative result occurs when a test fails to detect the presence of the virus. Studies have found that even a single base-pair change in the viral RNA under the labeled probe can cause failure of detection.[6] This specific source of the false-negative is not due to operator error on the part of the lab and is un-knowable at the time of testing.
The scenario that follows demonstrates how this hazard can result in risk to pork producers and laboratories:
→A strain of PRRS virus mutates during circulation within a herd. This strain spreads and becomes the predominant strain within the herd.
- →A veterinarian takes a random statistical sample of (let's say 30) animals within the herd, either in reaction to clinical signs or during routine health monitoring. Even though 30 animals are sampled, the mutant strain makes up the majority of PRRSV in all samples. The samples are submitted to the veterinary diagnostic lab for PRRS real-time PCR testing in order to get a quick diagnosis.
- →Mutation in viral RNA occurred in the small region(s) of the virus that the probe binds to, so the lab finds no signal and reports samples as 'negative' for absence of PRRS virus.
- →The veterinarian found evidence of other etiologic agents from related samples sent to the lab and assumes these must be the cause of clinical signs on farm. The animal owners are told that they can resume shipments of piglets from the sampled farm to another site where 5,000 PRRS-naive animals reside.
- →As the virus circulates in the new herd, more copies of the mutant virus are circulated. Further sampling continues to result in PRRS-negative results, and eventually clinical signs cause the veterinarian to explore other PRRSV test methods. The lab is contacted when other methods confirm that PRRSV is causing the signs on-farm.
- →At this point, the lab may attempt to isolate the virus (1 week at best), sequence the RNA from it (1 week), and analyze the sequence for miss-matches with the TaqMan probes used in the detection assay (1 week). Now the assay probe must be re-designed to allow detection of this new variant while still remaining sensitive to all other known strains. Optimization and validation of the re-designed assay can then take a substantial amount of time.
- →Meanwhile, the index case herd can no longer utilize PCR to determine which management options to use to control virus spread. Until the test is updated and implemented, the veterinarian cannot continue to use the diagnostic lab for testing, so samples are sent elsewhere and confidence in the laboratory is diminished.
This series of events is a frustrating and expensive event for veterinarian, diagnostic lab, and most of all the animal owners. Many labs in the United States each use their own real-time PCR method and communication among labs for new strains is inefficient at best, so information learned about the new strain is not leveraged across the many diagnostic labs. Due to the cost of testing and rapid detection of new virus introduction, PCR alone is often relied on as the primary screening tool. This over-reliance on a single diagnostic assay (of which none are 100% Sensitive and Specific) lead to longer interval of virus spread while the problem is being resolved.
Risk Management Options for Reliable Detection
Veterinarian and Producer Actions

Veterinarians can reduce the impact of this risk by paying close attention to clinical signs and utilizing more than one PRRS diagnostic test. Early communication with the lab is essential as often other methods can quickly be employed on existing samples. Given the rate of mutation for the PRRS virus, contingency plans should be developed for false-negative events that include selection of alternative labs and tests.
Diagnostic Laboratory Actions
Some laboratories have moved to the use of commercially-developed and maintained real-time PCR assays, which transfers the work of assay updates to a 3rd party albeit at a significant extra cost over in-house developed assays. In recent years, this strategy has allowed quicker response to new variants than would have been previously possible (unpublished). By commercial manufacturers leveraging assay updates across multiple labs, it is possible that detection capabilities for all client labs is improved. The flip-side of this approach is that if all labs run the same assay, there are limited options for veterinarians when an alternate assay is quickly needed.
Earlier technologies such as nested PCR are often called on during an investigation if the lab has retained the capability to perform them. By using these earlier methods the laboratory staff are more quickly able to identify the new strain due to their more robust detection capabilities.
References
- ^ a b c Murtaugh, Michael (2010). "The ever-expanding diversity of porcine reproductive and respiratory syndrome virus". Virus Research. 154: 18–30.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Prickett, John (2008). "Detection of Porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: a longitudinal study under experimental conditions". Journal of Veterinary Diagnostic Investigation. 20 (2): 156–163.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Yoon, KJ. "Diagnosis of PRRS Virus" (PDF). 2003 PRRS Compendium Producer Edition. www.pork.org. Retrieved 30 October 2011.
- ^ Christopher-Hennings, Jane (1995). "Detection of Porcine Reproductive and Respiratory Syndrome Virus in Boar Semen by PCR". Journal of Clinical Microbiology: 1730–1734.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Shi, Mang (2010). "Molecular epidemiology of PRRSV: A phylogenic perspective". Virus Research. 154: 7–17.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Klungthong, Chonticha (2010). "The impact of primer and probe-template mismatches on the sensitivity of pandemic influenza A/H1N1/2009 virus detection by real-time RT-PCR". Journal of Clinical Virology. 48: 91–95.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Pyne, Michael (2010). "Evaluation of the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 Test and Identification of Rare Polymorphisms Potentially Affecting Assay Peformance". Journal of Clinical Microbiology: 2852–2858.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Toplak, I. (ARTICLE IN PRESS). "Identification of genetically diverse sequence of PRRSV in Slovenia and the impact on the sensitivity of four molecular tests". Journal of Virological Methods. doi:10.1016/j.jviromet.2011.09.019.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help)
