Pearlite
| Steels |
|---|
 |
| Phases |
| Microstructures |
| Classes |
| Other iron-based materials |

This article needs additional citations for verification. (November 2010) |
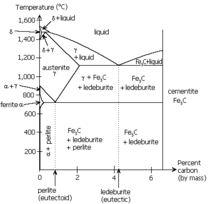
Pearlite is often said to be a two-phased, lamellar (or layered) structure composed of alternating layers of alpha-ferrite (88 wt%) and cementite (12%) that occurs in some steels and cast irons. In fact, the lamellar appearance is misleading since the individual lamellae within a colony are connected in three dimensions; a single colony is therefore an interpenetrating bicrystal of ferrite and cementite. In an iron-carbon alloy, during slow cooling pearlite forms by a eutectoid reaction as austenite is below 727 °C (1,341 °F) (the eutectoid temperature). Pearlite is a common microstructure occurring in many grades of steels.
The eutectoid composition of austenite is approximately 0.77% carbon; steel with less carbon content will contain a corresponding proportion of relatively pure ferrite crystallites that do not participate in the eutectoid reaction and cannot transform into pearlite. Likewise steels with higher carbon contents will form cementite before reaching the eutectoid point. The proportion of ferrite and cementite forming above the eutectoid point can be calculated from the iron/iron—carbide equilibrium phase diagram using the lever rule.
Pearlite was first identified by Henry Clifton Sorby and initially named sorbite, however the similarity of microstructure to nacre and especially the optical effect caused by the scale of the structure made the alternative name more popular.
Bainite is a similar structure with lamellae much smaller than the wavelength of visible light and thus lacks this pearlescent appearance. It is prepared by more rapid cooling. Unlike pearlite, whose formation involves the diffusion of all atoms, bainite grows by a displacive transformation mechanism.
Eutectoid steel
Eutectoid steel can in principle be transformed completely into pearlite; hypoeutectoid steels can also be completely pearlitic if transformed at a temperature below the normal eutectoid.[1] Pearlite can be hard and strong but is not particularly tough. It can be wear resistant because of a strong lamellar network of ferrite and cementite. Examples of applications include cutting tools, high strength wires, knives, chisels, and nails.
References
Further reading
- Comprehensive information on pearlite
- Introduction to Physical metallurgy by Sidney H. Avner, second edition, McGraw hill publications.
- Steels: Processing, Structure, and Performance, Chapter 15 High-Carbon Steels: Fully Pearlitic Microstructures and Applications by George Krauss, 2005 Edition, ASM International.
